Abstract
Purpose
The ZOTECT study assesses the effect of zoledronic acid (ZOL) on bone-marker levels and potential correlations with disease outcomes in bisphosphonate-naive patients.
Methods
This prospective, single-arm, open-label study in bisphosphonate-naive (≥6 months) patients with bone metastases from prostate cancer (PC; n=301) or breast cancer (BC; n=99) enrolled at 98 German sites (May 2006 to July 2008) investigated the effect of ZOL (4 mg intravenously every 4 weeks×4 months, with a final follow-up at 12 months) on bone-marker levels. Secondary assessments: skeletal-related event (SRE) rate, pain, quality of life (QoL), and prostate-specific antigen levels. Endpoints were assessed using summary statistics by visit/tumor type and Kaplan–Meier analyses.
Results
ZOL treatment significantly decreased bone-marker levels (amino-terminal propeptide of type I collagen [P1NP], C-terminal cross-linking telopeptide of type I collagen [CTX]; P<0.0001), and this decrease was maintained through the final 1-year follow-up visit. Baseline P1NP and CTX levels correlated with extent of bone disease (P<0.0001, each) and on-treatment decreases in marker levels. Skeletal disease burden and bone-marker levels were similar between PC and BC patients, and ZOL did not significantly influence osteoprotegerin/receptor activator of nuclear factor-κB ligand levels. Only 13 SREs occurred in 11 patients, supporting the known ZOL-mediated reduction in SREs. On-treatment bone-marker level changes did not correlate with SRE rate, pain scores, or QoL. Generally, ZOL was well tolerated and adverse events were consistent with its known safety profile.
Conclusions
This study confirms that ZOL therapy significantly reduces bone turnover (measured as P1NP and CTX levels) in patients with bone metastases from PC or BC.
Abbreviations: AE, adverse events; BC, breast cancer; CrCl, creatinine clearance; CTX, C-terminal cross-linking telopeptide of type I collagen; ECOG, Eastern Cooperative Oncology Group; EORTC, European Organisation for Research and Treatment of Cancer; ITT, intent-to-treat; NTX, urinary N-telopeptide; OPG, osteoprotegerin; P1NP, amino-terminal propeptide of type I collagen; PC, prostate cancer; PSA, prostate-specific antigen; QoL, quality of life; RANKL, receptor activator of nuclear factor-κB ligand; SRE, skeletal-related event; ULN, upper limit of normal; VAS, visual analogue scale; ZOL, zoledronic acid
Keywords: Bone marker, Bone metastases, Cancer, CTX, P1NP, Zoledronic acid
Highlights
► Bone metastases influence bone metabolism and increase SRE incidence. ► Elevated baseline bone marker levels correlate with burden of bone disease. ► Zoledronic acid significantly reduces bone turnover marker levels.
1. Introduction
Up to 75% of patients with advanced prostate cancer (PC) or breast cancer (BC) will develop bone metastases, which dysregulate normal bone metabolism [1]. Without antiresorptive therapies, most patients with bone metastases will experience potentially debilitating skeletal-related events (SREs: pathologic fractures, spinal cord compression, hypercalcemia, the need for surgery to bone, or severe bone pain requiring palliative radiotherapy) [1], [2]. Zoledronic acid (ZOL) is a nitrogen-containing bisphosphonate and potent osteoclast inhibitor. Treatment with ZOL reduces the risk of SREs and suppresses pathologic bone turnover in patients with multiple myeloma or bone metastases from solid tumors, including PC and BC [3], [4], [5].
Biochemical markers of bone turnover include enzymes and peptides released during the bone remodeling process, which can be measured in the urine or blood and are potentially useful for assessing the overall state of bone turnover [6]. For example, amino-terminal propeptide of type I collagen (P1NP), serum C-terminal cross-linking telopeptide of type I collagen (CTX), and urinary N-telopeptide (NTX) is physiologic byproducts of bone remodeling. Elevated levels of CTX or NTX are common in patients with osteolytic bone lesions; thus, these markers may reflect increased osteolysis (osteoclast-mediated bone resorption), which is more pronounced in BC. The bone formation marker P1NP is elevated in osteoblastic or mixed osteolytic–osteoblastic lesions, as occurs with PC. Other important markers of bone turnover include modulators of osteoclast activity, such as the receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG). Osteoprotegerin is a physiologic inhibitor of RANKL, an inducer of osteoclast activity.
Elevated levels of bone turnover markers (e.g., NTX and CTX) have been associated with poor clinical outcomes in patients with cancer [7], [8], [9], including increased SRE risk [7], [8]. Moreover, antiresorptive therapies have been shown to reduce bone turnover marker levels in patients with cancer [10], [11]. Retrospective analyses of data from phase 3 trials of ZOL versus control (i.e., placebo for PC, pamidronate for BC) in patients with bone metastases from castration-resistant PC (N=314) or BC (N=379) showed that ZOL normalized NTX levels within 3 months in most patients (70% PC, 81% BC) who had high baseline NTX levels (PC, n=193; BC, n=220) [11]. Moreover, ZOL-mediated normalization of NTX levels within 3 months of treatment was associated with decreased risks of first SRE (40% decrease; P≤0.04) and death (PC, 59% decrease; P<0.0001) versus persistently increased NTX levels [11]. Further retrospective analyses of this trial database also showed that ZOL therapy was associated with improved survival in patients with aggressive bone disease [12]. Thus, modulating bone turnover with ZOL may improve clinical outcomes in patients with advanced cancer. Here we present results from the ZOTECT study (NCT00334139), which assessed the effect of ZOL therapy on bone turnover and potential correlations with disease outcomes.
2. Patients and methods
2.1. Study design and treatment
This prospective, single-arm, open-label study examined the effect of ZOL on bone turnover marker levels in patients who were bisphosphonate naive for at least 6 months with bone metastases from PC or BC recruited between May 2006 and July 2008. The patients received four doses of ZOL (4 mg every 4 weeks); continued treatment with ZOL was recommended (but not mandatory) afterward. All patients were advised to take 500-mg calcium supplements and a multivitamin tablet daily. Patients also received concomitant anticancer therapy as determined by the treating physician.
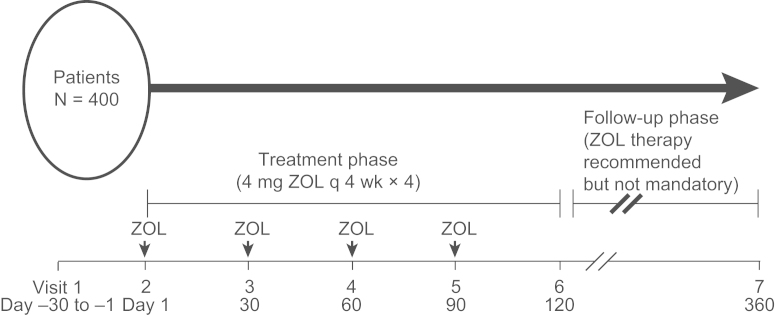
Bone turnover markers (CTX, P1NP, RANKL, and OPG) and prostate-specific antigen (PSA) levels were measured at baseline, monthly through 120 days (study end), and at the final 1-year follow-up visit (360 days; Fig. 1). No data were systematically collected between the 120-day visit and the 1-year follow-up (360-day visit). Laboratory measurements (PSA [patients with PC] and bone markers [all patients]) were performed at a single laboratory (Marburg, Germany). Bone scans were performed at baseline to determine the extent of bone disease, and SREs were assessed at baseline, on-study (visits 2–5), and at study end (visit 6). Pain was evaluated using visual analogue scale (VAS) and analgesic scores. Quality of life (QoL) was measured using the European Organisation for Research and Treatment of Cancer (EORTC) C-30 and BR-23 modules.
Fig. 1.
Trial visit schedule. Bone turnover marker levels and progression-free survival were measured at 1 day (visit 2), monthly through 120 day (visits 3–6), and at 360 day (final 1-year follow-up, visit 7); ZOL (4 mg intravenously q 4 weeks) was administered on visits 2–5. q 4 weeks, every 4 weeks; ZOL, zoledronic acid.
2.2. Patients
2.2.1. Inclusion criteria
All patients had histologically proven PC or BC with one or more cancer-related bone lesions, with or without hormonal therapy. Standard concomitant anticancer therapy was allowed, including prior surgery, chemotherapy, and radiotherapy (completed ≥4 weeks before enrollment). Additional requirements included Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, normal cardiac function, life expectancy of ≥6 months, total bilirubin ≤2.5 times the upper limit of normal (ULN), normal serum glutamic pyruvic transaminase, serum glutamic-oxaloacetic transaminase ≤2.5 times the ULN, and creatinine clearance (CrCl) ≥30 mL/min (Cockcroft–Gault formula). A negative pregnancy test at screening was required for women of childbearing potential. All patients were ≥18 years of age at study entry, signed informed consent before study entry, and were accessible for treatment during the study.
This clinical study was designed, implemented, and reported in accordance with the International Conference on Harmonisation's Harmonised Tripartite Guidelines for Good Clinical Practice, with applicable local regulations (including European Directive 2001/83/EC and US Code of Federal Regulations Part 21), and with the ethical principles laid down in the Declaration of Helsinki.
2.2.2. Exclusion criteria
Patients with prior bisphosphonate therapy within 6 months of enrollment, known hypersensitivity to ZOL or other bisphosphonates, or previous radiation therapy to bone (including therapeutic radioisotopes such as strontium-89) within 1 month were excluded. To reduce the risk of osteonecrosis of the jaw, patients with current or active dental problems or recent/planned dental surgery were excluded. Other exclusion criteria included no detectable cancer-related bone lesions (bone radiographs or bone scan) or the presence of symptomatic brain metastases, renal impairment (CrCl <30 mL/min), abnormal calcium levels (corrected serum calcium <8.0 mg/dL or ≥12.0 mg/dL), history of diseases that influence bone metabolism (e.g., Paget's disease, primary hyperparathyroidism), or osteoporosis (T-score≤−2.5) requiring antiresorptive therapy. Exclusions specific to women included breastfeeding, pregnancy, or failure to use at least one medically acceptable contraception method.
2.3. Key endpoints
The primary endpoint of this trial was the course of changes in bone turnover marker levels (CTX, P1NP, RANKL, OPG) during and after ZOL therapy. Secondary endpoints included pain (VAS and analgesic scores), rate of SREs (excluding hypocalcemia), PSA course (PC cohort), QoL, safety, tolerability, and potential relationships between baseline bone marker levels and clinical disease parameters (extent of disease, pain, and SREs).
2.4. Statistical analyses
Analyses of these data were exploratory (i.e., hypothesis generating rather than hypothesis confirming). Summary statistics (intent-to-treat [ITT] and per-protocol populations) for absolute values and changes from baseline were calculated by visit and tumor type. Data were transformed using natural log (original value +1) to adjust for skewed distributions. Time-to-event variables (time to first SRE and overall survival) were analyzed using the Kaplan–Meier method. All P values were calculated at a 5% significance level (two-sided). Appropriate two-sided 95% confidence intervals were calculated for means and proportions (Clopper and Pearson method). Missing values were replaced by the last observation carried forward. Safety assessments were based on frequency and type of adverse events (AEs) and changes in laboratory values.
3. Results
3.1. Patients
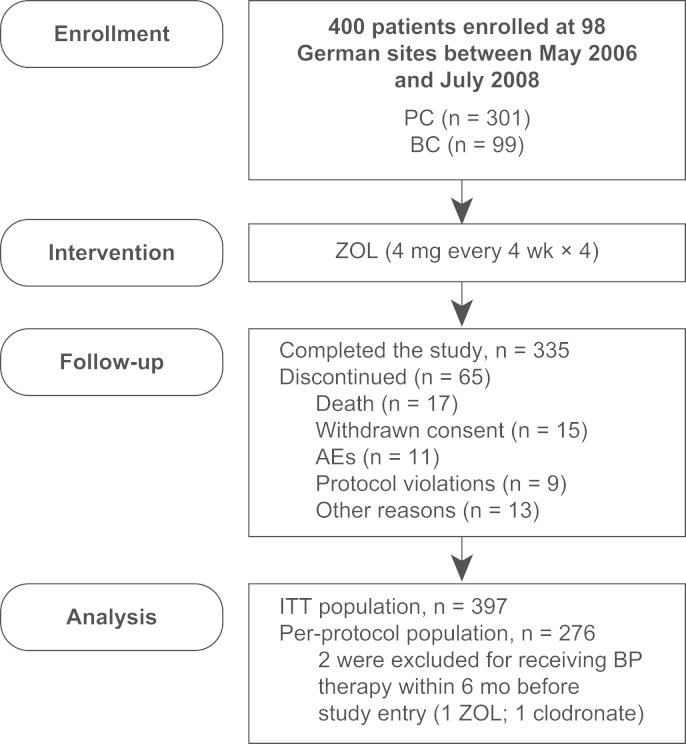
Patients with PC (n=301) or BC (n=99) were enrolled at 98 German sites between May 2006 and July 2008. Of the 400 treated patients, 335 completed the study (Fig. 2). A total of 65 patients discontinued the study because of death (n=17), withdrawn consent (n=15), AEs (n=11), protocol violations (n=9), or other reasons (n=13). The entire ITT population (n=397) was analyzed and is composed of patients who received at least one ZOL dose and had one postbaseline bone marker assessment. Furthermore, within the ITT population, 276 patients were treated per protocol (i.e., had no major protocol violations). Two patients were excluded for bisphosphonate use within 6 months before study entry (1 ZOL and 1 clodronate). Baseline patient demographics and disease characteristics are summarized in Table 1. Notably, at study entry 27 patients had experienced 30 SREs (PC: 15 patients with 17 SREs; BC: 12 patients with 13 SREs), and baseline serum CTX and P1NP levels were higher in patients with PC versus BC. Study-end (120-day) visit data were available for 94% (n=372) of the ITT population (n=397) and 96% (n=266) of the per-protocol population (n=276).
Fig. 2.
CONSORT diagram. AE, adverse event; BP, bisphosphonate; BC, breast cancer; ITT, intent-to-treat; PC, prostate cancer; ZOL, zoledronic acid.
Table 1.
Patient baseline demographics and disease characteristics (ITT).
| Total (N=397) | PC (n=299) | BC (n=98) | |
|---|---|---|---|
| Median age, years (range) | 70 (30–85) | 71 (48–85) | 61 (30–82) |
| Sex, n (%) | |||
| Male | 299 (75.3) | 299 (100) | 0 |
| Female | 98 (24.7) | 0 | 98 (100) |
| Race, n (%) | |||
| Caucasian | 383 (96.5) | 292 (97.7) | 91 (92.9) |
| Asian | 1 (0.3) | 0 | 1 (1.0) |
| Other | 13 (3.3) | 7 (2.3) | 6 (6.1) |
| Prior therapy, n (%) | |||
| Radiation | 129 (32.5) | 75 (25.1) | 54 (55.1) |
| Chemotherapy | 133 (33.5) | 65 (21.7) | 68 (69.4) |
| Hormone therapy | 342 (86.1) | 270 (90.3) | 72 (73.5) |
| Chemo- and hormone therapy | 111 (28) | 61 (20.4) | 50 (51.0) |
| Surgery | 215 (54.2) | 133 (44.2) | 82 (82.8) |
| Mean time from diagnosis to study entry, days (SD) | 155.4 (366.4) | 174.3 (382.2) | 97.7 (308.0) |
| Extent of bone disease, n (%) | |||
| Normal bone scan | 3 (0.8) | 2 (0.7) | 1 (1.0) |
| <6 Bone lesions | 195 (49.1) | 131 (43.8) | 64 (65.3) |
| 6–20 Bone lesions | 118 (29.7) | 93 (31.1) | 25 (25.5) |
| >20 Bone lesions but<super scan | 54 (13.6) | 48 (16.1) | 6 (6.1) |
| Super scan (75% of ribs, vertebrae, and pelvic bones have lesions) | 24 (6.0) | 23 (7.7) | 1 (1.0) |
| Sites of extraskeletal metastases, n (%) | |||
| Lung | 35 (8.8) | 12 (4) | 23 (23.5) |
| Liver | 24 (6.0) | 4 (1.3) | 20 (20.4) |
| Lymph nodes | 98 (24.7) | 80 (26.8) | 18 (18.4) |
| Other | 19 (4.8) | 10 (3.3) | 9 (9.2) |
| Soft tissue | 6 (1.5) | 3 (1.0) | 3 (3.1) |
| Not evaluated | 251 (63.2) | 207 (69.2) | 44 (44.9) |
| Bone turnover marker levels, mean (SD) | (n=387) | (n=294) | (n=93) |
| P1NP (ng/mL) | 227.1 (409.5) | 268 (458.8) | 97.9 (104) |
| CTX (ng/mL) | 0.5 (0.53) | 0.5 (0.58) | 0.3 (0.28) |
| OPG (pmol/L) | 5.6 (2.2) | 5.8 (2.4) | 5.1 (1.7) |
| RANKL (pmol/L) | 0.1 (0.27) | 0.1 (0.29) | 0.1 (0.20) |
| PSA levels, mean (SD) | (n=294) | (n=294) | N/A |
| PSA (μg/L) | 168.5 (531.1) | 168.5 (531.1) | N/A |
BC, breast cancer; CTX, serum C-terminal cross-linking telopeptide of type I collagen; ITT, intent-to-treat population; N/A, not applicable; OPG, osteoprotegerin; P1NP, amino-terminal propeptide of type I collagen; PC, prostate cancer; PSA, prostate-specific antigen; RANKL, receptor activator of nuclear factor-κB ligand; SD, standard deviation.
3.2. Bone turnover marker level changes
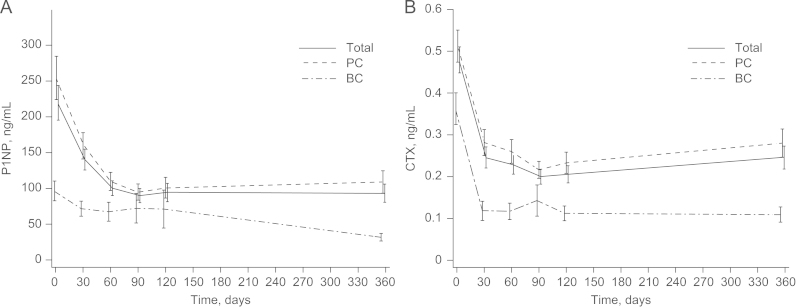
Therapy with ZOL decreased P1NP and CTX levels in 79% of patients (Table 2), and these reductions were significant (30 days, 60 days, or 90 days versus baseline; P<0.001 for all; 120 days versus baseline; P<0.0001). The decreases in P1NP and CTX levels were rapid and sustained through 360 days (Fig. 3), although final 1-year follow-up visit data were available for only 50% of the patients. No significant changes were observed in mean OPG (0.1±1.53 pmol/L) or RANKL (0±0.33 pmol/L) levels at 120 days (end of study) compared with baseline (Wilcoxon P=0.66, both). There were no statistically significant differences in bone marker levels between patients with PC or BC throughout the study.
Table 2.
Patients’ relative change in bone turnover marker levels at 120 days/LOCF versus baseline (ITT population).
| Change from baselinea | P1NP, n (%) | CTX, n (%) |
|---|---|---|
| PC | ||
| ↑ | 48 (16.1) | 45 (15.1) |
| ↓ | 235 (78.6) | 238 (79.6) |
| No change | 0 | 0 |
| BC | ||
| ↑ | 8 (8.2) | 3 (3.1) |
| ↓ | 81 (82.7) | 85 (86.7) |
| No change | 0 | 1 (1.0) |
BC, breast cancer; CTX, serum C-terminal cross-linking telopeptide of type I collagen; ITT, intent-to-treat; LOCF, last observation carried forward; PC, prostate cancer; P1NP, amino-terminal propeptide of type I collagen.
All values that were elevated relative to baseline were defined as an increase. All values that were reduced relative to baseline were defined as a decrease.
Fig. 3.
ZOL decreased (a) P1NP and (b) CTX levels in patients with PC or BC during 4 months of therapy (per-protocol population). (a) Mean change in P1NP levels at 120 days (end of study) versus baseline was −129.8 ng/mL (−42.3%). (b) Mean change in CTX levels at 120 days versus baseline was −0.3 ng/mL (−46.9%). These changes were statistically significant (P<0.0001, both). BC, breast cancer; CTX, serum C-terminal cross-linking telopeptide of type I collagen; PC, prostate cancer; P1NP, amino-terminal propeptide of type I collagen; ZOL, zoledronic acid.
3.3. Correlations between bone turnover marker levels and clinical parameters
3.3.1. Extent of disease at baseline
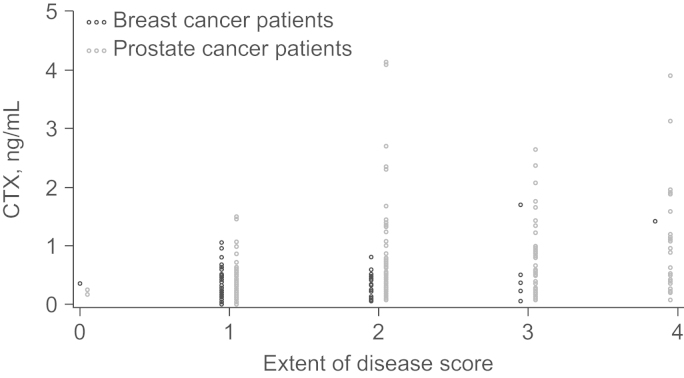
The extent of bone disease at baseline correlated with baseline levels of P1NP and CTX. Greater extent of bone disease was associated with elevated bone turnover marker levels (Fig. 4). Notably, patients with a high extent of bone disease at baseline also recorded the largest on-study reductions in bone marker levels (Table 3).
Fig. 4.
Correlation of baseline extent of disease and bone turnover markers P1NP and CTX in patients with PC or BC. Patients with a high extent of disease score at baseline also had the highest baseline levels of bone turnover markers P1NP and CTX. BC, breast cancer; CTX, serum C-terminal cross-linking telopeptide of type I collagen; PC, prostate cancer; P1NP, amino-terminal propeptide of type I collagen.
Table 3.
Correlation of extent of disease at baseline and change in bone turnover markers P1NP and CTX at 120 days versus baseline (per-protocol population).
| Extent of disease at baseline | Change in bone turnover markers at 120 days versus baseline | ||
|---|---|---|---|
| Patients (n) | P1NP (ng/mL) | CTX (ng/mL) | |
| PC | |||
| <6 Bone lesions | 96 | −57.0 | −0.2 |
| 6–20 Bone lesions | 68 | −234.5 | −0.3 |
| >20 Bone lesions but<super scan | 27 | −406.4 | −0.5 |
| Super scan (75% of ribs, vertebrae, and pelvic bones have lesions) | 15 | −33.6 | –0.2 |
| BC | |||
| <6 Bone lesions | 41 | −5.4 | −0.2 |
| 6–20 Bone lesions | 13 | −30.1 | −0.2 |
| >20 Bone lesions but <super scan | 5 | −113.7 | −0.4 |
| Super scan (75% of ribs, vertebrae, and pelvic bones have lesions) | 1 | −561.1 | −1.2 |
BC, breast cancer; CTX, serum C-terminal cross-linking telopeptide of type I collagen; PC, prostate cancer; P1NP, amino-terminal propeptide of type I collagen.
3.3.2. SREs, pain, and QoL
On-study SREs were infrequent, with 13 SREs recorded in 11 patients (2.8%). These SREs included spinal cord compression (4), radiation to bone (4), pathologic fractures (3), and surgery to bone (2). Patients reported small but significant increases in pain levels, as measured by analgesic scores (120 days versus baseline; P=0.008). Pain scores using the VAS remained nearly constant from visit 1 (26.1±26.6; n=158) to visit 6 (23.0±24.3; n=161). Significant, albeit numerically small, changes in reported QoL measures included decreased physical functioning (−2.7; Wilcoxon P=0.018) and increased global health status/QoL (+3.2; Wilcoxon P=0.019), nausea/vomiting (+2.3; Wilcoxon P=0.030), dyspnea (+5.0; Wilcoxon P=0.001), and financial problems (+3.1; Wilcoxon P=0.021). However, changes in bone turnover marker levels did not correlate with SRE rate, pain, or QoL.
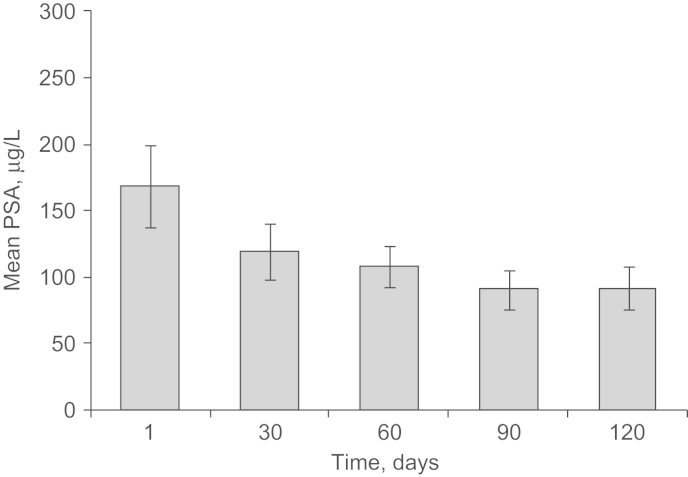
3.4. PSA
Prostate-specific antigen levels decreased with ZOL treatment on study (Fig. 5), and mean PSA levels were lower at study end (120 days; 92.5 μg/L) than at baseline (168.5 μg/L; Wilcoxon signed-rank test P=0.27). At the final 1-year follow-up visit, PSA levels increased to 218.7 μg/L (visit 7; 360 days). Data pertaining to bisphosphonate therapy and changes in anticancer therapies between 120 days (study end) and 360 days (final 1-year follow-up) were not collected. Therefore, it is not known whether ZOL was administered after completion of on-study therapy.
Fig. 5.
Course of PSA levels in patients with PC. Mean PSA levels steadily decreased during ZOL therapy in patients with PC, but were increased at the final 1-year follow-up. PC, prostate cancer; PSA, prostate-specific antigen; ZOL, zoledronic acid.
3.5. Safety
Generally, ZOL was well tolerated and AEs were consistent with its known safety profile. During the study, a total of 788 AEs were reported in 249 patients (Table 4). The most frequently reported AEs were bone pain (11.3%), nausea (8.3%), and fatigue (6.3%). Osteonecrosis of the jaw occurred in 1 patient (PC patient with a dental implant installed >12 months before beginning on-study therapy). Renal failure occurred in 3 patients (all with PC); none were suspected to be related to the study drug.
Table 4.
Adverse events (AEs) and serious adverse events (SAEs).a
| Patients, n (%) (N=400) | |
|---|---|
| All AEs | 249 (62.3) |
| Suspected relationship to study drug | 52 (13.0) |
| Led to dose adjustment or temporary treatment interruption | 4 (1.0) |
| Led to discontinuation | 16 (4.0) |
| Required concomitant medication/nondrug therapy | 155 (38.8) |
| SAEs | 75 (18.8) |
| Death | 17 (4.3) |
| SAEs with suspected relationship to study drug | 4 (1.0) |
| SAEs leading to discontinuation | 10 (2.5) |
| Frequent AEs (≥ 2.8%; by preferred term) | |
| Bone pain | 45 (11.3) |
| Nausea | 33 (8.3) |
| Fatigue | 25 (6.3) |
| Back pain | 19 (4.8) |
| Constipation | 18 (4.5) |
| Vomiting | 18 (4.5) |
| Chills | 18 (4.5) |
| Pain in extremity | 18 (4.5) |
| Pyrexia | 16 (4.0) |
| Diarrhea | 14 (3.5) |
| Tumor pain | 12 (3.0) |
| Anemia | 11 (2.8) |
| Arthralgia | 11 (2.8) |
| Malignant neoplasm progression | 11 (2.8) |
| Cough | 11 (2.8) |
Safety population includes all enrolled patients who received at least one dose of study drug.
4. Discussion
In the ZOTECT study, ZOL therapy was associated with significantly decreased P1NP and CTX levels within 1 month of initiating treatment (P<0.0001) in patients with bone metastases from PC or BC. These data support earlier evidence for ZOL-mediated normalization of bone marker levels [7], [8] and are consistent with exploratory analyses showing that ZOL therapy normalized elevated baseline NTX levels within 3 months in 70% and 81% of patients with PC or BC, respectively [11]. Furthermore, these data may be clinically relevant because elevated bone turnover marker levels have been associated with poor clinical outcomes [7], [8], whereas rapid normalization in marker levels has been associated with improved survival in patients with cancer [11]. Consistent with earlier reports [11], baseline CTX and P1NP levels were higher in PC patients than in BC patients, thereby supporting the mixed rather than purely osteoblastic nature of PC bone lesions in our study.
In the present study, ZOL did not significantly influence the levels of RANKL (inducer of osteoclasts) or OPG (physiologic inhibitor of RANKL). This is in contrast with data from a small trial in patients with newly diagnosed bone metastases from PC, BC, or lung cancer (N=49), wherein ZOL reduced RANKL and increased OPG levels at 12 months, although these changes were not statistically significant [14]. Because of our trial design (i.e., no assessments of bone marker levels and ZOL therapy not required between 120 days and 360 days), late or transient changes in RANKL or OPG levels may not have been detected.
In addition to decreased P1NP and CTX levels, PSA levels continuously decreased during ZOL treatment in patients with PC. Bone marker levels may be elevated in patients with PC receiving androgen-deprivation therapy for locally advanced disease as well as in those with bone metastases [11], [15], and elevated bone marker levels have been correlated with treatment failure (i.e., increasing PSA levels and progression of bone metastases) [16]. Exploratory analyses of a phase 3 study in men with bone metastases from castration-resistant PC [5], [13] suggest a trend for slower PSA progression with ZOL versus placebo [17]. Moreover, these analyses showed that PSA kinetics continued to correlate with clinical outcomes regardless of treatment modalities or the presence of bone disease [17]. Combined with our data showing decreased PSA levels during ZOL treatment, these observations suggest that ZOL-mediated normalization of bone turnover marker levels also might influence disease outcome. However, it should be noted that most patients with PC will not receive ZOL until they develop castration-resistant PC.
A limitation of this study was the single-arm trial design (i.e., no placebo or control arm). In addition, not recording data, particularly bisphosphonate use or anticancer therapy, between the last on-study ZOL dose and the final 1-year follow-up visit limits the potential to analyze correlations between on-study bone marker level changes and clinical outcomes. Despite these limitations, the very low incidence of SREs reported in this study (potentially a result of modern treatments for advanced BC and PC, which may offer better disease control overall and in bone compared with the treatment paradigms used in earlier trials of bisphosphonates [3], [4], [5]) is important and should be considered when interpreting ongoing studies or designing future trials.
5. Conclusions
These data confirm that ZOL decreases bone resorption levels in patients with bone metastases from PC or BC and support associations between bone marker levels and extent of bone disease. Our observations are also consistent with earlier reports of improved clinical outcomes with rapid normalization of bone marker levels during ZOL treatment.
Role of the funding source
The ZOTECT study received support toward research costs and support for medical editorial assistance from Novartis Pharmaceuticals Corporation. No payments were made to the authors for developing this manuscript. Novartis Pharmaceuticals Corporation did not influence the content or views expressed, except for contributions of coauthors Mathias Muth, Amelie Ruebel, Katrin Birkholz, and Christoph May, who are employees of Novartis Pharma GmbH.
Conflict of interest
Mathias Muth, Amelie Ruebel, Christoph May, and Katrin Birkholz are employees of Novartis Pharma GmbH.
Peyman Hadji has received honoraria and unrestricted educational grants from Amgen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, Roche, Sanofi-Aventis, and Wyeth.
Juergen Gschwend has received honoraria and unrestricted educational grants from Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Janssen-Cilag, Novartis, Pfizer, Roche, Sanofi-Aventis, and Wyeth.
May Ziller has received honoraria and unrestricted educational grants from Eli Lilly, Gedeon Richter Pharma GmbH, Jenapharm GmbH and Co. KG, Dr. Kade GmbH, Madaus/Rottapharm GmbH, Novartis, and Pfizer.
Peter Rothe has received honoraria from Novartis.
Michael Autenrieth has received honoraria and unrestricted educational grants from Bayer Healthcare, Pierre Fabre, Novartis, Pfizer, Roche, Sanofi-Aventis, and Wyeth.
Jochen Gleissner was a referee for Novartis and Amgen.
Tobias Maurer and Erhardt Diebel declare that they have no conflict of interest.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Duprane Pedaci Young, PhD, ProEd Communications, Inc., for her medical editorial assistance with this manuscript.
Contributor Information
Peyman Hadji, Email: hadji@med.uni-marburg.de.
May Ziller, Email: ziller@email.de.
Tobias Maurer, Email: T.Maurer@lrz.tu-muenchen.de.
Michael Autenrieth, Email: Michael.Autenrieth@lrz.tu-muenchen.de.
Mathias Muth, Email: mathias.muth@novartis.com.
Amelie Ruebel, Email: amelie.ruebel@novartis.com.
Christoph May, Email: christoph.may@novartis.com.
Katrin Birkholz, Email: katrin.birkholz@novartis.com.
Erhardt Diebel, Email: erhardiebel@t-online.de.
Jochen Gleissner, Email: jgleissner@dgu-team.de.
Peter Rothe, Email: info@vituro.de.
Juergen E. Gschwend, Email: Juergen.Gschwend@lrz.tu-muenchen.de.
References
- 1.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treatment Reviews. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. European Urology. 2011;59:572–583. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Novartis Pharmaceuticals Corporation. Zometa (package insert). East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- 4.Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 5.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. Journal of the National Cancer Institute. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R., Costa L., Saad F., Cook R., Hadji P., Terpos E. Consensus on the utility of bone markers in the malignant bone disease setting. Critical Reviews in Oncology/Hematology. 2011;80:411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Brown J.E., Cook R.J., Major P., Lipton A., Saad F., Smith M. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. Journal of the National Cancer Institute. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 8.Coleman R.E., Major P., Lipton A., Brown J.E., Lee K.A., Smith M. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. Journal of Clinical Oncology. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 9.Jung K., Miller K., Wirth M., Albrecht M., Lein M. Bone turnover markers as predictors of mortality risk in prostate cancer patients with bone metastases following treatment with zoledronic acid. European Urology. 2011;59:604–612. doi: 10.1016/j.eururo.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipton A., Cook R., Saad F., Major P., Garnero P., Terpos E. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 12.Body J-J, Cook RJ, Costa L, Brown JE, Terpos E, Saad F., et al. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumors and poor prognostic features. Poster Presented at The IX International Meeting on Cancer Induced Bone Disease, Arlington, VA, October 28–31, 2009.
- 13.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. Journal of the National Cancer Institute. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim T, Mercatali L, Sacanna E, Ricci R, Scarpi E, Serra P., et al. Circulating levels of RANK/RANKL and OPG in patients with bone metastasis treated with zoledronic acid: a prospective study. Poster Presented at 2011 ASCO Annual Meeting, Chicago, IL, June 3–7; 2011.
- 15.Michaelson M.D., Marujo R.M., Smith M.R. Contribution of androgen deprivation therapy to elevated osteoclast activity in men with metastatic prostate cancer. Clinical Cancer Research. 2004;10:2705–2708. doi: 10.1158/1078-0432.ccr-03-0735. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi M., Yahara J., Noda S. Serum levels of bone turnover markers parallel the results of bone scintigraphy in monitoring bone activity of prostate cancer. Urology. 2003;61:993–998. doi: 10.1016/s0090-4295(02)02583-9. [DOI] [PubMed] [Google Scholar]
- 17.Saad F, Segal S, Eastham J. Prostate-specific antigen kinetics and outcomes in patients with bone metastases from castration-resistant prostate cancer treated with or without zoledronic acid. European Urology 2012 Epub ahead of print. doi:10.1016/j.eururo.2012.05.007 [DOI] [PubMed]