Abstract
Background
Bone-targeted agents such as bisphosphonates and the RANKL antibody have revolutionised the care of patients with bone metastases. There has, however been increasing concern about the oral health of these patients and in particular osteonecrosis of the jaw (ONJ), especially with the increasing use of these agents at higher potencies for greater periods of time.
Methods
A review of the published data in PubMed and meeting abstracts was performed to examine incidence, risk factors, pathogenesis, clinical course and management of osteonecrosis of the jaw with focus on cancer patients treated with bone-targeted agents (BTA) for bone metastases. This manuscript takes the most frequent and pertinent questions raised by oncologists, dentists and oral and maxillofacial surgeons and tries to give a pragmatic overview of the literature.
Results
The incidence of ONJ varies depending on types of bone-targeted agents, duration of treatment and additional risk factors. The causes and pathogenesis of ONJ is not fully elucidated, however bone-targeted therapy induced impaired bone remodelling, microtrauma secondary to jaw activity, and oral bacterial infection seem to be important factors. Since the treatment options for ONJ are limited and not well established, preventive strategies have to be included in patients management.
Conclusions
Many unanswered questions remain about the optimal oral care of patients receiving bone-targeted agents. Prospective data collection will remedy this and help to provide practical guidelines for the management and treatment of those patients that require dental intervention.
Keywords: Bone metastasis, Bisphosphonate, ONJ, Bone targeted agent
1. Background
Few drugs have revolutionised the care of metastatic cancer patients as much as the bisphosphonates. These inhibitors of osteoclast function have undergone extensive clinical development over the last 20 years and have been shown to reduce hypercalcemia, bone pain, fractures, radiotherapy use, spinal cord compression and bone surgery in patients with bone metastases from a range of malignancies. They have also been shown to improve patient quality of life [1]. In addition, oncology patients will frequently receive bisphosphonates for osteoporosis, cancer treatment related bone loss and sometimes as an adjuvant therapy [2]. From an oncologist's standpoint these therapeutic benefits have been achieved with very few side effects when compared with most other systemic agents used in clinical practice. However, what has become evident is that as these increasingly potent bone-targeted agents are used for longer durations of time, less common side effects such as osteonecrosis of the jaw (ONJ) are presenting challenges not only to oncologists but also to the dental professionals who care for these patients.
This is becoming an increasingly important issue as the use of new agents such as the antibody to Receptor activator of nuclear factor kappa-B ligand (RANKL), denosumab, is also associated with this complication. Given that there is increasing awareness of this complication by oncologists, dental professionals and patients alike, we felt it was timely to offer a practical guide to assist all of us in the care of these patients. This article should not be viewed as a comprehensive review of the literature but more as a practical guide to assimilate the most up to date information.
2. Definition, diagnosis, incidence and risk factors for osteonecrosis of the jaw
2.1. What is osteonecrosis of the jaw and what other oral conditions can it look like?
While ONJ is also called bisphosphonate-related osteonecrosis of the jaw (BRONJ), in this article we will keep to the term ONJ as clearly BRONJ is a misnomer considering the condition is now described to also result from the use of non-bisphosphonate bone-targeted agents. Some are now calling this condition chemically-mediated ONJ, or chemo-necrosis of the jaw [3], [4]. The American Association of Oral and Maxillofacial Surgeons defines BRONJ as “exposed bone in the maxillofacial region that has persisted for more than 8 weeks, together with current or previous treatment with a bisphosphonate and without a history of radiation therapy to the jaw” [5]. This is a relatively rare clinical entity which was first described first in 2003 in cancer patients treated with potent bisphosphonates, following by increasing evidence on incidence, clinical pictures, staging, prophylaxis and treatment options [6]. ONJ can produce significant morbidity in affected patients, decreasing quality of life due to its chronic nature and relatively low recovery rate [7]. Approximately 30% of patients present with exposed bone and pain, and an additional 50% suffer from pain, gingival swelling and purulent discharge. In severe cases (about 20%), some patients can progress to pathologic fracture of the jaw, fistula formation, severe extended bone necrosis and an infection process in the soft tissues [8], [9]. Occasionally, pain in the jaw bone or tooth loosening may be the only symptom with no evidence of other clinical or radiological abnormalities [10].
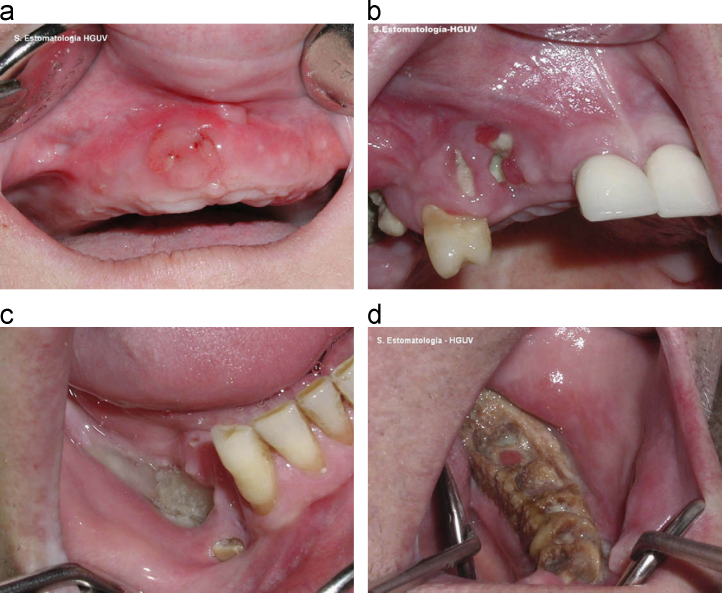
The diagnosis is made by a combination of clinical suspicion, clinical examination and radiological assessment. Clinical suspicion is going to be raised in those patients known to have been receiving treatment with highly potent bone-targeted agents. Clinical examination may reveal exposed jawbone, mucosal swelling, erythema, ulceration and tooth mobility as well as purulent discharge, intra- or extra-oral fistula and necrotic bone in the more advanced cases (Fig. 1). Panoramic and cross-sectional imaging will help determine the extent of necrosis and the presence of a sequestrum or osteomyelitis. Involvement of the mandible is more common than the maxilla, probably due to its more limited blood supply, and has been estimated as being involved in up to 70% of cases [11]. Radiology also helps to exclude other possible aetiologies such as unerupted teeth, bony cysts, sinusitis, temporo-mandibular joint (TMJ) pathology or metastatic disease [12], [13] (Table 1).
Fig. 1.
Stages of ONJ (adapted from Bagan et al. [78]) (with permission)): (a) an initial stage of osteonecrosis without visible necrotic bone. (b) Osteonecrosis of the upper jaw; stage 1. The patient had no symptoms. (c) Osteonecrosis of the jaw; stage 2. A more extensive area of necrosis and with symptoms. (d) Osteonecrosis of the jaw; stage 3. The patient had an extraoral (cutaneous) fistula.
Table 1.
Definition, diagnosis, risk factors of ONJ.
| Question | Answer | References |
|---|---|---|
| What is ONJ? | Exposed bone in the maxillofacial region that has persisted for more than 8 weeks, together with current or previous treatment with a bisphosphonate, without a history of radiation therapy to the jaws | [5] |
| What causes ONJ? | Bisphosphonate related factors: Impaired bone remodelling and [18], [19], [77], inhibition angiogenesis [20], [21] | |
| Patient related factors: Constant microtrauma due to jaw movement[14], Bone trauma due to surgical dental procedures [15], Oral microflora may inhibit healing process and super infect poorly healing wound [22], [23], [24] | ||
| Who gets ONJ? | Two main factors contribute to development of ONJ: Bone-targeted agent treatment and surgical procedures involving jaw bones | [28], [29], [30], [35], [36], [37] |
| Predisposing factors: Immunosuppressive status, increased patient's age, local oral inflammatory process, chronic corticosteroids use, concurrent chemotherapy, smoking | ||
| How do you diagnose ONJ? | Symptoms: pain, gingival swelling, purulent discharge, exposed, non-healing bone | [11] |
| Diagnostic procedure: Thorough dental examination, panoramic and cross-sectional imaging to determine the extent of necrosis and the presence of a sequestrum or osteomyelitis and to exclude other possible aetiologies for these manifestations. | ||
| Differential diagnosis of ONJ | Periodontal disease | [12], [13] |
| Gingivitis | ||
| Mucositis | ||
| Infectious osteomyelitis | ||
| Sinusitis | ||
| Periapical pathology caused by a carious infection | ||
| Temporomandibular joint disease | ||
| Osteoradionecrosis | ||
| Neuralgia-inducing cavitational osteonecrosis (NICO) | ||
| Bone tumors or metastases |
2.2. Causes and pathogenesis of osteonecrosis of the jaw
The mechanism for the development of ONJ is not fully understood, however, several hypotheses exist. The aetiology appears to be multi-factorial and includes bisphosphonate induced changes, patient related factors and dental surgical procedures.
Mandibular and maxillary bones, particularly the alveolar bone and periodontum, characterize by a high turnover rate throughout life as response to constant mechanical stress, tooth mobilisation, extraction or periodontal infections [14], [15].
Bone healing process requires involvement of multiple cytokines and growth factors including platelet-derived growth factors, bone morphogenic proteins, members of the transforming growth factor β superfamily, and members of the insulin-like growth factor family [16]. Human gingival fibroblast (HGF) and human periodontal ligament (HPDL) cells that present in periodontal tissue express RANKL and osteoprotegerin and provide balanced bone remodeling [17]. Bisphosphonate have a high affinity to bone tissue especially at high remodelling sites. Bone-targeted agents clearly interfere with bone remodelling through the osteoclast apoptosis [18], [19]. This results in accumulation of died bone tissue that cannot provide sufficient blood supply to overlying mucosa and lead to bone exposure. In addition, anti-angiogenic properties of bisphosphonates could contribute to vascular impairment [20], [21]. Mawardi et al. showed on the mouse bisphosphonate treated model that bacterial infection at the tooth extraction sites causes diminished keratinocyte-growth factor expression in gingival fibroblasts and therefore leads to a delay in wound-healing process [22]. Colonisation of oral microflora on exposed bone surface and surrounded mucosa might result in development of chronic infection and further impairment of wound healing [23], [24] (Table 1).
2.3. Incidence, prevalence and risk factors for osteonecrosis of the jaw
Oral or intravenous bisphosphonates use was found to be the most important risk factor for ONJ [25], [26].
Given the heterogeneous nature of the patient population treated by bone-targeted agents, there is considerable variability in the choice of agents, their dose, duration of therapy and schedule, and hence variability in the incidence and prevalence of ONJ. In general however, patients with metastatic bone disease receive considerably more treatment than patients treated for osteoporosis or cancer-treatment related bone loss and therefore it is the advanced cancer population that is more extensively studied. In addition, given the high incidence of bone involvement in metastatic breast, prostate cancers and multiple myeloma patients, together with the frequent use of bone-targeted agents in these patients for many years, it is not surprising that these are the patients at greatest risk for ONJ [27].
While the reported incidence of ONJ varies considerably, it tends to range between 1% and 12% based on results from particular case series, case-controlled and cohort studies [10] (Table 2). There is also variability in incidence across disease sites, with rates of approximately 0–6% in patients with prostate cancer, 1.2–2.9% in breast cancer patients and 2.4–9.9% in multiple myeloma patients. In other malignancies the incidence appears to be about 0–4% [28], [29]. It is this variability in incidence between cancer types that provides the greatest clues to risk factors for ONJ. These patients all receive high cumulative doses of potent bone-targeted agents for prolonged periods of time. The importance of potency of the bisphosphonate is reflected through the reported incidence with zoledronic acid (incidence of ONJ of 20% after 3 years of treatment), compared with pamidronate (7% after 4 years treatment), and oral bisphosphonates (0.00038–0.06%) [28], [29], [30], [31], [32]. The duration of bisphosphonate exposure is also important with cumulative rates of ONJ of 1% after 12 months of therapy rising to 11% after 4 years of therapy [28].
Table 2.
| General population | Metastatic breast, prostate cancer | Multiple myeloma | |
|---|---|---|---|
| Oral bisphosphonates | 0.00038–0.06% | Single reports | Single reports |
| Pamidronate | n/a | 0.5–1% | 1–4% |
| Zoledronic acid | 0.06% | 1.2–2.9% | 1–10% |
| Denosumab | 0% in 3 years | 2% | 1.1% |
As mentioned, ONJ is not just a result of bisphosphonate use. Denosumab use has also been associated with cases of ONJ. A recently published meta-analysis of 3 randomised trials compared the efficacy and safety of denosumab versus zoledronic acid in 5723 patients with metastatic breast cancer, prostate cancer or multiple myeloma, and prospectively evaluated the incidence of ONJ. Similar incidences of ONJ were observed with both treatments, with an incidence of 1.3% with zoledronic acid and 1.8% with denosumab. Interestingly the median time of drug exposure before ONJ was the same (14 months) in both groups [33]. However, it is important to realise that patients with any pre-existing dental problem were excluded from participating in the studies included in the meta-analysis and all patients received regular oral examinations. It is therefore likely, given the highly motivated nature of both the patients and the investigators in the trials, that the real world non-trial risks of ONJ would likely be higher.
While spontaneous cases of ONJ are reported, most (up to 80%) are related to recent dento-alveolar trauma, including tooth extractions, dental implant placement, periapical surgery and periodontal procedures involving osseous injury [9], [34].
The incidence of ONJ appears to increase for patients when they have additionally undergone an invasive dental procedure, and are approximately 9–50% in patients on intravenous bisphosphonate and 1–8% in patients receiving oral bisphosphonates. These procedures thus appear to result in an increase in the risk of developing ONJ by 5–21-fold [35]. It is important however to note that periodontal disease and bone exostosis (which in themselves increase the need for dental work) have also been reported to be significant independent risk factors for ONJ [28]. Other predisposing factors include: chronic steroid use in conjunction with bisphosphonates, particularly in the management of patients with multiple myeloma; and an immuno-compromised state secondary to concomitant chemotherapeutic agents and diabetes [25], [36], [37], [38].
There is a controversy for role of anti-angiogenic therapy on development of ONJ. Some reports support significant increase in ONJ incidence in patients treated with anti-angiogenic agents [39], [40]. Moreover, patients receiving anti-angiogenic agents including bevacizumab and sunitinib have doubled the risk of ONJ compared to patients who have not been exposed to such treatment following meta-analysis of denosumab trials [33]. On the other hand analysis of three large prospective trials of bevacizumab in metastatic breast cancer revealed 0.9–2.4% risk of ONJ in patients receiving concomitant bisphosphonates and bevacizumab, which was comparable with those receiving bisphosphonates alone [41]. In general, however, what is clear is that the more risk factors a patient has, the greater the risk of developing ONJ, which can reach 10–20% in patients with more than one risk factor [42].
A systematic review by Migliorati et al. showed variable prevalence of ONJ depending on study type, duration of follow up and type of bisphosphonate. While in studies with documented follow up prevalence was as high as 13.3%, in studies only 0.7%. Analysis of epidemiological studies resulted in prevalence 1.2%. As for type of bisphosphonate the overall prevalence for patients using zoledronic acid was 8.6%, for pamidronate 7.3%, and 21% for both. Prevalence was much higher in cancer patients (89%), and patients with multiple myeloma were affected more frequently than patients with solid cancers [43] (Table 3).
Table 3.
Prevalence of ONJ [43].
| Type of study | Type of bisphosphonate | ||
|---|---|---|---|
| Overall prevalence | 6.1% | Zoledronic acid | 8.6% |
| Documented Follow up | 13.3% | Pamidronate | 7.3% |
| No documented follow up | 0.7% | Both | 21% |
| Epidemiologic studies | 1.2% | Oral BP | 0.1–4% |
3. Preventive and prophylactic measures for patients about to start and those already established on bone-targeted therapies
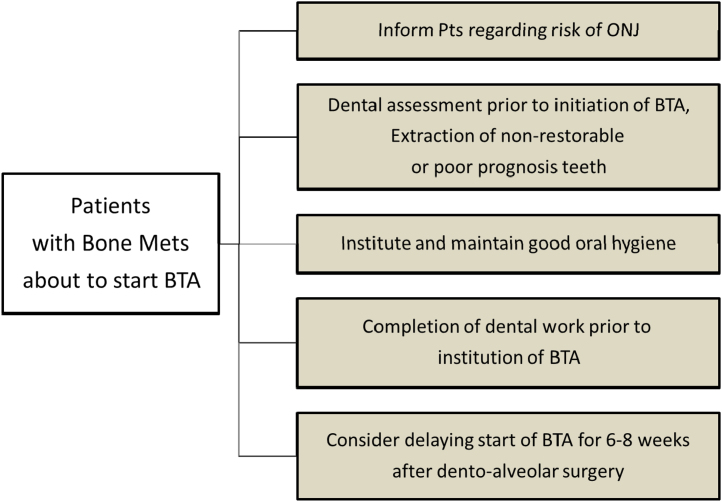
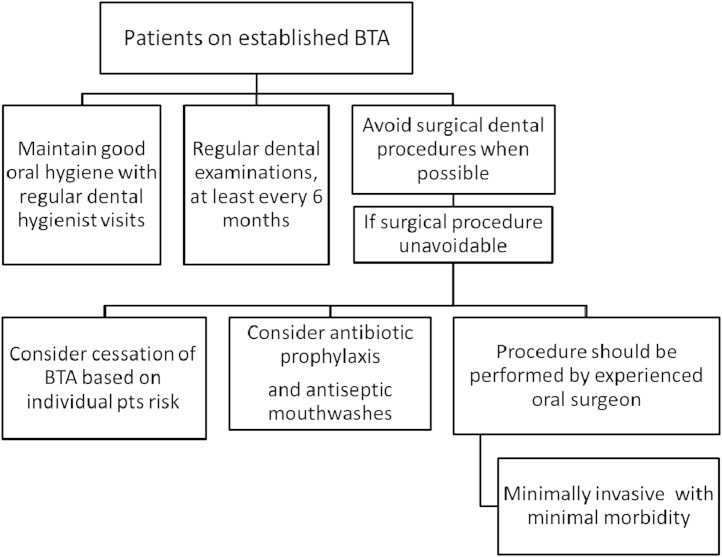
In this section we will try and address many of the frequently asked questions around preventive and prophylactic measures for patients either starting or already established on bone-targeted therapy (Fig. 2, Fig. 3). In view of the difficulties in treating ONJ, preventive strategies would seem to make the most sense. The efficacy of preventive strategies was investigated in two studies. Authors tried to compare the incidence of ONJ between the investigational group and a control group. The investigational group underwent preventive measures including dental assessment prior to bisphosphonate initiation so that any invasive dental procedures could be completed prior to initiation of bisphosphonate therapy. Bisphosphonate treatment was delayed for 6–8 weeks until complete wound healing after surgical procedures. Whenever possible minor dental interventions with preservation of dental roots, and avoidance of dento-alveolar surgery were preferred over tooth extraction and prophylactic antibiotics were used with invasive procedures. The retrospective control group of patients had received bisphosphonates before implementation of preventive measures. Incidence of ONJ was reduced by two-three times in patients on preventive measures [44], [45]. While this data is not from a randomized trial the authors did comment that given the effectiveness of ONJ preventive measures, performing a randomised study with a control group not receiving these measures would likely be considered unethical.
Fig. 2.
Preventive measures in patients with bone metastases about to start BTAs.
Fig. 3.
Prevention of ONJ in patients on established BTAs.
According to these studies and numerous recommendations, patients should be consulted about risk of developing ONJ prior to initiation of bone-targeted treatments and informed about the importance of maintaining their oral hygiene. Patients should be advised to have a dental examination, treatment existing dental problem and extraction of teeth that cannot be restored, preferably by a dental surgeon familiar with the risks of ONJ, prior to starting bisphosphonate therapy. Patients should also avoid dento-alveolar surgical procedures involving the mandibular or maxillary bone while actively receiving intravenous bisphosphonates, or for several months after completion of the therapy [5]. For patients receiving on-going bone-targeted therapies they too should continue to have frequent dental examinations. This allows the identification and early treatment of any dental disease, and the establishment of a suitable recall and oral hygiene program. Maintenance of good oral hygiene is essential to the prevention of dental disease and is therefore particularly important for this group of patients. Patients need to be kept aware of the ongoing risks of ONJ and their association with surgical dental procedures [46].
A meta-analysis of seven published single centre, non-randomised studies with a prospective interventional cohort and retrospective control group investigating the efficacy of preventive measures was presented recently at cancer-induced bone disease symposium in Lyon. It showed efficacy of preventive measures with a relative reduction of 68% of ONJ risk (RR 0.32; 95% CI 0.20–0.50; p<0.001).(Prevention of Osteonecrosis of the Jaw (ONJ) in Cancer Patients Receiving Bisphosphonates: Empty Promise or Effective strategy? Tim Van den Wyngaert et al., Antwerp University Hospital, Edegem, Belgium, 12th International Conference on Cancer-Induced Bone Disease, 15–17 November, 2012, poster P31, www.nature.com/bonekey)
Given that strategies to avoid ONJ are likely to be more effective than treating the condition once it occurs, it also seems sensible to reduce bisphosphonate exposure as much as possible. This can be accomplished by using less potent agents or less intensive infusion schedules, particularly in those patients who have been on bone-targeted agents for protracted periods of time. In multiple myeloma patients most guidelines recommend stopping bisphosphonates after two years. Recently emerging data in multiple myeloma patients suggests that the incidence of ONJ might be lowered by a reduced dosing schedule without affecting the incidence of skeletal-related events [47]. The risk of ONJ was eight-fold lower with the reduced schedule (monthly injection during the first year and every 3 months thereafter) than with the standard monthly schedule. However, this small retrospective study is not conclusive regarding the efficacy of less frequent dosing or its impact on the development of ONJ. However, a number of ongoing studies are investigating the efficacy of less intensive regimens of bisphosphonates in metastatic bone disease (NCT00320710, NCT00424983). The ZOOM trial, comparing a standard 4 weeks versus 12 weeks schedule of zoledronic acid for the prevention and delay of skeletal related events in metastatic breast cancer patients was presented at the ASCO meeting 2012 and showed equivalent results for these two regimens [48].
4. How do you manage patients on intravenous bone-targeted agents who require dental work?
For patients who are on IV bisphosphonate therapy and require dento-alveolar procedures there is a suggestion that prophylactic antibiotic use around the procedure may be helpful in reducing ONJ risk [45], [49]. A recent study by Lopez-Jornet et al. showed a statistically significant reduction of ONJ with pre and postoperative antibiotics for extraction procedures in an animal model [50]. If a surgical procedure is unavoidable, conservative surgical intervention is preferred in an attempt to minimize trauma to bone tissue. The procedure should be performed by experienced clinicians familiar with ONJ, ensuring that a minimally invasive, efficient procedure be performed with minimal morbidity.
5. Should patients on a bone-targeted agent requiring dental work stop their bone-targeted agent?
Recommendations on need of discontinuation of bisphosphonates in patients requiring dental work have not been created yet. Given the very long half-life of bisphosphonates in bone, with a 12-year terminal half-life even for oral agents like alendronate, effects of temporary cessation of the agents is questionable [31], [46]. On the other hand, temporary discontinuation of bisphosphonates may remove their acute toxic effect on soft tissue and could facilitate the healing process [5].
AAOMS recommends withholding oral bisphosphonates for up to 3 months before a surgical procedure and for up to 3 months thereafter [5]. This strategy also supported by a correlation of the level of the bone turnover marker, C-terminal telopeptide (CTX) with risk of development of ONJ. According to Marx et al. morning fasting serum CTX levels correlated with the duration of oral bisphosphonate use, with increased values for each month of a drug holiday when the oral bisphosphonate was discontinued, suggesting a recovery of bone remodelling during this time. A rising of CTX was associated with reduced risk of ONJ after surgical dental procedures [51]. On the other hand, other trials failed to show a correlation between level of biochemical markers (i.e. CTX, N-terminal telopeptide (NTX), or bone specific alkaline phosphatase) and risk of development of ONJ [52], [53], [54]. It however must be recognized that inter individual variability, gender, age, physical activity, and seasonal variation exist that can result in difficulty in interpreting these assays, hence more research is needed.
There is no conclusive data that stopping of intravenously given bisphosphonates or denosumab for 2–4-month prior to dental invasive procedure can reduce the risk of ONJ.
6. Management of patients with established osteonecrosis of the jaw
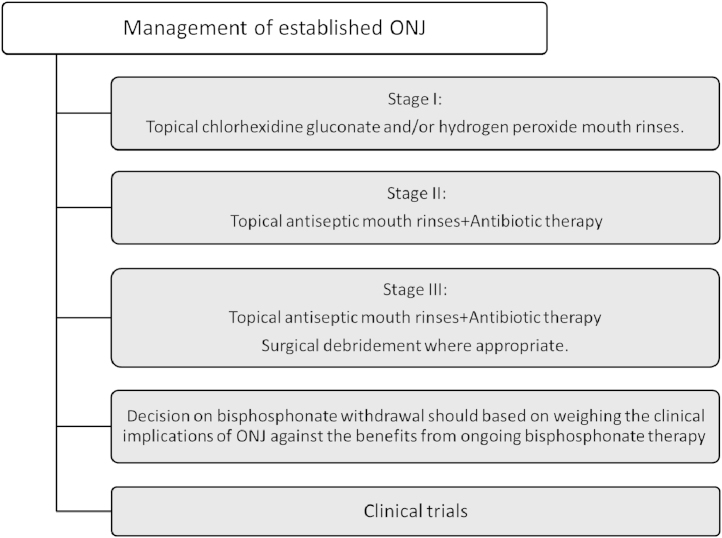
This section will deal with the care of patients on bone-targeted agents who then develop ONJ (Fig. 4). Although a number of clinical guidelines for management of patients with ONJ have been released by various oncology, oral surgical organizations and bisphosphonate manufacturers, there is no established gold standard, since most recommendations are based on case-control studies, retrospective analyses and expert opinions. For patients with established ONJ, treatment objectives are elimination of pain, control of infection in the soft and bone tissue, and minimization of the progression or occurrence of bone necrosis.
Fig. 4.
Management of established ONJ.
In general, patients with ONJ should be evaluated and managed by a team including an oral and maxillofacial surgeon and an oncologist [5], [46]. Several staging systems of ONJ have been developed by different dental and oncology organizations to help facilitate treatment decisions. The most useful system had been proposed by Ruggiero and subsequently revised by the American Association of Oral and Maxillofacial Surgeons (Table 4 and Fig. 1). According to this classification, Stage 0 defines patients presenting with non-specific symptoms such as tooth pain, sinus pain, and unexplained tooth mobility but without significant clinical findings on examination. For these patients conservative management with topical mouth rinses (chlorhexidine gluconate or hydrogen peroxide) and analgesia is recommended. This is to decrease and prevent further progression of infection in the exposed bone [5], [11].
Table 4.
Staging of ONJ [3].
| Stage | Clinical picture |
|---|---|
| Stage 0 | Tooth and jaw pain with no findings on examination, unexplained tooth mobility |
| Stage I | Asymptomatic exposed and necrotic bone without infection |
| Stage II | Exposed and necrotic bone with pain and infection |
| Stage III | Exposed and necrotic bone with pain and infection plus |
| Pathologic fracture or extra-oral fistula/communication or necrosis extending beyond the region of alveolar bone or oro-antral/oro-nasal communication |
Among patients with established infection in the bone or surrounding tissue (Stage II) antibiotic penicillin-based therapy in addition to mouthwash may result in healing in patients with minor ONJ lesions. However, a large proportion of cases tend to show chronically infected necrotic process in jaw bones with very limited response to any treatment. Prior to commencement of antimicrobial (penicillin-based) and antifungal therapy, wound and pus culture samples, including those for Actinomyces species should be taken [55], [56].
Generally, surgical debridement has been variably effective in eradicating necrotic bone and is not recommended for early stages of ONJ due to the concern of possible exacerbation of the necrotic process [57]. However, in patients with advanced process (Stage III) with pathological fracture, extra oral fistula, osteolysis extending to the base of the jaw or recurrent infections, complete removal of the necrotic bone and where possible immediate reconstruction in addition to systemic antibiotic treatment is indicated [58], [59].
Several authors reported successful outcome with surgical treatment of ONJ [9], [60], [61].
As alternative for conventional conservative surgery, laser applications at low intensity (low level laser therapy—LLLT) have been reported in the literature for the treatment of ONJ with promising results [54], [62]. Biostimulatory effects of laser improve reparative processes, increase inorganic matrix of bone and stimulate lymphatic and blood capillary growth, as well as having a bactericidal effect.
Hyperbaric oxygenation therapy has shown inconsistent results and is now under investigation in an ongoing randomised trial as an addition to surgical or non-surgical treatment [63]. As a result, its use is not presently recommended outside of clinical trials.
Pentoxifylline (blood viscosity reducer agent) and oral vitamin E has shown efficacy in a small case series [64].
Ozone (O3) therapy in the management of bone necrosis or in extractive sites during and after oral surgery in patients treated with bisphosphonate may stimulate cell proliferation and soft tissue healing resulting in alleviation of symptoms [6]. However, several case reports and small uncontrolled studies reported controversial efficacy of O3 gas formulation in addition to conventional in treatment of ONJ [65], [66]
In a phase I and II study, medical O3 has been shown to heal ONJ if antibiotic therapy (azithromycin) is administered 10 days prior to O3 oil formulation, where more than half of the patients showing a complete response with radiologic lesion disappearance following reconstruction of the oral tissue [67].
Teriparatide (synthetic peptide that corresponds to the N-terminal residues of human parathyroid hormone) therapy has been recommended to adjust the mechanisms of failed bone remodelling and have anabolic effects on osteoblasts. Teriparatide has been shown to help remove necrotic bone for new healthy bone to be laid down in order to resolve periodontal osseous defects [68]. Subramanian et al. [69] reported on the off-label use of teriparatide for ONJ in 6 patients treated with bisphosphonates for osteoporosis. In this report healing occurred in all cases within 5 months of initiating treatment. In contrast, Narvaez [70] reported a case of lack of response to teriparatide treatment. Of note, teriparatide is contraindicated in patients with osteosarcoma or metastatic bone disease following reports that osteosarcoma has occurred in rats and people who took the drug [71].
Other treatments such as administration of platelet rich plasma, and bone morphogenic proteins have been published as possible procedures in ONJ treatment in small case reports. Clearly the efficacy of these strategies needs to be established in additional prospective studies [72].
7. Should bisphosphonate therapy be discontinued if osteonecrosis of the jaw is present?
Decisions about to continue or stop bisphosphonates in face of established osteonecrosis of the jaw remains controversial.
Although bisphosphonates have not been shown to improve cancer-specific survival patients with metastatic bone disease patients significantly benefits from bisphosphonate treatment through reduced bone pain and a lower incidence of skeletal related events. Their use is therefore recommended in most international guidelines, starting once bone metastases are diagnosed [73], [74], [75], however, none give comprehensive recommendations about when to stop treatment.
Recently emerging data suggest that lower frequency of IV bisphosphonates in metastatic breast cancer has the same efficacy as monthly regimen [48]. ONJ on the other hand can be extremely symptomatic with a severely detrimental effect on quality of life.
A case-control study based on risk factors for ONJ confirms that ONJ is associated with the duration of bisphosphonate treatment. A higher risk of ONJ began within 2 years of bisphosphonate treatment and increased four-fold 2 years later, showing that even less potent bisphosphonates remain linked to ONJ after brief treatment therapy [25].
There are several reports that long term discontinuation of IV bisphosphonates in patients with ONJ may be beneficial in stabilizing established sites of osteonecrosis and provide improvement of clinical symptoms [5], [46]. However, there have been some cases of spontaneous resolution during ongoing monthly bisphosphonate therapy [28], [29]. Moreover, Wilde et al. [60] reported favorable outcome with surgical treatment of ONJ irrespective of whether bisphosphonates were discontinued or not. In another report, the patients who developed ONJ after dental procedures safely restarted bisphosphonate therapy, but those who developed ONJ without a predisposing cause were at increased risk of recurrence after initial healing, especially when these agents were reintroduced [76]. Since bisphosphonates are incorporated into the mineral matrix of bone, it is unknown as to whether or not stopping bisphosphonate therapy would be beneficial in managing ONJ [7]. Nevertheless, stopping the bisphosphonate would remove any acute influences on the periosteum and soft tissues, and could potentially improve the healing process. [5], [31].
Therefore decisions around bisphosphonate withdrawal and reintroduction after ONJ are complex and should be made using a multidisciplinary team including oncologist, oral and maxillo-fascial surgeon and patient and based on weighing the severity of ONJ symptoms against the benefits from ongoing bisphosphonate therapy including patients' overall prognosis and symptoms of bone metastases [46].
8. Discussion
Bone-targeted agents are established standard of care treatments for patients with metastatic bone disease. Generally they are well tolerated with few side effects, however, in recent years cases of ONJ have been reported as a rare but serious complication of treatment. In light of the palliative intent of bisphosphonate administration on the one hand and the serious implications of ONJ on patient's well-being and quality of life on the other, it has become an important issue for oncologists, dentists, and oral and maxillofacial surgeons. Given the increasingly widespread use of highly potent bone-targeted agents such as bisphosphonates and inhibitors of RANKL function, the prevalence of ONJ is likely to continue to increase.
We simply have to accept that while there is growing data on incidence and risk factors of ONJ, the current data about the prevention and treatment of ONJ is relatively poor and is based mainly on case reports, case-controlled series, retrospective studies and expert opinions. Ongoing prospective trials in metastatic and adjuvant (D-CARE trial, http://www.clinicaltrials.gov/ct2/show/NCT01077154) settings with accurate ONJ monitoring will help us answer many more questions about the prevention and management of this condition. In particular strategy looking at de-escalating regimens in patients with bone metastases that can reduce exposure of the jaw to bisphosphonates and therefore reduced risk of ONJ and improve patient care [48].
References
- 1.Ross J.R., Saunders Y., Edmonds P.M., Patel S., Wonderling D., Normand C. A systematic review of the role of bisphosphonates in metastatic disease. Health Technology Assessment (Winchester, England) 2004;8:1–176. doi: 10.3310/hta8040. [DOI] [PubMed] [Google Scholar]
- 2.Hadji P. Managing bone health with zoledronic acid: a review of randomized clinical study results. Climacteric: The Journal of the International Menopause Society. 2011;14:321–332. doi: 10.3109/13697137.2010.529966. [DOI] [PubMed] [Google Scholar]
- 3.Hegarty A., Georgakopolou E., Porter S. Bisphosphonate-related osteochemonecrosis of the jaws. British Journal of Hospital Medicine (London, England: 2005) 2008;69:158–162. doi: 10.12968/hmed.2008.69.3.28753. [DOI] [PubMed] [Google Scholar]
- 4.Magremanne M., Vervaet C., Dufrasne L., Declercq I., Legrand W., Daelemans P. Bisphosphonates and maxillo-mandibular osteo(chemo)necrosis. Revue de Stomatologie et de Chirurgie Maxillo-faciale. 2006;107:423–428. doi: 10.1016/s0035-1768(06)77081-4. [DOI] [PubMed] [Google Scholar]
- 5.Ruggiero S.L., Dodson T.B., Assael L.A., Landesberg R., Marx R.E., Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw-2009 update. Australian Endodontic Journal: The Journal of the Australian Society of Endodontology Inc. 2009;35:119–130. doi: 10.1111/j.1747-4477.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 6.Vescovi P., Nammour S. Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) therapy. A critical review. Minerva Stomatologica. 2010;59(181–203):4–13. [PubMed] [Google Scholar]
- 7.Ruggiero S., Gralow J., Marx R.E., Hoff A.O., Schubert M.M., Huryn J.M. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. Journal of Oncology Practice/American Society of Clinical Oncology. 2006;2:7–14. doi: 10.1200/jop.2006.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outcomes of bisphosphonate-related osteonecrosis of the jaw—importance of staging and management guidelines: a large single institutional update. Abstract no. 20526; 2008.
- 9.Jacobsen C., Metzler P., Obwegeser J., Zemann W., Gra Tz.K. Osteopathology of the jaw associated with bone resorption inhibitors: what have we learned in the last 8 years? Swiss Medical Weekly. 2012;142:0. doi: 10.4414/smw.2012.13605. [DOI] [PubMed] [Google Scholar]
- 10.Mehrotra B, Ruggiero S. Bisphosphonate complications including osteonecrosis of the jaw. Hematology/The Education Program of the American Society of Hematology American Society of Hematology Education Program. 2006;356–60:515. doi: 10.1182/asheducation-2006.1.356. [DOI] [PubMed] [Google Scholar]
- 11.Krueger C.D., West P.M., Sargent M., Lodolce A.E., Pickard A.S. Bisphosphonate-induced osteonecrosis of the jaw. The Annals of Pharmacotherapy. 2007;41:276–284. doi: 10.1345/aph.1H521. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi P., Pai P.S., Chaukar D.A., Gupta S., D'Cruz A.K. Bisphosphonate induced osteonecrosis of the jaw masquerading as tumor: a word of caution for oral surgeons and oncologists. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2010;36:541–545. doi: 10.1016/j.ejso.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Khosla S., Burr D., Cauley J., Dempster D.W., Ebeling P.R., Felsenberg D. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 14.Bonafe-Oliveira L., Faltin R.M., Arana-Chavez V.E. Ultrastructural and histochemical examination of alveolar bone at the pressure areas of rat molars submitted to continuous orthodontic force. European Journal of Oral Sciences. 2003;111:410–416. doi: 10.1034/j.1600-0722.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu M., Sasaki T., Ishihara A., Furuya R., Kawawa T. Bone wound healing after maxillary molar extraction in ovariectomized aged rats. Journal of Electron Microscopy. 1998;47:517–526. doi: 10.1093/oxfordjournals.jmicro.a023623. [DOI] [PubMed] [Google Scholar]
- 16.Linkhart T.A., Mohan S., Baylink D.J. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19:1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 17.Bertoldo F., Santini D., Lo Cascio V. Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nature Clinical Practice Oncology. 2007;4:711–721. doi: 10.1038/ncponc1000. [DOI] [PubMed] [Google Scholar]
- 18.Fleisch H. Bisphosphonates: mechanisms of action. Endocrine Reviews. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 19.Migliorati C.A., Siegel M.A., Elting L.S. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. The Lancet Oncology. 2006;7:508–514. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- 20.Vincenzi B., Napolitano A., Zoccoli A., Iuliani M., Pantano F., Papapietro N. Serum VEGF levels as predictive marker of bisphosphonate-related osteonecrosis of the jaw. Journal of Hematology & Oncology. 2012;5:56. doi: 10.1186/1756-8722-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood J., Bonjean K., Ruetz S., Bellahcene A., Devy L., Foidart J.M. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. The Journal of Pharmacology and Experimental Therapeutics. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 22.Mawardi H., Giro G., Kajiya M., Ohta K., Almazrooa S., Alshwaimi E. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. Journal of Dental Research. 2011;90:1339–1345. doi: 10.1177/0022034511420430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen T., Kunkel M., Springer E., Walter C, Weber A., Siegel E. Actinomycosis of the jaws—histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Archiv: An International Journal of Pathology. 2007;451:1009–1017. doi: 10.1007/s00428-007-0516-2. [DOI] [PubMed] [Google Scholar]
- 24.Sedghizadeh P.P., Kumar S.K., Gorur A., Schaudinn C., Shuler C.F., Costerton J.W. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. Journal of the American Dental Association (1939) 2009;140:1259–1265. doi: 10.14219/jada.archive.2009.0049. [DOI] [PubMed] [Google Scholar]
- 25.Barasch A., Cunha-Cruz J., Curro F.A., Hujoel P., Sung A.H., Vena D. Risk factors for osteonecrosis of the jaws: a case-control study from the CONDOR dental PBRN. Journal of Dental Research. 2011;90:439–444. doi: 10.1177/0022034510397196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fellows J.L., Rindal D.B., Barasch A., Gullion C.M., Rush W., Pihlstrom D.J. ONJ in two dental practice-based research network regions. Journal of Dental Research. 2011;90:433–438. doi: 10.1177/0022034510387795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. Journal of Cancer Research and Clinical Oncology. 2010;136:1117–1124. doi: 10.1007/s00432-010-0907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoff A.O., Toth B.B., Altundag K., Johnson M.M., Warneke C.L., Hu M. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bamias A., Kastritis E., Bamia C., Moulopoulos L.A., Melakopoulos I., Bozas G. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 30.Mavrokokki T., Cheng A., Stein B., Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. Journal of Oral and Maxillofacial Surgery: Official journal of the American Association of Oral and Maxillofacial Surgeons. 2007;65:415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 31.Woo S.B., Hellstein J.W., Kalmar J.R. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 32.Assael L.A. Oral bisphosphonates as a cause of bisphosphonate-related osteonecrosis of the jaws: clinical findings, assessment of risks, and preventive strategies. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67:35–43. doi: 10.1016/j.joms.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2011 doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 34.Thumbigere-Math V., Tu L., Huckabay S., Dudek A.Z., Lunos S., Basi D.L. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. American Journal of Clinical Oncology. 2012;35:386–392. doi: 10.1097/COC.0b013e3182155fcb. [DOI] [PubMed] [Google Scholar]
- 35.Lazarovici T.S., Mesilaty-Gross S., Vered I., Pariente C., Kanety H., Givol N. Serologic bone markers for predicting development of osteonecrosis of the jaw in patients receiving bisphosphonates. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2010;68:2241–2247. doi: 10.1016/j.joms.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 36.Khamaisi M., Regev E., Yarom N., Avni B., Leitersdorf E., Raz I. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. The Journal of Clinical Endocrinology and Metabolism. 2007;92:1172–1175. doi: 10.1210/jc.2006-2036. [DOI] [PubMed] [Google Scholar]
- 37.Marx R.E., Sawatari Y., Fortin M., Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Zahrowski JJ. Osteonecrosis of the jaws is associated with high-dose bisphosphonate treatment in patients with cancer. Journal of the American Dental Association (1939) 2010;141:887–888. doi: 10.14219/jada.archive.2010.0288. [DOI] [PubMed] [Google Scholar]
- 39.Christodoulou C., Pervena A., Klouvas G., Galani E., Falagas M.E., Tsakalos G. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 40.Aragon-Ching J.B., Ning Y.M., Chen C.C., Latham L., Guadagnini J.P., Gulley J.L. Higher incidence of Osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Investigation. 2009;27:221–226. doi: 10.1080/07357900802208608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarneri V., Miles D., Robert N., Dieras V., Glaspy J., Smith I. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Research and Treatment. 2010;122:181–188. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 42.Gebara S.N., Moubayed H. Risk of osteonecrosis of the jaw in cancer patients taking bisphosphonates. American Journal of Health-system Pharmacy: AJHP: Official Journal of the American Society of Health-System Pharmacists. 2009;66:1541–1547. doi: 10.2146/ajhp080251. [DOI] [PubMed] [Google Scholar]
- 43.Migliorati C.A., Woo S.B., Hewson I., Barasch A., Elting L.S., Spijkervet F.K. A systematic review of bisphosphonate osteonecrosis (BON) in cancer. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1099–1106. doi: 10.1007/s00520-010-0882-1. [DOI] [PubMed] [Google Scholar]
- 44.Dimopoulos M.A., Kastritis E., Bamia C., Melakopoulos I., Gika D., Roussou M. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2009;20:117–120. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 45.Ripamonti C.I., Maniezzo M., Campa T., Fagnoni E., Brunelli C., Saibene G. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2009;20:137–145. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 46.Fehm T., Felsenberg D., Krimmel M., Solomayer E., Wallwiener D., Hadjii P. Bisphosphonate-associated osteonecrosis of the jaw in breast cancer patients: recommendations for prevention and treatment. Breast. 2009;18:213–217. doi: 10.1016/j.breast.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Corso A., Varettoni M., Zappasodi P., Klersy C., Mangiacavalli S., Pica G. A different schedule of zoledronic acid can reduce the risk of the osteonecrosis of the jaw in patients with multiple myeloma. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007;21:1545–1548. doi: 10.1038/sj.leu.2404682. [DOI] [PubMed] [Google Scholar]
- 48.Amadori D., Aglietta M., Alessi B., Gianni L., Ibrahim T., Farina G. ZOOM: A prospective, randomized trial of zoledronic acid (ZOL; q 4 wk vs. q 12 wk) for long-term treatment in patients with bone-metastatic breast cancer (BC) after 1 yr of standard ZOL treatment. Journal of Clinical Oncology. 2012;30:9005. [Google Scholar]
- 49.Montefusco V., Gay F., Spina F., Miceli R., Maniezzo M., Teresa Ambrosini M. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leukemia & Lymphoma. 2008;49:2156–2162. doi: 10.1080/10428190802483778. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Jornet P., Camacho-Alonso F., Martinez-Canovas A., Molina-Minano F., Gomez-Garcia F., Vicente-Ortega V. Perioperative antibiotic regimen in rats treated with pamidronate plus dexamethasone and subjected to dental extraction: a study of the changes in the jaws. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2011;69:2488–2493. doi: 10.1016/j.joms.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 51.Marx R.E., Cillo J.E., Jr., Ulloa J.J. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2007;65:2397–2410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Kunchur R., Need A., Hughes T., Goss A. Clinical investigation of C-terminal cross-linking telopeptide test in prevention and management of bisphosphonate-associated osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67:1167–1173. doi: 10.1016/j.joms.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Morris P.G., Fazio M., Farooki A., Estilo C., Mallam D., Conlin A. Serum N-telopeptide and bone-specific alkaline phosphatase levels in patients with osteonecrosis of the jaw receiving bisphosphonates for bone metastases. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2012 doi: 10.1016/j.joms.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 54.Atalay B., Yalcin S., Emes Y., Aktas I., Aybar B., Issever H. Bisphosphonate-related osteonecrosis: laser-assisted surgical treatment or conventional surgery? Lasers in Medical Science. 2011;26:815–823. doi: 10.1007/s10103-011-0974-2. [DOI] [PubMed] [Google Scholar]
- 55.Naik N.H., Russo TA. Bisphosphonate-related osteonecrosis of the jaw: the role of actinomyces. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2009;49:1729–1732. doi: 10.1086/648075. [DOI] [PubMed] [Google Scholar]
- 56.Saussez S., Javadian R., Hupin C., Magremanne M., Chantrain G., Loeb I. Bisphosphonate-related osteonecrosis of the jaw and its associated risk factors: a Belgian case series. The Laryngoscope. 2009;119:323–329. doi: 10.1002/lary.20076. [DOI] [PubMed] [Google Scholar]
- 57.Lazarovici T.S., Yahalom R., Taicher S., Elad S., Hardan I., Yarom N. Bisphosphonate-related osteonecrosis of the jaws: a single-center study of 101 patients. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67:850–855. doi: 10.1016/j.joms.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Kademani D., Koka S., Lacy M.Q., Rajkumar S.V. Primary surgical therapy for osteonecrosis of the jaw secondary to bisphosphonate therapy. Mayo Clinic Proceedings Mayo Clinic. 2006;81:1100–1103. doi: 10.4065/81.8.1100. [DOI] [PubMed] [Google Scholar]
- 59.Carlson E.R., Basile J.D. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67:85–95. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Wilde F., Heufelder M., Winter K., Hendricks J., Frerich B., Schramm A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2011;111:153–163. doi: 10.1016/j.tripleo.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 61.Voss P.J., Joshi Oshero J., Kovalova-Muller A., Veigel Merino E.A., Sauerbier S., Al-Jamali J. Surgical treatment of bisphosphonate-associated osteonecrosis of the jaw: Technical report and follow up of 21 patients. Journal of Cranio-maxillo-facial Surgery: Official Publication of the European Association for Cranio-Maxillo-Facial Surgery. 2012 doi: 10.1016/j.jcms.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Vescovi P., Manfredi M., Merigo E., Meleti M., Fornaini C., Rocca J.P. Surgical approach with Er:YAG laser on osteonecrosis of the jaws (ONJ) in patients under bisphosphonate therapy (BPT) Lasers in Medical Science. 2010;25:101–113. doi: 10.1007/s10103-009-0687-y. [DOI] [PubMed] [Google Scholar]
- 63.Freiberger J.J. Utility of hyperbaric oxygen in treatment of bisphosphonate-related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67:96–106. doi: 10.1016/j.joms.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Epstein M.S., Wicknick F.W., Epstein J.B., Berenson J.R., Gorsky M. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2010;110:593–596. doi: 10.1016/j.tripleo.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 65.Agrillo A., Ungari C., Filiaci F., Priore P., Iannetti G. Ozone therapy in the treatment of avascular bisphosphonate-related jaw osteonecrosis. The Journal of Craniofacial Surgery. 2007;18:1071–1075. doi: 10.1097/scs.0b013e31857261f. [DOI] [PubMed] [Google Scholar]
- 66.Ripamonti C.I., Maniezzo M., Pessi M.A., Boldini S. Treatment of osteonecrosis of the jaw (ONJ) by medical ozone gas insufflation. A case report. Tumori. 2012;98:72e–75ee. doi: 10.1700/1125.12414. [DOI] [PubMed] [Google Scholar]
- 67.Ripamonti C.I., Cislaghi E., Mariani L., Maniezzo M. Efficacy and safety of medical ozone (O(3)) delivered in oil suspension applications for the treatment of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: Preliminary results of a phase I–II study. Oral oncology. 2011;47:185–190. doi: 10.1016/j.oraloncology.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Bashutski J.D., Eber R.M., Kinney J.S., Benavides E., Maitra S., Braun T.M. Teriparatide and osseous regeneration in the oral cavity. The New England Journal of Medicine. 2010;363:2396–2405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subramanian G., Cohen H.V., Quek S.Y. A model for the pathogenesis of bisphosphonate-associated osteonecrosis of the jaw and teriparatide's potential role in its resolution. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2011;112:744–753. doi: 10.1016/j.tripleo.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 70.Narvaez J. Lack of response to teriparatide therapy for bisphosphonate-associated osteonecrosis of the jaw: reply to Subramanian and Quek. Osteoporosis International: A Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2012. [DOI] [PubMed]
- 71.Miller C.S. Teriparatide's potential role in jaw bone related diseases. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2011;112:703–705. doi: 10.1016/j.tripleo.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Lee C.Y., David T., Nishime M. Use of platelet-rich plasma in the management of oral biphosphonate-associated osteonecrosis of the jaw: a report of 2 cases. The Journal of Oral Implantology. 2007;33:371–382. doi: 10.1563/1548-1336(2007)33[371:UOPPIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 73.Van Poznak C.H., Temin S., Yee G.C., Janjan N.A., Barlow W.E., Biermann J.S. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 74.Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar RN, et al. NCCN task force report: bone health in cancer care. Journal of the National Comprehensive Cancer Network 2009;7 (Suppl. 3):S1–32; quiz S3–5. [DOI] [PMC free article] [PubMed]
- 75.Berenson JR, Hillner BE, Kyle RA, Anderson K, Lipton A, Yee GC. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 76.Badros A., Terpos E., Katodritou E., Goloubeva O., Kastritis E., Verrou E. Natural history of osteonecrosis of the jaw in patients with multiple myeloma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26:5904–5909. doi: 10.1200/JCO.2008.16.9300. [DOI] [PubMed] [Google Scholar]
- 77.Van Beek E.R., Lowik C.W., Papapoulos S.E. Bisphosphonates suppress bone resorption by a direct effect on early osteoclast precursors without affecting the osteoclastogenic capacity of osteogenic cells: the role of protein geranylgeranylation in the action of nitrogen-containing bisphosphonates on osteoclast precursors. Bone. 2002;30:64–70. doi: 10.1016/s8756-3282(01)00655-x. [DOI] [PubMed] [Google Scholar]
- 78.Bagan J., Scully C., Sabater V., Jimenez Y. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): a concise update. Oral Oncology. 2009;45:551–554. doi: 10.1016/j.oraloncology.2009.01.002. [DOI] [PubMed] [Google Scholar]