Abstract
Background
Bone-targeted agents are widely used for the treatment of osteoporosis, the prevention of cancer-therapy induced bone loss, and for reducing the risk of skeletal related events in patients with metastatic disease. Despite widespread use, relatively little is known about the in vivo effect of these agents on bone homeostasis, bone quality, and bone architecture in humans. Traditionally bone quality has been assessed using a transiliac bone biopsy with a 7 mm “Bordier” core needle. We examined the possibility of using a 2 mm “Jamshidi” core needle as a more practical and less invasive method to assess bone turnover and potentially other tumor effects.
Methods
A pilot study on the feasibility of assessing bone quality and microarchitecture and tumor invasion using a 2 mm bone marrow trephine was conducted. Patients underwent a posterior trans-iliac trephine biopsy and bone marrow aspirate. Samples were analyzed for bone microarchitecture, bone density, and histomorphometry. The study plan was to accrue three patients with advanced breast cancer to assess the feasibility of the study before enrolling more patients.
Results
The procedure was well tolerated. The sample quality was excellent to analyze bone trabecular microarchitecture using both microCT and histomorphometry. Intense osteoclastic activity was observed in a patient with extensive tumor burden in bone despite intravenous bisphosphonate therapy.
Discussion
Given the success of this study for assessing bone microarchitecture, bone density, and histomorphometry assessment using a 2 mm needle the study will be expanded beyond these initial three patients for longitudinal assessment of bone-targeted therapy.
Keywords: Histomorphometry, Microarchitecture, MicroCT, Breast cancer, Bisphosphonate
1. Introduction
Bone-targeted agents aimed at reducing osteoclastogenesis including bisphosphonates and denosumab are widely used in cancer patients. Their uses include; the treatment of osteoporosis, prevention of cancer-therapy induced bone loss, reducing the risk of skeletal related events, reducing pain, and improving quality of life in patients with metastatic disease [1]. In cancer patients these agents are administered systemically at high and frequent, often monthly, doses and for extended periods of time relative to their use in the treatment of osteoporosis. Despite this there is a paucity of data about the in vivo effect of these agents on bone homeostasis, bone quality, and microarchitecture in humans. This topic has gained increasing attention recently with the awareness of long term toxicities of bisphosphonates such as osteonecrosis of the jaw (ONJ) and atypical fractures.[2], [3].
Traditionally bone strength has been viewed as an integration of bone quantity and bone quality [4]. Clinically, bone strength and susceptibility to fracture is based on bone mineral density (BMD) as reflected through dual energy X-ray absortiometry (DEXA) scans [5]. This, however, has only modest sensitivity/specificity [5], [6], and only a small proportion of fracture risk reduction is actually explained by bone density increases [7], [8].
Clinical and laboratory evidence suggest mechanical properties, in addition to BMD, play an important role in bone strength [9], [10], [11]. However, assessing bone quality requires invasive testing, traditionally with a 7–8 mm “Bordier” trephine of transiliac bone [12]. The size of the needle poses two problems. First, it is uncomfortable and painful for the patient. Second, it is difficult technically in achieving an adequate sample in cancer patients on bone-targeted agents as anecdotally the procedure is more challenging than in patients with osteoporosis [13]. Previous data from cadavers has demonstrated that the 2 mm trephine provides comparable results to a 7 mm trephine [14]. A review of the literature revealed no studies comparing the two trephine sizes in living cancer patients.
Given the widespread use of outpatient posterior iliac crest bone marrow biopsies in hematology and oncology using a 2 mm Jamshidi needle, we wanted to see if this would allow a more practical, technically easier sampling amenable to routine screening of bone status in cancer patients.
2. Methods
2.1. Subjects and design
This study was a pilot feasibility study performed in three patients prior to starting a larger prospective, single-arm, non-interventional feasibility study using a 2 mm trephine to assess bone quality in breast cancer patients. Patients were required to have: advanced breast cancer, with or without bone metastases with or without bisphosphonate treatment, and an ECOG ≤2. All patients enrolled underwent a bone mineral density assessment using DEXA. The study was approved by the research ethics committee at the Ottawa Hospital Cancer Centre, and all patients provided written informed consent.
2.2. Bone specimen collection
Trans-iliac crest bone biopsy specimens were obtained from the posterior iliac crest using a Jamshidi bone biopsy trephine (diameter 2 mm) (Cardinal Health, Dublin, Ohio, United States of America) (see Appendix 1). The samples underwent histomorphometric analysis, microarchitectural analysis, and routine pathologic assessment.
2.3. Histological and histomorphometrical analysis of bone
Hematoxyline and Eosin (HE), von Kossa and van Gieson (VKVG), and tartrate-resistant acid phosphatase (TRAP) staining of plastic sections of posterior iliac bone samples from three patients were performed on plastic sections. As described elsewhere, for plastic sectioning, bone samples fixed in 4% PFA/PBS were embedded in methyl methacrylate, and sectioned (7-μm thickness), and von Kossa and van Gieson staining was applied [15]. Unmineralized bone sections were analyzed using Osteomeasure software (Osteometrics, Inc.). Images were taken at room temperature using a light microscope (DM200; Leica) with a 20×(numerical aperture of 0.40) or 40×(numerical aperture of 0.65) objective. All histological images were captured using a camera (DP72; Olympus), acquired with a DP2-BSW software (XV3.0; Olympus), and processed using Photoshop (Adobe).
2.4. Three dimensional micro-computed tomography (3D microCT) of bone samples
The core biopsy samples were scanned wet in 70% ethanol by micro-CT at 40X magnification with a SkyScan 1072 (Skyscan, Aartselaar, Belgium) and analyzed with a bone analysis software (ver2.2f, Aartselaar, Belgium). Trabecular bone structure was measured. Parameters were acquired with a rotation of 0.9° between each picture and the x-ray source set at 100 kV and 98 μA. The segmentation of the image was made by a global threshold and a voxel size of 21.90×21.90×21.90 μm³; the same threshold setting was used for all the samples. Architectural measurements were made as previously described [16], [17].
2.5. Bone mineral density measurements by DEXA
Core biopsies were subjected to bone mineral density (BMD) analysis using a PIXIMUS bone densitometer (PIXIMUS TM, GE medical systems, Schenectady, NY, USA). A quality control phantom was used to calibrate the densitometer prior to each experiment. All patients also underwent DEXA scanning.
2.6. Statistical analysis
The primary endpoint of this pilot study was to assess the feasibility of obtaining sufficient tissue using bone marrow aspirates and trephine biopsy for histomorphometry. The secondary endpoint was comparison between current bone mineral density and pathological analysis of bone marrow trephine biopsy looking at histomorphometry, and micro-architecture analysis. Given this was a pilot study simple descriptive statistics were used.
3. Results
Between January and July 2011 three patients consented and underwent outpatient biopsies. Patient number one and number two received two biopsies from separate areas of the posterior iliac crest. Patient three received a single biopsy. The demographic characteristics of each patient can be found in Table 1. Notably, all patients were post-menopausal, had metastatic disease, and two were on long-term bisphosphonate therapy.
Table 1.
Demographic data of study patients.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age | 64 | 56 | 59 |
| Menopause status | Postmenopausal | Postmenopausal | Postmenopausal |
| BMI (kg/m²) | 34.0 | 19.5 | 27.9 |
| Smoking history | None | None | None |
| BMD (t-score) | −1.9 [L1–L2] | −1.3 [L1–L4] | −2.2 [L1–L4] |
| Breast cancer | Metastatic to soft tissues | Extensive bone metastases | Limited bone metastases |
| Current therapy | Aromatase inhibitor | Aromatase inhibitor | Aromatase inhibitor |
| Bisphosphonate use and duration | Nil | Yes (3 years) | Yes (2 years) |
BMI=body mass index, BMD= bone mineral density and L=lumbar spine (i.e. L1=first lumbar vertebrae).
4. 3D microCT data
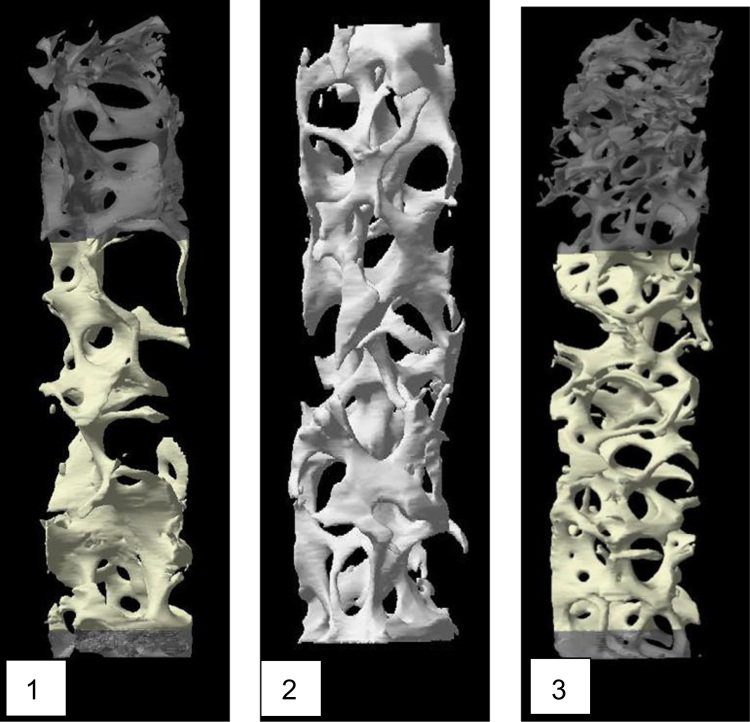
Bone volume (BV/TV) ranged from 10.8 to 13.5% consistent with the published literature [18]. Trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N) and bone surface per tissue area (BS/TV) values were also consistent with the range published in the literature in post-menopausal women [18]. 3D microCT data are located in Table 2. The accompanying figures for each biopsy are located in Fig. 1.
Table 2.
Microarchitectural parameters and measurement of cellular activities in the poster iliac crest assessed by histomorphometry and 3D microCT.
| Sample ID | Patient1 | Patient 2 | Patient 3 |
|---|---|---|---|
| BV/TV (%) | 10.9 | 13.5 | 10.8 |
| Tb. Th (mm) | 0.14814 | 0.173 | 0.132 |
| Tb. Sp (mm) | 0.726 | 0.570 | 0.575 |
| Tb.N (mm) | 0.741 | 0.781 | 0.819 |
| Ct. Wi (μm) | N/A | N/A | N/A |
| BS/TV (1/mm) | 2.707 | 3.049 | 2.98 |
- BV/TV: bone volume.
- Tb.Sp: trabecular separation.
- Ct.Wi: cortical width.
- N/A: non-applicable.
- Tb.Th: trabecular thickness.
- Tb.N: trabecular number.
- BS/TV: bone surface per tissue area.
Fig. 1.
MicroCT images, for patient #1, #2, and #3.
4.1. Bone histomorphometry
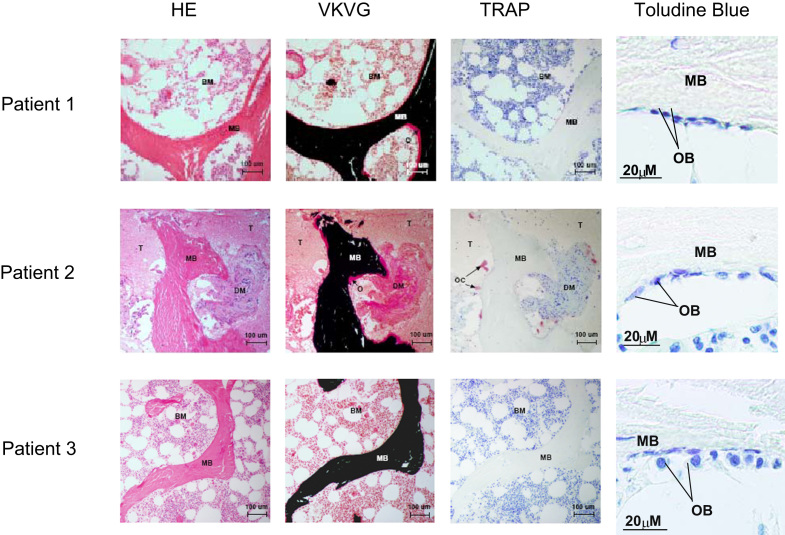
Hematoxylin–Eosin (HE) stain of the hisotology sections shows the presence of tumor (T) in sample of patient 1 and 2 (Fig. 2). Samples from patient 1 and patient 2 shows the presence of osteoid (O) by von Kossa and van Gieson (VKVG) stain. Among the microscopic field presented only sample from patient 2 shows the presence of osteoclasts (C) as evident by tartrate-resistant acid phosphatase (TRAP) staining (third panel). Osteoblasts were stained with toludine blue. Osteoblast and osteoclast numbers per tissue area and bone perimeter are displayed in Table 3.
Fig. 2.
Histological and histomorphometric analysis of bone. Hematoxyline and Eosin (HE), von Kossa and van Gieson (VKVG), and tartrate-resistant acid phosphatase (TRAP) staining of plastic sections of bone samples from three patients. HE stain shows the presence of tumor (T) in patient #2. Patient #1 and #2 shows the presence of osteoid volume (O) by VKVG stain (middle panel). No osteoclast is seen in the field shown for sample of patient #1. BM: bone marrow and MB: mineralized bone. Among the microscopic field presented only sample from patient #2 show the presence of osteoclasts (C) by TRAP stain (right panel). Osteoblasts were stained with toludine blue.
Table 3.
Osteoblast and osteoclast count data.
| Patient | N.Ob/T.Ar | N.Ob/B.Pm | N.Oc/T.Ar | N.Oc/B.Pm |
|---|---|---|---|---|
| 1 | 142.57 | 14.55 | 2.22 | 0.22 |
| 2 | 142.57 | 16.98 | 6.68 | 0.80 |
| 3 | 100.25 | 10.91 | 0 | 0 |
N.Ob/T.Ar: number of osteoblasts/ tissue area N.Ob/B.PM: number of osteoblasts/bone perimeter Oc/T.Ar: number of osteoclasts/ tissue area and N.Oc/B.PM: number of osteoclasts/bone perimeter in different patient bone sections.
5. Discussion
Despite the widespread use of bone-targeted agents in breast cancer patients, there is a relative paucity of data about the in vivo effect of these agents on bone homeostasis, bone quality, and microarchitecture in humans [1]. This is not withstanding that we are using increasingly potent agents at higher doses and increased frequencies, relative to their use in the treatment of osteoporosis. This is important as long-term bisphosphonate use confers prolonged suppression of bone remodeling sites which can have deleterious clinical effects [3], [19], [20], [21], [22].
Newer methods for assessing bone quality are under investigation, such as microindentation technique and high-resolution peripheral quantitative CT [23], [24], [25], but are regarded as exploratory. High resolution-peripheral quantitative CT appears to be better than DEXA at predicting fracture risk, but has not yet reached widespread clinical use [24], [25], [26], [27]. Further, the use of biochemical turnover markers [8] are primarily used for monitoring treatment efficacy and compliance, and do not directly assess bone quality. Bone histomorphometry remains the gold standard in evaluating bone status prior to and following therapeutic interventions. However, this invasive procedure is not amenable to routine use. In cancer patients bone marrow biopsy is frequently used for pathological assessment and we explored the possibility to extend its use to bone histomorphometric parameters. We therefore assessed the feasibility of using 2 mm core biopsies to obtain additional information on in vivo bone biology indices.
The current study demonstrates that using a 2 mm Jamshidi is feasible, practical and yields a sample sufficient to determine trabecular microarchitecture and bone turnover indices by both microCT and histomorphometry. The benefit of this technique is that it provides qualitative information on the in vivo effect of bone-targeted agents on bone quality.This technique may also provide a feasible means of assessing bone quality in cancer patients on therapies known to affect bone quality (e.g., tamoxifen, aromatase inhibitors). Previous studies have used BMD in this setting, but it has its limitations and is not able to assess bone quality [28], [29], [30]. The importance of assessing bone quality and balancing the potential risks against the benefits of bone toxic agents will be of paramount importance if such agents (e.g., exemestane) are used for primary prevention of breast cancer [23], [31].
The findings of this prospective, pilot study are limited by the small sample size, but recruitment is currently under way to expand the study cohort. Another important limitation is that preclinical models have demonstrated that the effect of bisphosphonates on bone turnover is highly site specific [32]. The implications are that data from a single biopsy site (i.e. iliac crest) may not be representative of global bone quality [32], [33]. There were, however, several findings that merit consideration. Patient #1 had metastatic cancer to soft tissues but no bone metastasis and was treated with aromatase inhibitors but not with bisphosphonates. Bone histomorphometry revealed active bone turnover as indicated in Table 3. In contrast patient #3 who had limited bone metastatic disease was treated with both aromatase inhibitors and bisphosphonates and had evidence of bone suppression with no active osteoclast detected. Patient #2 was of particular interest because of extensive bone metastasis by skeletal survey and its confirmation by the presence of metastatic tumor on histomorphometric sections (Fig. 2). Interestingly this patient had very active osteoclastic activitiy as indicated in Table 3 despite treatment with intravenous bisphosphonates for three years suggesting that bisphosphonates were unable to completely suppress turnover when tumor burden is high. Given our sample size, it is premature to generalize our findings but it provides a rationale to extend our study with a larger sample size to assess the reproducibility of our findings and whether or not a 2 mm core will be able to replace the use of 7 mm core biopsies.
6. Conclusion
The 2 mm Jamshidi is feasible, practical, and yields an adequate sample to assess bone structure and quality using microCT and histomorphometry in breast cancer patients. Additional samples are required to assess reproducibility, effect of bisphosphonates on bone quality and novel information on bone/tumor interaction. Further research is required to determine whether this technique may be to longitudinally assess bone quality in cancer patients on long-term bone-targeted therapies.
Acknowledgments
We acknowledge support from Renee Amours-Ouellette and her family and “Tina and her Angels of Hope Fund” (MC). This research was also supported by grants from Canadian Institutes for Health Research(CIHR) MOP-10839 and the Susan G Komen Foundation grant KG 100766(to RK).
Appendix A. Transilliac crest bone marrow aspirate and trephine biopsy
Transilliac crest bone marrow aspirate and trephine biopsy will be performed as an outpatient procedure with proper anesthesia
-
•
The patient is placed in the lateral decubitus position, with the top leg flexed and the lower leg straight, or alternatively in the supine position.
-
•
Palpate the iliac crest, and mark the preferred sampling site with a pen.
-
•
Aseptic technique is employed, including sterile gloves and gown.
-
•
The site is prepared with an antiseptic (eg, povidone-iodine or chlorhexidine gluconate), scrubbed, and draped, exposing only the site to be sampled.
-
•
The skin and the underlying tissue to the periosteum are infiltrated with a local anesthetic (eg, approximately 10 mL of 1% Xylocaine). A 10 mL syringe with a 25-gauge needle is used to inject an initial 0.5 mL directly under the skin, raising a wheal. A 22-gauge needle is used to penetrate deeper into the subcutaneous tissue and the underlying periosteum, an area roughly 1 cm in diameter.
-
•
A skin incision is made with a small surgical blade, through which the bone marrow aspiration needle, with a stylet locked in place, is inserted.
-
•
Once the needle contacts the bone, it is advanced by slowly rotating clockwise and counterclockwise until the cortical bone is penetrated and the marrow cavity is entered. Contact with the marrow cavity is usually noted by a sudden reduction in pressure. The depth of the penetration should not extend beyond an initial 1 cm.
-
•
Once within the marrow cavity, the stylet is removed. Using a 20 mL syringe, approximately 0.3 mL of bone marrow is aspirated. The material collected for bone marrow slides is not mixed with an anticoagulant, and it is processed immediately by a technologist.
Bone marrow biopsy:
-
•
Patient preparation is to be followed in the manner previously described for bone marrow aspiration.
-
•
The needle, with stylet locked in place, is held with the palm and index finger and repositioned so that a new insertion site is created for biopsy sampling. Once the needle touches the bone surface, the stylet is removed.
-
•
Using firm pressure, slowly rotate the needle in an alternating clockwise-counterclockwise motion, and advance it into the bone marrow cavity to obtain an adequate bone marrow specimen measuring approximately 1.6–3 cm in length.
-
•
Rotate the needle along its axis to help loosen the sample, pull back approximately 2–3 mm, and advance the needle again slightly, at a different angle, to help secure the specimen.
-
•
Following this procedure, slowly pull the needle out, while rotating in an alternating clockwise and counterclockwise motion.
-
•
Remove the specimen from the needle and introduce a probe through the distal cutting end.
Refrences
- 1.Coleman R.E., McCloskey E.V. Bisphosphonates in oncology. Bone. 2011;49(1):71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Lewiecki E.M. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs. 2011;71(6):791–814. doi: 10.2165/11585470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Schilcher J., Michaelsson K., Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. New England Journal of Medicine. 2011;364(18):1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. Journal of the American Medical Association 2001;285(6):785–795.
- 5.Rivadeneira F., Zillikens M.C., De Laet C.E., Hofman A., Uitterlinden A.G., Beck T.J. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. Journal of Bone and Mineral Research. 2007;22(11):1781–1790. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 6.Yang L., Peel N., Clowes J.A., McCloskey E.V., Eastell R. Use of DXA-based structural engineering models of the proximal femur to discriminate hip fracture. Journal of Bone and Mineral Research. 2009;24(1):33–42. doi: 10.1359/jbmr.080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings S.R., Karpf D.B., Harris F., Genant H.K., Ensrud K., LaCroix A.Z. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. American Journal of Medicine. 2002;112(4):281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu S.K., Hampson G. The pathogenesis, diagnosis, investigation and management of osteoporosis. Journal of Clinical Pathology. 2011;64(12):1042–1050. doi: 10.1136/jcp.2010.077842. [DOI] [PubMed] [Google Scholar]
- 9.Chavassieux P., Seeman E., Delmas P.D. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocrine Reviews. 2007;28(2):151–164. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- 10.Currey J.D. Changes in the impact energy absorption of bone with age. Journal of Biomechanics. 1979;12(6):459–469. doi: 10.1016/0021-9290(79)90031-9. [DOI] [PubMed] [Google Scholar]
- 11.Vashishth D. Age-dependent biomechanical modifications in bone. Critical Reviews in Eukaryotic Gene Expression. 2005;15(4):343–358. doi: 10.1615/critreveukargeneexpr.v15.i4.40. [DOI] [PubMed] [Google Scholar]
- 12.Bilezkian J.P., Raisz L.G., Martin T.J. Elsevier; Oxford, United Kingdom: 2008. Principles of bone biology. [Google Scholar]
- 13.Hilton J.F., Amir E., Hopkins S., Nabavi M., DiPrimio G., Sheikh A. Acquisition of metastatic tissue from patients with bone metastases from breast cancer. Breast Cancer Research and Treatment. 2011;129(3):761–765. doi: 10.1007/s10549-010-1264-6. [DOI] [PubMed] [Google Scholar]
- 14.Moore R.J., Durbridge T.C., Woods A.E., Vernon-Roberts B. Comparison of two bone trephine instruments used for quantitative histomorphometry. Journal of Clinical Pathology. 1989;42(2):213–215. doi: 10.1136/jcp.42.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khavandgar Z., Poirier C., Clarke C.J., Li J., Wang N., McKee M.D. A cell-autonomous requirement for neutral sphingomyelinase 2 in bone mineralization. Journal of Cell Biology. 2011;194(2):277–289. doi: 10.1083/jcb.201102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duque G., Macoritto M., Dion N., Ste-Marie L.G., Kremer R. 1, 25(OH)2D3 acts as a bone-forming agent in the hormone-independent senescence-accelerated mouse (SAM-P/6) American Journal of Physiology Endocrinology and Metabolism. 2005;288(4):E723–30. doi: 10.1152/ajpendo.00180.2004. [DOI] [PubMed] [Google Scholar]
- 17.El Abdaimi K., Dion N., Papavasiliou V., Cardinal P.E., Binderup L., Goltzman D. The vitamin D analogue EB 1089 prevents skeletal metastasis and prolongs survival time in nude mice transplanted with human breast cancer cells. Cancer Research. 2000;60(16):4412–4418. [PubMed] [Google Scholar]
- 18.Recker R.R., Kimmel D.B., Parfitt A.M., Davies K.M., Keshawarz N., Hinders S. Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. Journal of Bone and Mineral Research. 1988;3(2):133–144. doi: 10.1002/jbmr.5650030203. [DOI] [PubMed] [Google Scholar]
- 19.Whyte M.P., Wenkert D., Clements K.L., McAlister W.H., Mumm S. Bisphosphonate-induced osteopetrosis. New England Journal of Medicine. 2003;349(5):457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]
- 20.Odvina C.V., Zerwekh J.E., Rao D.S., Maalouf N., Gottschalk F.A., Pak C.Y. Severely suppressed bone turnover: a potential complication of alendronate therapy. Journal of Clinical Endocrinology and Metabolism. 2005;90(3):1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 21.Seeman E., Delmas P.D. Bone quality—the material and structural basis of bone strength and fragility. New England Journal of Medicine. 2006;354(21):2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein R.S., Roberson P.K., Manolagas S.C. Giant osteoclast formation and long-term oral bisphosphonate therapy. New England Journal of Medicine. 2009;360(1):53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung A.M., Tile L., Cardew S., Pruthi S., Robbins J., Tomlinson G. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncology. 2012;13(3):275–284. doi: 10.1016/S1470-2045(11)70389-8. [DOI] [PubMed] [Google Scholar]
- 24.Sornay-Rendu E., Boutroy S., Munoz F., Delmas P.D. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. Journal of Bone and Mineral Research. 2007;22(3):425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 25.Stein E.M., Liu X.S., Nickolas T.L., Cohen A., Thomas V., McMahon D.J. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. Journal of Bone and Mineral Research. 2010;25(12):2572–2581. doi: 10.1002/jbmr.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama K.K., Macdonald H.M., Moore S.A., Fung T., Boyd S.K., McKay H.A. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: An HR-pQCT study. Journal of Bone and Mineral Research. 2011:25. doi: 10.1002/jbmr.552. [DOI] [PubMed] [Google Scholar]
- 27.Varga P., Pahr D.H., Baumbach S., Zysset P.K. HR-pQCT based FE analysis of the most distal radius section provides an improved prediction of Colles' fracture load in vitro. Bone. 2010;47(5):982–988. doi: 10.1016/j.bone.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Van Poznak C. Managing bone mineral density with oral bisphosphonate therapy in women with breast cancer receiving adjuvant aromatase inhibition. Breast Cancer Research. 2010;12(3):110. doi: 10.1186/bcr2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lester J.E., Dodwell D., Purohit O.P., Gutcher S.A., Ellis S.P., Thorpe R. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clinical Cancer Research. 2008;14(19):6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 30.Brufsky A., Bundred N., Coleman R., Lambert-Falls R., Mena R., Hadji P. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13(5):503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 31.Goss P.E., Ingle J.N., Ales-Martinez J.E., Cheung A.M., Chlebowski R.T., Wactawski-Wende J. Exemestane for breast-cancer prevention in postmenopausal women. New England Journal of Medicine. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 32.Allen M.R., Kubek D.J., Burr D.B. Cancer treatment dosing regimens of zoledronic acid result in near-complete suppression of mandible intracortical bone remodeling in beagle dogs. Journal of Bone and Mineral Research. 2010;25(1):98–105. doi: 10.1359/jbmr.090713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen M.R., Burr D.B. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: What we think we know and what we know that we do not know. Bone. 2011;49(1):56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]