Abstract
The bone microenvironment provides a fertile soil for cancer cells. It is therefore not surprising that the skeleton is a frequent site of cancer metastasis. It is believed that reciprocal interactions between tumour and bone cells, known as the “vicious cycle of bone metastasis” support the establishment and orchestrate the expansion of malignant cancers in bone. While the full range of molecular mechanisms of cancer metastasis to bone remain to be elucidated, recent research has deepened our understanding of the cell-mediated processes that may be involved in cancer cell survival and growth in bone. This review aims to address the importance of the bone microenvironment in skeletal cancer metastasis and discusses potential therapeutic implications of novel insights.
Keywords: Bone metastasis, Cancer, Bone microenvironment, Bone remodelling
1. Introduction
Bone metastases are a major cause of cancer-related pain and can result in pathological fractures, paralysis and life-threatening hypercalcaemia. Less than 20% of patients survive for five years after the discovery of bone metastasis [1], [2], [3], [4]. In other types of cancers, such as liver and lung malignancies, the incidence of bone metastasis has increased in recent years, possibly due to the effect of improved treatment regimens on life expectancy [5], [6].
Metastasis of tumour cells to bone depends on a complex cascade of events which includes the detachment of individual cancer cells from the primary tumour site; invasion into the vasculature; migration and adherence to distant capillaries within the bone; extravasation and initial survival within the new environment; proliferation to micrometastases; recruitment of blood supply to the tumour for further expansion; and invasion beyond the adjacent tissues [3], [4], [7]. The ability of cancer cells to survive and expand in the bone marrow cavity has long been based on the “seed and soil” theory: In 1889, Sir James Paget proposed that bone acts as a fertile environment (‘soil’) for cancer cell (‘seed’) colonization and growth [8]. Many years later, Mundy and colleagues greatly broadened our understanding of the mechanisms that govern the growth of bone metastases by developing a concept widely known as the “vicious cycle” [7], [9], [10], [11]. This theory elegantly explains how cancer metastases, once established in bone, modify their immediate environment to support their own survival and growth. Thus, tumour-derived factors such as parathyroid hormone-related protein (PTHrP) up-regulate the expression of Receptor Activator of Nuclear Factor KB Ligand (RANKL) by cells of the osteoblast lineage (i.e., osteoblast precursors, osteoblasts and osteocytes). RANKL then binds to the Receptor Activator of Nuclear Factor KB (RANK) on osteoclasts and osteoclast precursors to increase osteoclast recruitment and formation, and to activate bone resorption. Accelerated bone resorption then triggers the release of growth factors embedded in the bone matrix, which in turn act on cancer cells to promote their further growth [7], [10], [12] (Fig. 1). This model has been extremely useful in elucidating some of the mechanisms that support and maintain established cancer metastases in bone. It is, however, less clear how individual cancer cells survive and proliferate within the bone environment at the very early stages of colonisation, i.e., before reaching a critical mass that allows them to manipulate resident bone cells in a significant way. We would therefore predict that additional mechanisms are at work at the early stages of bone metastases that involve more direct signalling pathways than those described by the classical vicious pathway.
Fig. 1.
Schematic representation of the ‘vicious cycle’. Up-regulation of RANKL in bone cells and subsequent osteoclast activation is driven primarily by tumour-derived factors such as PTHrP and IL-6. Accelerated bone resorption then triggers the release of growth factors from the degraded bone matrix, which in turn promote further tumour growth.
Numerous animal studies have demonstrated beyond doubt that effective inhibition of osteoclastogenesis or osteoclast function significantly reduces metastatic tumour growth in bone [13], [14], [15], [16], [17], [18], [19], [20]. Likewise, clinical trials in patients with non-metastatic or metastatic cancers established that treatment with “anti-resorptive” agents such as bisphosphonates or the anti-RANKL antibody, denosumab, resulted in significant reductions in the incidence, progress or complications of bone metastases [21], [22], [23]. Despite these significant developments, complications of bone metastases still occur in up to 50% of patients even whilst receiving anti-resorptive therapy [1], [4], indicating that there are still significant unmet needs in the prevention and treatment of metastatic bone disease.
2. Types of bone metastasis
Bone metastases have generally been characterized as osteolytic or osteoblastic based on their radiographic appearance [1]. Osteolytic lesions are caused by increased osteoclast activity accompanied by a concomitant absolute or relative decrease in osteoblast number or activity. This results in net bone resorption [7], [24] with little or no associated bone repair. In contrast, osteoblastic lesions are characterized by abnormal bone formation around tumour cell foci, but this typically also co-exists with increased osteoclast activity. Thus, both types of cancer metastasis to bone are characterised by significantly accelerated bone resorption with the radiographic appearance depending on the concurrent levels of bone formation. These tumour-induced changes in bone metabolism can clinically be identified and monitored through the measurement of bone turnover markers, which correlate with both tumour burden and therapy-induced reductions in skeletal related events [1], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. Thus, the classification of metastatic bone lesions into osteolytic and osteoblastic represent no less than the two extremes of a continuum in which the normal bone remodelling process becomes dysfunctional. Furthermore, patients can present with both osteolytic and osteoblastic lesions, and in fact, many bone metastases are mixed in nature, containing both lytic and blastic elements [12]. For example, breast cancer predominantly causes osteolytic metastases but at least 20% of patients present with mixed osteolytic-osteosclerotic lesions [2]. Conversely, prostate cancer presents mostly with osteoblastic lesions although a concurrent increase in bone resorption invariably occurs [2], [4], [36]. In patients with advanced bone metastases, high circulating levels of bone resorption markers, such as the aminoterminal telopeptide of type I collagen (NTX), were seen regardless of whether the lesions were radiographically lytic, blastic or “mixed” [30], [37], [38]. This indicates that all types of bone metastases contain an element of osteoclast activation, and this has been confirmed histologically. The role of osteoclasts in the spectrum of metastatic bone lesions is also supported by the fact that anti-resorptive therapy effectively reduces skeletal related events independent of whether there is predominantly lytic or blastic metastatic bone disease [23], [39], [40].
Within the bone microenvironment, the establishment of a tumour thus results in a disruption of the normally well-coordinated coupling of osteoblast and osteoclast functions. The resulting abnormal and accelerated bone remodelling then offers a fertile soil for further tumour expansion. Therefore, when it comes to the understanding of the mechanisms that enable cancers to grow in bone, the role of the bone microenvironment and its manipulation by the cancer cannot be underestimated.
3. The bone microenvironment
The term ‘bone microenvironment’ attempts to describe a complex structural and biological system which contains both haematopoietic and mesenchymal cells of multiple lineages, a sinusoidal blood supply, the bone marrow stroma and the bone extracellular matrix. In the context of skeletal cancer metastases, the bone matrix serves as a rich source of growth factors, while a number of different cells types inside, or recruited to the bone marrow cavity function to orchestrate the bone-tumour interactions. The cells within the bone microenvironment include resident bone cells (osteoclasts, osteoblasts and osteocytes) as well as various other cell types such as myeloid and immune cells, platelets, bone marrow endothelial and haematopoietic cells and bone marrow-derived mesenchymal stem cells, all of which may engage with the metastatic process to varying degrees.
3.1. Role of the bone matrix
Over the past 30 years it has become apparent that the bone matrix is extremely rich in growth factors. Many of these, including TGFβ, IGFs, FGFs, PDGF and BMPs not only promote the growth of metastatic cancer cells in bone, but also increase the production and release of cytokines and other bone resorbing factors from tumour cells [1], [41]. Growth factors released by the bone matrix are able to change the phenotype of tumour cells to cause more aggressive metastatic lesions [3], [7]. To again use Paget's analogy: The bone ‘soil’ is ‘fertilized’ by matrix-derived growth factors to facilitate the growth of the cancer ‘seed’ [1], [3], [7]. These factors can be released into the bone microenvironment during bone remodelling [7], [12], [42], [43]. Physical properties of the bone matrix, its structure and local changes associated with remodelling activity, including hypoxia, acidosis, and high extracellular calcium concentrations, create an environment favourable for tumour cells and their growth [12], [44], [45]. Finally, the bone matrix contains numerous non-collagenous proteins (e.g., osteopontin, vitronectin) which are able to interact directly with adhesion molecules on cancer cells, commonly via RGD sequences, and thus alter cancer cell behaviour.
3.2. Bone remodelling and bone cells
Bone is continuously moulded, shaped and repaired through the actions of different bone cells [46]. During development and growth, the skeleton is built up to achieve its shape and size by the removal of bone from some sites and deposition (synthesis) of bone at other sites; this process is called bone modelling [47], [48]. Once the skeleton has reached its mature size and structure, another life-long process termed remodelling commences, resulting in the continuous replacement of ‘old’ bone by newly formed tissue in the same location. Bone remodelling generally occurs at a micro-scale throughout the skeleton, carried out by the coordinated activity of juxtaposed osteoblasts and osteoclasts, an entity also known as a ‘basic multicellular unit (BMU)’ [47].
During bone remodelling, osteoblasts and osteoclasts are in intimate contact with the bone marrow, from which their precursors are derived. The differentiation of osteoclasts from the macrophage/monocyte lineage and their subsequent activation initially result in bone resorption, either on the bone surface or by tunnelling into the bone matrix [49]. A reversal phase then follows and a cement line is deposited. During the final stages of bone remodelling, osteoblasts (cells of mesenchymal origin) lay down new bone matrix which subsequently becomes mineralised [42]. Osteocytes are cells derived from osteoblasts [50] which become embedded in the newly formed bone matrix. Bone surfaces then remain in a resting state of variable duration until the next remodelling cycle begins [48].
During adult life, balanced bone remodelling is the major process by which healthy bone structure and function are maintained. In the young adult, several million bone remodelling units work their way through the skeleton at any one time, resorbing old bone and replacing it with an equal amount of new bone, such that total bone mass remains unchanged. During aging (which for the skeleton starts around 40 years of age), bone remodelling becomes increasingly imbalanced, and a shift in favour of net bone resorption occurs [46], [48].
The bone remodelling process is under the control of osteoblasts which integrate the signalling input from systemic hormones, locally acting growth factors and cytokines, and mechanical stress. Formation of osteoclasts occurs through a sequence of events that includes proliferation, differentiation, fusion and activation [12], [43], [51]. These events are regulated via the RANKL/ RANK/OPG signalling system (see below) [49].
3.3. The RANKL/RANK/OPG system
The receptor activator of NF Kappa B (RANK) ligand (RANKL) belongs to the TNF super family and is expressed by several cell types within the bone environment, including osteoblasts, other cells of the osteoblast lineage and T-cells. RANKL has been identified as the key signal in the regulation of osteoclastogenesis and bone resorption [52], [53], [54], [55]. Specifically, RANKL binds to its receptor, RANK, a transmembrane signalling receptor expressed by haematopoietic osteoclast precursor cells [52] and induces their differentiation into functional, multinucleated osteoclasts. RANKL also promotes osteoclast activation and survival [1], [7]. Gene knockout experiments further reveal the physiological importance of RANK and RANKL and their interactions. Thus, mice deficient in either RANK or RANKL are phenotypically identical, each presenting with profound osteopetrosis and an absence of osteoclasts. These phenotypes clearly demonstrate the essential role of this receptor-ligand pair in bone modelling and remodelling [56], [57].
The RANK–RANKL system is further regulated through osteoprotegerin (OPG), which acts as a decoy receptor to RANKL, preventing RANKL from binding to RANK. Interestingly, OPG is expressed also by osteoblasts as a secreted soluble protein. Through its ability to block osteoclast differentiation and activation (i.e., bone resorption), OPG becomes an important counter-regulator of bone metabolism [54], [58]. When administered systemically, OPG has been shown to inhibit both physiologic and pathologic bone resorption in various animal models, including those of metastatic bone disease [15], [20], [58], [59], [60], [61]. However, it is the ratio of RANKL to OPG rather than the absolute levels of either that determines the level of osteoclastogenesis in vivo [54], [58].
Numerous osteotropic hormones and cytokines are able to influence the expression levels of both OPG and RANKL [62], [63]. Systemic factors such as parathyroid hormone (PTH), interleukins and tumour necrosis factor α (TNF-α) increase the osteoblastic expression of RANKL relative to that of OPG, thereby promoting osteoclast activity [7]. Conversely, treatment with OPG has been shown to effectively inhibit bone resorption in humans [64], [65]. Importantly, the same RANKL/RANK/OPG pathways are operational in humans and rodents.
4. The ‘vicious cycle’ of metastatic tumour growth in bone
The concept of a ‘vicious cycle’ supporting and maintaining metastatic tumour growth in bone was first introduced by Mundy and Guise in 1997 [66]. The model successfully explains how bone and cancer cells interact in a feed-forward loop to allow and perpetuate cancer cell growth within the bone microenvironment. In its essence, the model describes how tumour cells communicate with osteoblasts, which ultimately leads to osteoclast activation and accelerated bone resorption. This not only makes room for the cancer to grow, but also triggers the release of growth factors embedded into the degraded bone matrix. These growth factors then promote further tumour growth, resulting in the production of more pro-resorptive signals by the cancer (Fig. 1).
4.1. The vicious cycle
Factors secreted by tumour cells play a critical role in cancer bone metastasis. Thus, it has been well established that breast or prostate cancer cells are able to produce and release signalling molecules that have the potential to modulate normal bone remodelling. These include parathyroid hormone-related protein (PTHrP), interleukins 6 (IL-6), 8 (IL-8) and 11 (IL-11), as well as vascular endothelial growth factor (VEGF) [3], [12], [67], [68], [69], [70], [71]. Among these factors, the most extensively studied is PTHrP, which initially was identified as the causal factor in humoral hypercalcemia of malignancy (HMM) [69], [72], [73], [74], [75]. PTHrP shares a common receptor with parathyroid hormone (PTH) [76], and although the protein sequence of PTHrP is different from PTH, there is an approximately 70% sequence homology between the two hormones across the first 13 amino acids at the N-terminus, resulting in similar biological activities [77]. Tumour-derived PTHrP can indirectly activate osteoclastogenesis via osteoblasts by stimulating osteoblasts and stromal cells to increase RANKL and suppress OPG expression [78], [79], [80]. Mouse models of bone metastasis provide solid evidence that PTHrP plays a critical role in breast cancer bone metastasis [81] (Fig. 2). Blocking PTHrP with neutralizing antibodies reduced osteolytic lesions in the MDA-MB-231 mouse model [82]. Interestingly, however, patients with PTHrP-positive primary breast tumours were found to be at lower risk of developing bone metastasis and to have a better overall prognosis than patients with tumours that express no or little PTHrP [71], [83]. These intriguing results suggest that increased expression of PTHrP by breast cancer is correlated with a less invasive phenotype, indicating that PTHrP may have other effects on tumour cell behaviour which are independent of their local effects on bone following bone metastasis [39], [71].
Fig. 2.
Overexpressing PTHrP in MDA-MB-231 cells accelerates bone metastases. MDA/TβRIIΔcyt cells were created in MDA-MB-231 cells that expressed the dominant–negative of the TGF-β type II receptor rendered the human breast cancer cell line MDA-MB-231 unresponsive to TGF-β. MDA-MB-231 and MDA/TβRIIΔcyt cell clones that overexpress PTHrP (TβRIIΔcyt+PTHrP; two clones) or the empty vector (TβRIIΔcyt+pcDNA3.1zeo) were used. (A) Representative radiographs of hindlimbs from mice bearing two different TβRIIΔcyt+PTHrP clones or TβRIIΔcyt+pcDNA3.1zeo control 31 days after tumour inoculation. Osteolytic lesions are indicated by the arrows. (B) Osteolytic lesion number and area on radiographs as measured by computerized image analysis of forelimbs and hindlimbs. Respective tumour cells were inoculated on day 0. Values represent the mean±SEM (n=5) per group.
From Ref. [81] with permission from the Publisher.
Other factors able to stimulate osteoclast formation and subsequently osteolytic activity include interleukins 6, 8, 11, TNF-α, M-CSF and endothelial growth factor (VEGF) [12], [84]. IL-6, IL-11 and VEGF all increase osteoclast formation and activity via the RANK ligand pathway, while macrophage colony stimulating factor (M-CSF) and IL-8 act directly to stimulate human osteoclast formation [85], [86], [87].
Up-regulation of RANKL in osteoblasts and other cells of the osteoblast lineage leads to osteoclast activation and increased bone resorption. This has two effects: First, it removes existing bone, thus eliminating an important barrier to further tumour expansion. Second, and as mentioned above, the bone microenvironment is a fertile soil for metastatic tumour cells, given its abundance in growth factors and cytokines. Breakdown of the bone matrix during bone resorption results in the release of potent growth factors, which in turn stimulate tumour growth. Among the factors, TGF-β is of particular importance as it has been shown to increase the production of PTHrP in breast cancer cells [70], [81]. Preclinical studies indicate that blocking TGF-β signalling may have clinical benefits in patients with bone metastases [88], [89], [90], [91]. Likewise, various other studies suggest that targeting bone-derived IGF-I, PDGF and BMP and their associated signalling pathways may offer potential therapeutic value in the treatment of bone metastasis [92], [93], [94], [95], [96].
It is clear that disruption of the vicious cycle at any level should result in an inhibition of metastatic tumour growth, and in contrast, increased bone resorption is likely to enhance cancer growth in bone [7], [24].
4.2. Anti-resorptive treatments reduce metastatic tumour growth in bone
Xenograft models of malignant bone disease have provided clear evidence that inhibiting osteoclast activity and hence bone resorption strongly affects the ability of cancer cells to grow within the bone environment [13], [14], [15], [16], [17], [18], [19], [20]. Currently, two main classes of anti-resorptive treatments are available: Bisphosphonates and the anti-RANKL antibody, denosumab. While initially developed for the management of osteoporosis, these agents were subsequently found to also reduce skeletal-related events in patients with metastatic bone disease. Bisphosphonates have successfully been used in the treatment of malignant hypercalcemia and skeletal metastasis in breast and prostate cancers [18], [97]. Both animal and human studies have demonstrated that bisphosphonates not only reduce osteolysis and bone pain associated with cancer metastasis but also decrease skeletal tumour burden. One area of contention is whether these effects are solely due to inhibiting bone resorption, and thus through altering the “vicious cycle”, or whether any of these treatments have direct cytotoxic effects on cancer cells. As discussed below, bisphosphonates and OPG (or indeed denosumab) inhibit bone resorption by different mechanisms, and may therefore possess different anti-tumour potential. Earlier studies with OPG had already demonstrated a functional role for RANKL-induced osteoclastogenesis in humoral hypercalcemia of malignancy and solid malignancies of bone [13], [58] (Fig. 3). Beneficial effects of anti-resorptive treatments on malignant bone lesions have been reported across the continuum of lytic to sclerotic lesions for diverse tumour types including prostate, breast, lung and other epithelial tumours [12], [98], [99], [100], [101], [102].
Fig. 3.
Effect of OPG treatment on histomorphometric indices of skeletal Colon-26 tumour burden. OPG treatment (1 mg/kg and 3 mg/kg) significantly reduced the average tumour area at each dose. *, Significantly different from 0 mg/kg OPG. Data represent the means±SE (n=10 mice/group).
From Ref. [13] with permission from the Publisher.
4.2.1. Bisphosphonates
Bisphosphonates are analogues of the naturally occurring compound pyrophosphate (P–O–P) in which the oxygen in ‘P–O–P’ has been replaced by a carbon atom, resulting in a ‘P–C–P’ structure [103], [104], [105]. Bisphosphonates bind preferentially to bone minerals at sites of active bone resorption, where they are taken up by resorbing osteoclasts. Once within the cell, the newer nitrogen-containing bisphosphonates inhibit farnesyl pryrophosphate synthase and prevent protein prenylation, which interferes with normal cell metabolism and induces a profound decline in osteoclast-mediated bone resorption [104], [106], [107]. Some bisphosphonates (e.g., ibandronate) have also been shown to exert direct anti-tumour effects in vitro, albeit at micromolar, i.e., exceedingly high concentrations [108], [109]. However, since sequestered bisphosphonate can be released from the bone matrix during bone resorption, some cancer cells may indeed be exposed to relatively high BP concentrations in vivo. For example, alendronate is believed to reach concentrations of greater than 100 μM within the sealed zone of an osteoclast during bone resorption [110], potentially producing transient high levels that may affect cancer cells immediately adjacent to osteoclasts. Whether bisphosphonates do posses direct anti-tumour effects in vivo remains an open question.
Alternatively, it has been suggested that the anti-resorptive activity of bisphosphonates mediate an indirect anti-tumour effect via the vicious cycle. In a study comparing ibandronate and OPG in a breast cancer bone metastasis in mice, we found similar inhibition of tumour growth with both agents, suggesting that their common anti-resorptive actions are dominant in their anti-tumour effects [15] (Fig. 4).
Fig. 4.
Osteoprotegerin and ibandronate treatment completely inhibits the enlargement of osteolytic bone lesions. (A) Representative radiographs of osteolytic lesions in tibiae of nude mice before treatment (Day 10; a–c) or after treatment (Day 17; d–f) with vehicle (PBS) osteoprotegerin (OPG) or ibandronate (IBN). At day 10, small but distinct osteolytic lesions (arrows) are detected in the tibiae (a–c). The size of these osteolytic lesions in untreated bones is markedly increased 7 days later, at day 17 (e). In contrast, in all treated bones, increase in size of these lytic lesions is inhibited (e–f). (B) Effects of osteoprotegerin (OPG) and ibandronate (IBN) treatment on the progression of established osteolytic bone lesions. Data are mean±SD and n=10 in each group. *significantly different from vehicle-treated group at Day 17 (p<0.01), #different to Day 10 (p<0.01).
From Ref. [15] with permission from the Publisher.
For more than two decades, bisphosphonates have been used as highly effective therapies for the treatment of skeletal malignancies and the prevention of secondary complications [97], [111], [112], [113]. Anti-resorptive treatment of patients with early, non-metastatic breast cancer with clodronate has been reported to have beneficial effects on both the development of bone metastases and patient survival [38], [114], [115], [116], [117], [118]. Furthermore, oral clodronate, when given as adjuvant therapy over 5 years, was shown to significantly reduce the rate of bone metastasis in women with breast cancer receiving standard treatment [115], [119]. Although one clinical trial reported that clodronate had no effect on metastases and even a negative effect on survival [118], [120], bisphosphonates have been widely adopted in clinical practice [121], [122] based on further positive outcomes from studies with zoledronic acid [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134] and ibandronate [135], [136] suggesting that bisphosphonates limit the progression of breast or prostate cancer in bone and other tissues [104], [114].
Recent results from clinical trials (e.g., AZURE, ABCSG-12, ZO-FAST) suggest that zoledronic acid may have more pronounced effects on the prevention and treatment of breast cancer patients within a low-estrogen environment, i.e., in postmenopausal women [126], [127], [128], [129], [130], [131], [132], [133], [134]. This effect is likely due to increases in local and systemic bone resorption in the setting of sex hormone deficiency, as high bone turnover is potentially associated with cancer metastasis (see 4.3 below). In addition to its effects on bone resorption, zoledronic acid may or may not have direct anticancer activity. This, however, is a complex question requiring further research. Indeed, clinical data [130], [132], [133], [137] suggest that both hormone suppression and a reduction in bone turnover may be required to achieve sufficient suppression of dormant micrometastases in patients with early-stage breast cancer in the menopausal women.
4.2.2. Anti-RANKL treatments
As a decoy receptor for RANKL, OPG has potent anti-resorptive effects without direct cytotoxic actions [13], [20], [98]. Binding of RANKL to its cognate receptor RANK on the surface of osteoclast precursor cells is essential for osteoclast differentiation. By sequestering RANKL, OPG inhibits osteoclastogenesis and thus bone resorption in vitro and in vivo [55], [56], [58], [138], [139]. In clinical trials, recombinant OPG constructs and anti-RANKL antibodies (denosumab) were demonstrated to reduce bone resorption effectively in patients with multiple myeloma or bone metastasis from breast cancer[140], [141]. In prostate cancer, treatment with denosumab has been reported to delay the appearance of bone metastases [142]. Further studies demonstrated that denosumab significantly decreased skeletal complications and reduced bone pain [23]. Interestingly, a recent paper highlights the bone-independent role of RANKL in mammary gland development in mice, where it appears to mediate progesterone-induced proliferation. This data implies that denosumab may be effective in directly targeting subtypes of breast and prostate cancers that express RANKL [143]. Both bisphosphonates and denosumab have been shown to be effective in reducing cancer-induced bone pain [34], [144], [145]. It is likely that these effects, too, are related to the strong anti-resorptive activity of these agents.
4.3. High bone turnover is causally associated with cancer metastasis
While many studies demonstrated that blocking bone resorption inhibits or even prevents the establishment and growth of tumour cells in the bone environment, investigations into the effects of accelerated bone resorption on tumour growth are scarce. One study reported enhanced cancer cell growth in bone following ovariectomy [146]. Other groups found faster tumour growth during treatment with G-CSF [147] or 17-allylamino-17-demethoxygeldanamycin (17-AAG) [148], although whether the effects on tumour growth were caused by an increase in bone resorption or mediated via other effects related to the bone marrow environment remained uncertain.
Clinical studies have reported that cancer patients with high levels of bone resorption at baseline or on treatment are at higher risk for adverse clinical outcomes, such as SREs or tumour progression [26], [27], [28], [29], [30], [31], [32], [37], [149], [150], [151], [152], [153], [154]. In this context, it is interesting to note that calcium and/or vitamin D deficiency have their own and well established effects on bone turnover. Both conditions, which are common in the general but particularly in the older population, are often associated with hyperparathyroidism and, consequently, accelerated bone turnover. Of note, vitamin D deficiency has been identified in epidemiological studies as a risk factor for breast and prostate cancer progression [31], [32], [155], [156], [157]. However, very little was known about the effects of calcium deficiency on skeletal cancer progression.
Over the past years, we have therefore investigated the complex relationship between tumour growth in bone, vitamin D or calcium deficiency, and bone turnover. To this aim, we first created a model of calcium deficiency by restricting dietary calcium intake in young growing nude mice [158]. Within 3 days, these mice develop secondary hyperparathyroidism (Table 1) and accelerated bone turnover, resulting in significant bone loss (Table 2). Using this model, we were able to demonstrate that calcium deficiency in mice significantly stimulated the growth of two human breast cancer cell lines (MDA-MB-231 and MCF-7) implanted intra-tibially into bone (Fig. 5) [158], [159]. Since vitamin D is a major regulator of calcium homeostasis, we proceeded to develop a rodent model of vitamin D deficiency [60]. After weaning, 3-week-old nude mice were provided with either normal chow (1000 IU/kg cholecalciferol) or chow deficient in vitamin D. Mice on the latter diet developed marked vitamin D-deficiency within 6 weeks, as indicated by serum 25-hydroxyvitamin D3 levels of less than 20 nmol/l (normal: >100 nmol/l) (Fig. 6A). Similar to calcium deficiency, these mice developed secondary hyperparathyroidism and accelerated bone resorption as indicated by increased serum bone resorption and formation markers (Fig. 6B and C). Importantly, vitamin D deficiency significantly stimulated skeletal tumour growth in a number of different cancer models, including human breast (MDA-MB-231 cells [60], MCF-7 cells [160] and prostate cancer (PC3 cells) (Fig. 7) [24], [161].
Table 1.
Levels of serum calcium, parathyroid hormone and bone markers in mice maintained on a normal or a low calcium diet.
| Serum assay | Normal-Ca | Low-Ca |
|---|---|---|
| Day 0 (n=5) | ||
| Calcium (mmol/l) | 2.22±0.04 | 2.11±0.02* |
| PTH (pg/ml) | 31.01±4.50 | 63.13±16.99* |
| mTRAP5b (b U/l) | 8.66±0.61 | 11.78±1.17* |
| Osteocalcin (ng/ml) | 179.24±13.92 | 267.81±16.91* |
PTH, serum intact parathyroid hormone; mTRAP5b, serum mouse tartrate-resistant acid phosphatase 5b; OPG, osteoprotegerin.
Data are expressed as mean±SE.
p<0.05 v.s. Normal-Ca.
From Ref. [158] with permission from the Publisher.
Table 2.
Bone histomorphometry of the tibiae of mice maintained on a normal or low calcium diet.
| Normal-Ca | Low-Ca | |
|---|---|---|
| BV/TV (%) | 11.67±0.47 | 9.03±0.62* |
| No/BS | 7.19±0.23 | 9.33±0.35* |
| Oc.S/BS (%) | 40.41±1.46 | 52.68±1.53* |
| Ob.S/BS (%) | 24.60±1.37 | 38.03±2.31* |
BV/TV: Bone volume % tissue volume.
N.Oc/BS: Osteoclast number per mm bone surface.
Oc.S/BS: Osteoclast surface % bone surface.
Ob.S/BS: Osteoblast surface % bone surface.
Data are expressed as mean±SE, * p<0.05 v.s. Normal-Ca, n=5/group. From Ref. [158] with permission from the publisher.
Fig. 5.
Low dietary calcium promotes breast cancer growth in bone. Mice fed a low calcium diet and injected intratibially with breast cancer MDA-MB-231 cells develop larger lytic lesions (left and centre) and larger tumours (right) compared to mice on a normal diet. *p<0.01.
From Ref. [158] with permission from the Publisher.
Fig. 6.
Biochemical assessment of mice receiving vitamin D deficient or vitamin D sufficient diets. (A) Plasma 25(OH)D levels are profoundly reduced at 6 and 11 weeks. (B and C) Plasma levels of PINP and TRAcP5b were significantly increased in vitamin D deficient mice at week 6. At week 11, plasma PINP levels were still significantly higher in vitamin D deficient compared to vitamin D sufficient mice. There was no difference between TRAcP5b levels.
Data are shown as mean±SD for group sizes of n =9.
*, P<0.05, **, P<0.01, compared to vitamin D sufficient mice.
From Ref. [161] with permission from the Publisher.
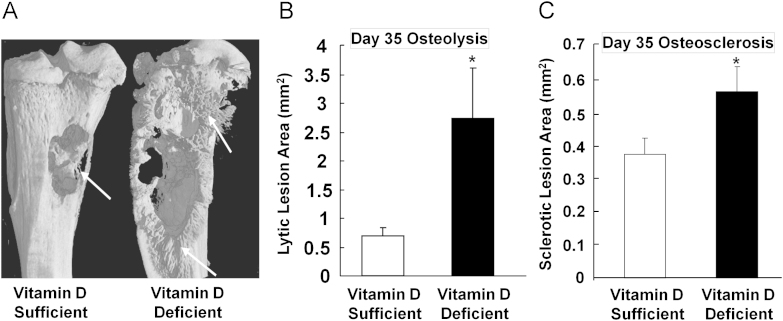
Fig. 7.
Radiographic assessment of osteolytic and osteosclerotic lesions in vitamin D sufficient and vitamin D deficient mice. Vitamin D deficient mice had developed significantly larger osteolytic (B) and osteosclerotic lesions (C) than vitamin D sufficient mice (arrows indicate sclerotic lesions, (A)), when implanted with prostate cancer PC-3 cell into tibiae of mice.
Data are shown as mean±SE for group sizes of n =9.
*, P<0.05, compared to vitamin D sufficient mice.
From Ref. [161] with permission from the Publisher.
In the same experiments, we inhibited bone resorption by administration of OPG to provide further evidence that accelerated bone resorption was indeed responsible for the enhanced tumour growth. Mice on OPG maintained normal calcium levels at the expense of further increases in PTH levels. However, tumour growth was significantly reduced or even abolished by OPG treatment independent of whether the animals were fed a normal diet, or a chow deficient in either calcium or vitamin D [24], [60], [158], [159], [160], [161]. In contrast, when breast or prostate cancer cells were implanted in the subcutaneous soft tissues away from bone, tumours grew similarly in all test groups (controls and calcium or vitamin D deficient mice), indicating that the effects of vitamin D or calcium deficiency on skeletal tumour growth are not systemic but related to changes in the bone microenvironment [24], [60], [158], [159], [160], [161]. Taken together, it seems clear that accelerated bone turnover, and particularly increased bone resorption are the dominant factors in promoting breast and prostate cancer growth in bone. These results are consistent with the concept that the enhancement of a “vicious cycle” by increased bone resorption supports tumour growth in bone [7], [10], [12], which further lays a solid ground for anti-resorptive treatment targeting the bone microenvironment for patients who have bone metastasis.
These studies directly link vitamin D deficiency to enhanced tumour expansion in bone metastatic growth, providing support for a causal association between low vitamin D status and enhanced breast and prostate cancer progression as observed in clinical observational studies. These results also provide a clinical and therapeutic rational for maintaining vitamin D sufficiency, or correcting vitamin D deficiency in patients with breast or prostate cancer-induced bone metastases.
5. The role of other bone marrow cells in metastatic cancer growth
Other cell types also that take part in the regulation of the bone microenvironment, including myeloid and immune cells (T cells), platelets, bone marrow endothelial and haematopoietic cells, as well as bone marrow-derived mesenchymal stem cells [3], [162]. Some of these cells are likely to participate in the creation of the pre-metastatic niche [3], [162].
Haematopoietic cells (other than osteoclasts) have the ability to potentially affect bone metabolism, and in particular bone resorption. For example, T cells produce osteoclast-activating factors such as RANKL, tumour necrosis factor (TNF) and TGF-β [91], [163], [164], It is via this link that these cells may influence cancer growth in bone. Furthermore, tumour cells are able to activate platelets to release lysophosphatidic acid (LPA), which in turn promotes breast cancer growth and skeletal metastasis in mice via production of IL-6 and IL-8, again potentially augmenting the vicious cycle [67].
In addition, myeloid-derived suppressor cells (MDSCs), platelets, bone marrow endothelial and haematopoietic cells as well as bone marrow-derived mesenchymal stem cells may all be involved in tumour neovascularisation [162]. These cells may interact with other bone cells at various levels and participate in the process of bone metastasis [3], [162]. While most of these events affect tumour growth by changing the bone microenvironment, some may have additional effects on cancer cell metastasis by co-operating with osteoblasts, osteocytes and osteoclasts in creating what is known as the pre-metastatic niche.
5.1. The pre-metastatic niche
The vicious cycle, with its associated changes in the bone microenvironment, has been extremely useful in elucidating some of the mechanisms that support established cancer metastases in bone [7], [8]. However, being mono-directional and depending on three different cell types to become, and remain activated, the model is less suitable to explain tumour growth at early stages of the metastatic process. It is conceivable that such growth kinetics warrant additional pathways in the form of amplifying elements, which would initiate, sustain and accelerate cell growth and expansion within the bone environment. Different modes of action to achieve amplification could involve direct communication between tumour cells and other bone cells including osteoclasts, osteoblasts and other bone marrow cells, via the various signalling pathways.
Recent advances in preclinical melanoma and lung cancer studies have demonstrated that the bone microenvironment may act as a pre-metastatic niche, through which the primary tumour is able to prime distant organs to become receptive to metastasising tumour cells early during tumourigenesis. For example, vascular endothelial growth factor receptor 1 (VEGFR1)-positive bone marrow-derived haematopoietic progenitor cells are able to travel to the sites of future metastasis before tumour cell arrival to facilitate tumour cell metastasis by increasing production of fibronectin or inflammatory chemoattractants in tumour target sites [165], [166], [167], [168]. Bone is rich in the chemokine SDF1 and its neutralisation has been reported to reduce prostate cancer metastasis to bone [169].
In the context of the bone microenvironment, the pre-metastatic niche may function via endocrine-like actions. Perhaps similar to the so-called priming in organs such as lung, primary tumours may be able to set up a pre-conditioning microenvironment through the production of circulating factors, which signal to various cells in the bone microenvironment. In this way the primary tumour could make the bone microenvironment conducive to tumour localization and colonization. There are a few tumour derived factors such as PTHrP [71], [170], heparanase [171] and osteopontin [172], [173] which have been reported to increase bone resorption and promote tumour formation. Intriguingly, matrix metalloproteinase (MMP) production from osteoclasts can also support prostate cancer skeletal metastasis [174]. However, the concept of the pre-metastatic niche itself as a potential therapeutic target requires further investigation but is provoking interest in the field.
Furthermore, the possibility of direct crosstalk between tumour cells and osteoblasts has not been well explored. Direct interactions, between these cells could short-cut the vicious cycle and also provide mechanisms relevant to initiation and/or amplification of cancer growth in bone.
Acknowledgements
This work has been supported in part by the following grants: Prostate Cancer Foundation of Australia (PCFA) (M.J.S, Y.Z, H.Z, C.R.D), University of Sydney Cancer Research Fund (M.J.S), National Health and Medical Research Council, Australia (NHMRC) (R.L.S, Program Grant 535903 and Fellowship 427601; C.R.D, M.J.S, and H.Z, Project Grant 352332; Y.Z, Early Career Fellowship 596870), Australian Cancer Research Foundation (R.L.S), RT Hall Trust (R.L.S), Petre Foundation (R.L.S).
Contributor Information
Yu Zheng, Email: yzheng@anzac.edu.au.
Markus J. Seibel, Email: markus.seibel@sydney.edu.au.
References
- 1.Roodman GD. Mechanisms of bone metastasis. New England Journal of Medicine. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treatment Reviews. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 3.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Natural Review of Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipton A, Berenson JR, Body JJ, Boyce BF, Bruland OS, Carducci MA. Advances in treating metastatic bone cancer: summary statement for the First Cambridge Conference. Clinical Cancer Research. 2006;12:6209s–6212ss. doi: 10.1158/1078-0432.CCR-06-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukutomi M, Yokota M, Chuman H, Harada H, Zaitsu Y, Funakoshi A. Increased incidence of bone metastases in hepatocellular carcinoma. European Journal of Gastroenterology and Hepatology. 2001;13:1083–1088. doi: 10.1097/00042737-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Husaini HA, Wheatley-Price P, Clemons M, Shepherd FA. Prevention and management of bone metastases in lung cancer a review. Journal of Thoracic Oncology. 2009;4:251–259. doi: 10.1097/JTO.0b013e31819518fc. [DOI] [PubMed] [Google Scholar]
- 7.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Natural Review of Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 8.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Natural Review of Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 9.Guise TA. The vicious cycle of bone metastases. Journal of Musculoskeletal Neuronal Interaction. 2002;2:570–572. [PubMed] [Google Scholar]
- 10.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochemical and Biophysical Research Communications. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80:1546–1556. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clinical Cancer Research. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 13.Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ. Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Research. 2001;61:4432–4436. [PubMed] [Google Scholar]
- 14.Neudert M, Fischer C, Krempien B, Bauss F, Seibel MJ. Site-specific human breast cancer (MDA-MB-231) metastases in nude rats: model characterisation and in vivo effects of ibandronate on tumour growth. International Journal of Cancer. 2003;107:468–477. doi: 10.1002/ijc.11397. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Zhou H, Brennan K, Blair JM, Modzelewski JR, Seibel MJ. Inhibition of bone resorption, rather than direct cytotoxicity, mediates the anti-tumour actions of ibandronate and osteoprotegerin in a murine model of breast cancer bone metastasis. Bone. 2007;40:471–478. doi: 10.1016/j.bone.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Guise TA, Chirgwin JM. Role of bisphosphonates in prostate cancer bone metastases. Seminars in Oncology. 2003;30:717–723. doi: 10.1016/s0093-7754(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 17.Hiraga T, Williams PJ, Mundy GR, Yoneda T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Research. 2001;61:4418–4424. [PubMed] [Google Scholar]
- 18.Mundy GR, Yoneda T, Hiraga T. Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment. Seminars in Oncology. 2001;28:35–44. doi: 10.1016/s0093-7754(01)90263-5. [DOI] [PubMed] [Google Scholar]
- 19.Yoneda T, Sasaki A, Dunstan C, Williams PJ, Bauss F, De Clerck YA. Inhibition of osteolytic bone metastasis of breast cancer by combined treatment with the bisphosphonate ibandronate and tissue inhibitor of the matrix metalloproteinase-2. Journal of Clinical Investigation. 1997;99:2509–2517. doi: 10.1172/JCI119435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. Journal of Clinical Investigation. 2001;107:1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. Journal of Bone and Mineral Research. 2009;25:440–446. doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 22.Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. Journal of Urology. 2009;182 doi: 10.1016/j.juro.2009.04.023. 509-15; discussion 15-6. [DOI] [PubMed] [Google Scholar]
- 23.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. Journal of Clinical Oncology. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 24.Ooi LL, Zheng Y, Stalgis-Bilinski K, Dunstan CR. The bone remodeling environment is a factor in breast cancer bone metastasis. Bone. 2011;48:66–70. doi: 10.1016/j.bone.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Yonou H, Ochiai A, Ashimine S, Maeda H, Horiguchi Y, Yoshioka K. The bisphosphonate YM529 inhibits osteoblastic bone tumor proliferation of prostate cancer. Prostate. 2007;67:999–1009. doi: 10.1002/pros.20592. [DOI] [PubMed] [Google Scholar]
- 26.Pecherstorfer M, Seibel MJ, Woitge HW, Horn E, Schuster J, Neuda J. Bone resorption in multiple myeloma and in monoclonal gammopathy of undetermined significance: quantification by urinary pyridinium cross-links of collagen. Blood. 1997;90:3743–3750. [PubMed] [Google Scholar]
- 27.Diel IJ, Solomayer EF, Seibel MJ, Pfeilschifter J, Maisenbacher H, Gollan C. Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clinical Cancer Research. 1999;5:3914–3919. [PubMed] [Google Scholar]
- 28.Woitge HW, Horn E, Keck AV, Auler B, Seibel MJ, Pecherstorfer M. Biochemical markers of bone formation in patients with plasma cell dyscrasias and benign osteoporosis. Clinical Chemistry. 2001;47:686–693. [PubMed] [Google Scholar]
- 29.Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005;2:504–517. doi: 10.1038/ncponc0320. quiz 1 p following 33. [DOI] [PubMed] [Google Scholar]
- 30.Ali SM, Demers LM, Leitzel K, Harvey HA, Clemens D, Mallinak N. Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastasis. Annals of Oncology. 2004;15:455–459. doi: 10.1093/annonc/mdh089. [DOI] [PubMed] [Google Scholar]
- 31.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. Journal of Clinical Oncology. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 32.Cook RJ, Coleman R, Brown J, Lipton A, Major P, Hei YJ. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clinical Cancer Research. 2006;12:3361–3367. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 33.Lipton A, Costa L, Ali S, Demers L. Use of markers of bone turnover for monitoring bone metastases and the response to therapy. Seminars in Oncology. 2001;28:54–59. doi: 10.1016/s0093-7754(01)90233-7. [DOI] [PubMed] [Google Scholar]
- 34.Coleman RE, Guise TA, Lipton A, Roodman GD, Berenson JR, Body JJ. Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clinical Cancer Research. 2008;14:6387–6395. doi: 10.1158/1078-0432.CCR-08-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton A. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 36.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Natural Review of Cancer. 2005;5:21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 37.Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. Journal of the National Cancer Institute. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 38.Costa L, Demers LM, Gouveia-Oliveira A, Schaller J, Costa EB, de Moura MC. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. Journal of Clinical Oncology. 2002;20:850–856. doi: 10.1200/JCO.2002.20.3.850. [DOI] [PubMed] [Google Scholar]
- 39.Siclari VA, Guise TA, Chirgwin JM. Molecular interactions between breast cancer cells and the bone microenvironment drive skeletal metastases. Cancer and Metastasis Reviews. 2006;25:621–633. doi: 10.1007/s10555-006-9023-1. [DOI] [PubMed] [Google Scholar]
- 40.Aft R. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncology. 2010;11:421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. Journal of Biological Chemistry. 1986;261:12665–12674. [PubMed] [Google Scholar]
- 42.Aubin JE. Advances in the osteoblast lineage. Biochemistry and Cell Biology. 1998;76:899–910. [PubMed] [Google Scholar]
- 43.Mundy GR, Chen D, Zhao M, Dallas S, Xu C, Harris S. Growth regulatory factors and bone. Reviews in Endocrinology & Metabolism Disorders. 2001;2:105–115. doi: 10.1023/a:1010015309973. [DOI] [PubMed] [Google Scholar]
- 44.Vessella RL, Corey E. Targeting factors involved in bone remodeling as treatment strategies in prostate cancer bone metastasis. Clinical Cancer Research. 2006;12:6285s–6290ss. doi: 10.1158/1078-0432.CCR-06-0813. [DOI] [PubMed] [Google Scholar]
- 45.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Molecular Cancer Therapy. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 46.Lemaire V, FLFL Tobin, Greller LD, Cho CR, Suva LJ. Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. Journal of Theoretical Biology. 2004;229:293–309. doi: 10.1016/j.jtbi.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Frost HM. Metabolism of bone. New England Journal of Medicine. 1973;289:864–865. [PubMed] [Google Scholar]
- 48.Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000;26:319–323. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- 49.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 50.Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. Journal of Cellular Biochemistry. 1994;55:287–299. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- 51.Smith R. Physiology of bone. Oxford Textbook of Medicine. 2002:p.3.133.
- 52.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 53.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. Journal of Biological Chemistry. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 56.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 59.Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey DL. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1beta, TNF-alpha, PTH, PTHrP, and 1, 25(OH)2D3. Journal of Bone and Mineral Research. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- 60.Ooi LL, Zhou H, Kalak R, Zheng Y, Conigrave AD, Seibel MJ. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Research. 2010;70:1835–1844. doi: 10.1158/0008-5472.CAN-09-3194. [DOI] [PubMed] [Google Scholar]
- 61.Quinn JE, Brown LG, Zhang J, Keller ET, Vessella RL, Corey E. Comparison of Fc-osteoprotegerin and zoledronic acid activities suggests that zoledronic acid inhibits prostate cancer in bone by indirect mechanisms. Prostate Cancer Prostatic Disease. 2005;8:253–259. doi: 10.1038/sj.pcan.4500815. [DOI] [PubMed] [Google Scholar]
- 62.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. Journal of Bone and Mineral Research. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 63.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annual Review of Immunology. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 64.Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. Journal of Bone and Mineral Research. 2001;16:348–360. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- 65.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. Journal of Bone and Mineral Research. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 66.Mundy GR, Guise TA. Hypercalcemia of malignancy. American Journal of Medicine. 1997;103:134–145. doi: 10.1016/s0002-9343(97)80047-2. [DOI] [PubMed] [Google Scholar]
- 67.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. Journal of Clinical Investigation. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Research and Treatment. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 69.Burtis WJ, Brady TG, Orloff JJ, Ersbak JB, Warrell RP, Jr, Olson BR. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. New England Journal of Medicine. 1990;322:1106–1112. doi: 10.1056/NEJM199004193221603. [DOI] [PubMed] [Google Scholar]
- 70.Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. Journal of Biological Chemistry. 2002;277:24571–24578. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. Journal of Clinical Investigation. 2011;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall RE, Kemp BE, Suva LJ. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:5048–5052. doi: 10.1073/pnas.84.14.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suva LJ, Winslow GA, Wettenhall RE, Hammonds RG, Moseley JM, Diefenbach-Jagger H. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237:893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 74.Strewler GJ, Stern PH, Jacobs JW, Eveloff J, Klein RF, Leung SC. Parathyroid hormonelike protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. Journal of Clinical Investigation. 1987;80 doi: 10.1172/JCI113275. 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kemp BE, Moseley JM, Rodda CP, Ebeling PR, Wettenhall RE, Stapleton D. Parathyroid hormone-related protein of malignancy: active synthetic fragments. Science. 1987;238:1568–1570. doi: 10.1126/science.3685995. [DOI] [PubMed] [Google Scholar]
- 76.Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horiuchi N, Caulfield MP, Fisher JE, Goldman ME, McKee RL, Reagan JE. Similarity of synthetic peptide from human tumor to parathyroid hormone in vivo and in vitro. Science. 1987;238:1566–1568. doi: 10.1126/science.3685994. [DOI] [PubMed] [Google Scholar]
- 78.Guise TA. Parathyroid hormone-related protein and bone metastases. Cancer. 1997;80:1572–1580. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- 79.Capparelli C, Kostenuik PJ, Morony S, Starnes C, Weimann B, Van G. Osteoprotegerin prevents and reverses hypercalcemia in a murine model of humoral hypercalcemia of malignancy. Cancer Research. 2000;60:783–787. [PubMed] [Google Scholar]
- 80.Morony S, Warmington K, Adamu S, Asuncion F, Geng Z, Grisanti M. The inhibition of RANKL causes greater suppression of bone resorption and hypercalcemia compared with bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology. 2005;146:3235–3243. doi: 10.1210/en.2004-1583. [DOI] [PubMed] [Google Scholar]
- 81.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. Journal of Clinical Investigation. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 83.Henderson M, Danks J, Moseley J, Slavin J, Harris T, McKinlay M. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. Journal of the National Cancer Institute. 2001;93:234–237. doi: 10.1093/jnci/93.3.234. [DOI] [PubMed] [Google Scholar]
- 84.Guise TA, O'Keefe R, Randall RL, Terek RM. Molecular biology and therapeutics in musculoskeletal oncology. Journal of Bone and Joint Surgery. American Volume. 2009;91:724–732. doi: 10.2106/JBJS.I.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Research. 2005;65:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 86.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of steoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 87.Clines GA, Guise TA. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocrine-Related Cancer. 2005;12:549–583. doi: 10.1677/erc.1.00543. [DOI] [PubMed] [Google Scholar]
- 88.Hu Z, Gupta J, Zhang Z, Gerseny H, Berg A, Chen YJ. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. Human Gene Therapy. 2012;23:871–882. doi: 10.1089/hum.2012.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-beta/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Research. 2011;71:5606–5610. doi: 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Research. 2011;71:175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 2011;48:23–29. doi: 10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Hiraga T, Myoui A, Hashimoto N, Sasaki A, Hata K, Morita Y. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Research. 2012;72:4238–4249. doi: 10.1158/0008-5472.CAN-11-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim SJ, Uehara H, Yazici S, Langley RR, He J, Tsan R. Simultaneous blockade of platelet-derived growth factor-receptor and epidermal growth factor-receptor signaling and systemic administration of paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Research. 2004;64:4201–4208. doi: 10.1158/0008-5472.CAN-03-3763. [DOI] [PubMed] [Google Scholar]
- 94.Russell MR, Jamieson WL, Dolloff NG, Fatatis A. The alpha-receptor for platelet-derived growth factor as a target for antibody-mediated inhibition of skeletal metastases from prostate cancer cells. Oncogene. 2009;28:412–421. doi: 10.1038/onc.2008.390. [DOI] [PubMed] [Google Scholar]
- 95.Uehara H, Kim SJ, Karashima T, Shepherd DL, Fan D, Tsan R. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. Journal of the National Cancer Institute. 2003;95:458–470. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]
- 96.Sethi N, Kang Y. Dysregulation of developmental pathways in bone metastasis. Bone. 2011;48:16–22. doi: 10.1016/j.bone.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Clezardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Research. 2005;65:4971–4974. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 98.Holen I, Shipman CM. Role of osteoprotegerin (OPG) in cancer. Clinical Science (London) 2006;110:279–291. doi: 10.1042/CS20050175. [DOI] [PubMed] [Google Scholar]
- 99.Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM. Metastasis and bone loss: advancing treatment and prevention. Cancer Treatment Reviews. 2010;36:615–620. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin JK, Dayyani F, Gallick GE. Steps in prostate cancer progression that lead to bone metastasis. International Journal of Cancer. 2011;128:2545–2561. doi: 10.1002/ijc.26024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncology. 2011;7:1285–1297. doi: 10.2217/fon.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Natural Review of Clinical Oncology. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 103.Russell RG, Smith R, Preston C, Walton RJ, Woods CG. Diphosphonates in Paget’s disease. Lancet. 1974;1:894–898. doi: 10.1016/s0140-6736(74)90347-x. [DOI] [PubMed] [Google Scholar]
- 104.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 105.Fleisch H, Russell RG, Francis MD. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science. 1969;165:1262–1264. doi: 10.1126/science.165.3899.1262. [DOI] [PubMed] [Google Scholar]
- 106.Padalecki SS, Guise TA. Actions of bisphosphonates in animal models of breast cancer. Breast Cancer Research. 2002;4:35–41. doi: 10.1186/bcr415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Epstein S, Zaidi M. Biological properties and mechanism of action of ibandronate: application to the treatment of osteoporosis. Bone. 2005;37:433–440. doi: 10.1016/j.bone.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Fromigué O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. Journal of Bone and Mineral Research. 2000;15:2211–2221. doi: 10.1359/jbmr.2000.15.11.2211. [DOI] [PubMed] [Google Scholar]
- 109.Bauss F, Body JJ. Ibandronate in metastatic bone disease: a review of preclinical data. Anticancer Drugs. 2005;16:107–118. doi: 10.1097/00001813-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 110.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. Journal of Clinical Investigation. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gainford MC, Dranitsaris G, Clemons M. Recent developments in bisphosphonates for patients with metastatic breast cancer. BMJ. 2005;330:769–773. doi: 10.1136/bmj.330.7494.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coleman RE. The role of bisphosphonates in breast cancer. Breast. 2004;13(Suppl 1):S19–S28. doi: 10.1016/j.breast.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 113.Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 Update on the role of bisphosphonates and bone health issues in women with breast cancer 10.1200/JCO.2003.08.017. Journal of Clinical Oncology 2003;21:4042–4057. [DOI] [PubMed]
- 114.Paterson AH. The role of bisphosphonates in early breast cancer. Oncologist. 2006;11(Suppl 1):13–19. doi: 10.1634/theoncologist.11-90001-13. [DOI] [PubMed] [Google Scholar]
- 115.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. New England Journal of Medicine. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 116.Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, Sokal M. Oral sodium clodronate for nonmetastatic prostate cancer–results of a randomized double-blind placebo-controlled trial: medical research council PR04 (ISRCTN61384873) Journal of the National Cancer Institute. 2007;99:765–776. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 117.Powles T, Paterson S, Kanis JA, McCloskey E, Ashley S, Tidy A. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. Journal of Clinical Oncology. 2002;20:3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 118.Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncologica. 2004;43:650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 119.Powles T, Paterson A, McCloskey E, Schein P, Scheffler B, Tidy A. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Research. 2006;8:R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. Journal of Clinical Oncology. 2001;19:10–17. doi: 10.1200/JCO.2001.19.1.10. [DOI] [PubMed] [Google Scholar]
- 121.Body JJ. Bisphosphonates in the treatment of metastatic breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2001;6:477–485. doi: 10.1023/a:1014795216669. [DOI] [PubMed] [Google Scholar]
- 122.Conte PF, Latreille J, Mauriac L, Calabresi F, Santos R, Campos D. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: results from a multinational randomized controlled trial. The Aredia Multinational Cooperative Group. Journal of Clinical Oncology. 1996;14:2552–2559. doi: 10.1200/JCO.1996.14.9.2552. [DOI] [PubMed] [Google Scholar]
- 123.Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncology. 2010;11:421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F. Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Annals of Oncology. 2006;17:986–989. doi: 10.1093/annonc/mdl041. [DOI] [PubMed] [Google Scholar]
- 125.Li EC, Davis LE. Zoledronic acid: a new parenteral bisphosphonate. Clinical Therapeutics. 2003;25:2669–2708. doi: 10.1016/s0149-2918(03)80327-2. [DOI] [PubMed] [Google Scholar]
- 126.Hadji P, Coleman R, Gnant M, Green J. The impact of menopause on bone, zoledronic acid, and implications for breast cancer growth and metastasis. Annals of Oncology. 2012;23:2782–2790. doi: 10.1093/annonc/mds169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncology. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 128.Pfeiler G, Konigsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. Journal of Clinical Oncology. 2011;29:2653–2659. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 129.Coleman R, Woodward E, Brown J, Cameron D, Bell R, Dodwell D. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01-04) for women with stage II/III breast cancer. Breast Cancer Research and Treatment. 2011;127:429–438. doi: 10.1007/s10549-011-1429-y. [DOI] [PubMed] [Google Scholar]
- 130.Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M. Breast-cancer adjuvant therapy with zoledronic acid. New England Journal of Medicine. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 131.Gnant M. Intravenous bisphosphonates for breast cancer: impact on patient outcomes and scientific concepts. Breast Disease. 2011;33:71–81. doi: 10.3233/BD-2010-0325. [DOI] [PubMed] [Google Scholar]
- 132.Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008;112:1001–1010. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 133.Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Annals of Oncology. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 134.Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J. Immediate administration of zoledronic acid reduces aromatase inhibitoraAssociated bone loss in postmenopausal women with early breast cancer: 12-month analysis of the E-ZO-FAST trial. Clinical Breast Cancer. 2012;12:40–48. doi: 10.1016/j.clbc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 135.Barrett J, Worth E, Bauss F, Epstein S. Ibandronate: a clinical pharmacological and pharmacokinetic update. Journal of Clinical Pharmacology. 2004;44:951–965. doi: 10.1177/0091270004267594. [DOI] [PubMed] [Google Scholar]
- 136.Russell RG. Ibandronate: pharmacology and preclinical studies. Bone. 2006;38 doi: 10.1016/j.bone.2006.01.151. S7-12. [DOI] [PubMed] [Google Scholar]
- 137.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. New England Journal of Medicine. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 138.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes and Development. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. American Journal of Pathology. 2000;157:435–448. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 141.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clinical Cancer Research. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 142.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;Epub ahead of print. [DOI] [PubMed]
- 144.Goblirsch MJ, Zwolak PP, Clohisy DR, Roodman, Guise, Coleman Biology of bone cancer pain. Clinical Cancer Research. 2006;12:6231s–6235ss. doi: 10.1158/1078-0432.CCR-06-0682. [DOI] [PubMed] [Google Scholar]
- 145.Honore P, Luger NM, Sabino MAC, Schwei MJ, Rogers SD, Mach DB. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nature Medicine. 2000;6:521–528. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 146.Libouban H, Moreau MF, Basle MF, Bataille R, Chappard D. Increased bone remodeling due to ovariectomy dramatically increases tumoral growth in the 5T2 multiple myeloma mouse model. Bone. 2003;33:283–292. doi: 10.1016/s8756-3282(03)00196-0. [DOI] [PubMed] [Google Scholar]
- 147.Hirbe AC, Uluckan O, Morgan EA, Eagleton MC, Prior JL, Piwnica-Worms D. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007;109:3424–3431. doi: 10.1182/blood-2006-09-048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Price JT, Quinn JM, Sims NA, Vieusseux J, Waldeck K, Docherty SE. The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line. Cancer Research. 2005;65:4929–4938. doi: 10.1158/0008-5472.CAN-04-4458. [DOI] [PubMed] [Google Scholar]
- 149.Woitge HW, Pecherstorfer M, Horn E, Keck AV, Diel IJ, Bayer P. Serum bone sialoprotein as a marker of tumour burden and neoplastic bone involvement and as a prognostic factor in multiple myeloma. British Journal of Cancer. 2001;84:344–351. doi: 10.1054/bjoc.2000.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE. Bone resorption predicts for skeletal complications in metastatic bone disease. British Journal of Cancer. 2003;89:2031–2037. doi: 10.1038/sj.bjc.6601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blair JM, Zhou H, Seibel MJ, Dunstan CR. Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Natural Clinical Practice Oncology. 2006;3:41–49. doi: 10.1038/ncponc0381. [DOI] [PubMed] [Google Scholar]
- 152.Fohr B, Dunstan CR, Seibel MJ. Clinical review 165: markers of bone remodeling in metastatic bone disease. Journal of Clinical Endocrinology and Metabolism. 2003;88:5059–5075. doi: 10.1210/jc.2003-030910. [DOI] [PubMed] [Google Scholar]
- 153.Seibel M. Bone remodelling markers and bone cancer. In: Heyman E, editor. Recent advances in bone cancer: progression and therapeutic approaches. Elsevier; 2011. pp. 253–264. [Google Scholar]
- 154.Seibel MJ. The use of molecular markers of bone turnover in the management of patients with metastatic bone disease. Clin ical Endocrinology (Oxford) 2008;68:839–849. doi: 10.1111/j.1365-2265.2007.03112.x. [DOI] [PubMed] [Google Scholar]
- 155.Lagunova Z, Porojnicu AC, Dahlback A, Berg JP, Beer TM, Moan J. Prostate cancer survival is dependent on season of diagnosis. Prostate. 2007;67:1362–1370. doi: 10.1002/pros.20577. [DOI] [PubMed] [Google Scholar]
- 156.Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Medicine. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Grant WB. Geographic variation of prostate cancer mortality rates in the United States: implications for prostate cancer risk related to vitamin D. International Journal of Cancer. 2004;111:470–471. doi: 10.1002/ijc.20220. author reply 2. [DOI] [PubMed] [Google Scholar]
- 158.Zheng Y, Zhou H, Modzelewski JR, Kalak R, Blair JM, Seibel MJ. Accelerated bone resorption, due to dietary calcium deficiency, promotes breast cancer tumor growth in bone. Cancer Research. 2007;67:9542–9548. doi: 10.1158/0008-5472.CAN-07-1046. [DOI] [PubMed] [Google Scholar]
- 159.Zheng Y, Zhou H, Fong-Yee C, Modzelewski JR, Seibel MJ, Dunstan CR. Bone resorption increases tumour growth in a mouse model of osteosclerotic breast cancer metastasis. Clinical and Experimental Metastasis. 2008;25:559–567. doi: 10.1007/s10585-008-9172-4. [DOI] [PubMed] [Google Scholar]
- 160.Ooi LL, Zheng Y, Zhou H, Trivedi T, Conigrave AD, Seibel MJ. Vitamin D deficiency promotes growth of MCF-7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone. 2010;47:795–803. doi: 10.1016/j.bone.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 161.Zheng Y, Zhou H, Ooi LL, Snir AD, Dunstan CR, Seibel MJ. Vitamin D deficiency promotes prostate cancer growth in bone. Prostate. 2011;15:1012–1021. doi: 10.1002/pros.21316. [DOI] [PubMed] [Google Scholar]
- 162.Park SI, Soki FN, McCauley LK. Roles of bone marrow cells in skeletal metastases: no longer bystanders. Cancer Microenviron. 2011;4:237–246. doi: 10.1007/s12307-011-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nature Reviews Rheumatology. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 164.Sato K. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. Journal of Experimetnal Medicine. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer and Metastasis Reviews. 2006;25:521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 166.Kaplan RN. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Natural Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 168.Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Research. 2009;69:7529–7537. doi: 10.1158/0008-5472.CAN-08-4382. [DOI] [PubMed] [Google Scholar]
- 169.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Cook K. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. Journal of Bone and Mineral Research. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 170.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. Journal of Clinical Investigation. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kelly T. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Research. 2005;65:5778–5784. doi: 10.1158/0008-5472.CAN-05-0749. [DOI] [PubMed] [Google Scholar]
- 172.Pazolli E. Senescent stromal-derived osteopontin promotes preneoplastic cell growth. Cancer Research. 2009;69:1230–1239. doi: 10.1158/0008-5472.CAN-08-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McAllister SS. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lynch CC. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]