Abstract
Bisphosphonates are the gold standard for preventing skeletal-related events in patients with bone-metastatic cancer and have been investigated for reducing cancer treatment-induced bone loss. Evidence suggests bisphosphonates also offer anticancer benefits in adjuvant and advanced cancer settings. We conducted a retrospective analysis of data from a single-center, unselected cohort of women with early breast cancer (N=1646: 962 received adjuvant bisphosphonates, 684 did not) to assess the impact of bisphosphonates on disease-free and overall survival. The bisphosphonate group comprised all women who started bisphosphonate treatment within 1 year of breast cancer diagnosis and received ≥3 months of bisphosphonate treatment (zoledronic acid, clodronate, ibandronate, or alendronate; majority received zoledronic acid). Disease-free survival was defined as the time from breast cancer diagnosis until first disease recurrence or death. Treatment groups were balanced for cancer stage, hormone receptor expression, and human epidermal growth factor receptor-2 expression. Patients in the no-bisphosphonate group were more likely to be ≥75 years of age, node-negative, and have histologic grade 3 tumors. In patients treated with adjuvant bisphosphonates, disease-free survival was significantly longer than in those who did not receive bisphosphonates (P=0.0017). Both disease-free and overall survival were significantly longer in patients with hormone receptor-positive disease irrespective of lymph node status (disease-free survival: P=0.0038; overall survival: P<0.0026). No significant disease-free survival difference was detected in patients with hormone receptor-negative disease. This large, retrospective study demonstrates a significant survival benefit with adjuvant bisphosphonates in patients with early breast cancer, particularly in patients with node-positive and hormone receptor-positive disease.
Keywords: Adjuvant therapy, Bisphosphonates, Breast cancer, Disease-free survival, Zoledronic acid
Highlights
► Adjuvant bisphosphonate (BP) use increased from 10% in 1998 to >90% in 2008. ► This analysis supports clinical evidence of adjuvant BP benefit in breast cancer. ► Adjuvant BPs were associated with prolonged disease-free survival and OS vs. no BPs. ► Adjuvant BP clinical benefits were more pronounced in HR+ tumors (vs. HR−). ► Adjuvant BP clinical benefits were also pronounced with more lymph node involvement.
1. Introduction
Third-generation aromatase inhibitors (letrozole, anastrozole, and exemestane) have now largely replaced tamoxifen as the standard of care for postmenopausal women with hormone receptor-positive breast cancer because of their greater efficacy [1], [2]. Aromatase inhibitors block the production of peripheral estradiol precursors and reduce endogenous estrogen to levels substantially below those naturally occurring in healthy postmenopausal women [3]. Because estrogen is a negative regulator of bone turnover, its depletion during aromatase inhibitor therapy results in increased bone turnover and osteoclast activity, and leads to rapid reduction in bone mineral density and increased risk of fractures [4], [5]. Bisphosphonates (BPs)4 inhibit osteoclast-mediated bone resorption, and thus can reduce or prevent bone loss during aromatase inhibitor therapy. Intravenous (IV) and oral (PO) BPs such as zoledronic acid, pamidronate, clodronate, and ibandronate are approved for reducing the risk of skeletal-related events (SREs) in patients with metastatic bone disease, and have demonstrated efficacy for preventing cancer treatment-induced bone loss in patients with breast cancer [5]. Denosumab, a monoclonal antibody against the receptor activator of nuclear factor kappaB ligand (RANKL), has emerged as an alternative to BPs for reducing the risk of SREs in patients with bone metastases from solid tumors, including breast cancer [6], [7]. However, the long-term side-effect profile of denosumab is still unknown, and no guidance currently exists for managing the risk of hypocalcemia. Because denosumab has a short history of clinical use and has not demonstrated anticancer activity, it was not included in this study.
A growing body of evidence supports the anticancer benefits of BPs above and beyond their bone-conserving effects. In recent retrospective (N=154,768) and population-based (N=9950) studies in healthy women, BP treatment was associated with reduced risk of breast cancer [8], [9], [10]. Preclinical and clinical data suggest that BPs may provide anticancer benefits such as inducing cancer cell apoptosis; inhibiting cancer cell proliferation, migration, invasiveness, and tumor-associated angiogenesis; activating anticancer immune activity; and potential synergy with anticancer agents [5], [11], [12], [13]. Several pilot clinical studies in women with early breast cancer demonstrated that adding (neo)adjuvant zoledronic acid (4 mg every 3–4 weeks) to hormonal therapy or chemotherapy reduced the number of persistent disseminated tumor cells in the bone marrow compared with regular anticancer therapy alone [14], [15], [16], [17]. Such reductions in disseminated tumor cells might lead to reduced tumor recurrences in these patients. Two clinical trials that included patients with early breast cancer who were either postmenopausal or premenopausal and received ovarian suppression showed that adding twice-yearly zoledronic acid (4 mg) to standard adjuvant endocrine therapy significantly improved disease-free survival (DFS) compared with endocrine therapy alone [18], [19]. Moreover, in patients with breast cancer and bone marrow micrometastases, overall survival (OS) improved with adjuvant clodronate compared with placebo [20], [21]. In a meta-analysis of randomized, controlled clinical trials of BPs, adjuvant zoledronic acid reduced the risk of breast cancer recurrence; however, overall, adjuvant BPs did not reduce mortality, disease recurrence rates, or relapse rates in this population [22]. Recent results from the Adjuvant Zoledronic acid to redUce REcurrence (AZURE) trial, conducted in women with early breast cancer, showed no significant benefit of adjuvant zoledronic acid in the overall population, whereas significant DFS and OS benefits were observed in a subset of women with established postmenopausal status (i.e., >5 years postmenopause at baseline) [23]. These results suggest that the anticancer effects of BPs may be dependent on a low-estrogen environment.
In the current retrospective analysis, we assessed DFS and OS in unselected patients with early breast cancer who received BPs or no BPs at the St. Josefs Breast Centre in the St. Josefsklinik in Offenburg, Germany, from 1997 to 2009. We report here the results of the overall population and subgroups stratified by hormone receptor status and nodal involvement.
2. Materials and methods
2.1. Data source
Clinical data were obtained between 1997 and 2009 from all women who received treatment for early breast cancer at the St. Josefs Breast Centre in the St. Josefsklinik in Offenburg, Germany.
2.2. Patient selection
This retrospective analysis included all patients with invasive breast cancer and documented tumor-node-metastasis (TNM) stage and hormone receptor status. Additionally, women included in the BP cohort of this study must have initiated BP therapy <1 year after diagnosis of breast cancer and must have received BPs for ≥3 months. These selection criteria were chosen to allow standard therapy for breast cancer (i.e., surgery, chemotherapy, radiotherapy) before initiation of BP therapy and to eliminate women with early relapse or early noncompliance with BP therapy.
Patients included in the BP group received one of the following: zoledronic acid 4 mg IV two to three times per year; ibandronate 50 mg/day PO, 150 mg/month PO, or 4 mg to 6 mg IV every 3 months; clodronate 1600 mg/day PO; or alendronate 70 mg/week PO. Some patients switched from one BP to another, especially among those receiving oral agents.
2.3. Statistical analyses
Disease-free survival was defined as the time from breast cancer diagnosis until first disease recurrence or death. Disease-free survival and OS were estimated using the Kaplan–Meier method and were compared between the BP and no-BP groups using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for DFS and OS in BP- vs. no-BP-treated patients were obtained via Cox regression models/multivariate analyses adjusted for age, Body Mass Index, tumor stage, nodal status, grade, estrogen receptor (ER) status, and progesterone receptor (PgR) status. Data were stratified by hormone receptor and nodal status. Tumors were considered to be hormone receptor-positive if ≥10% of cells were ER-positive (ER+), and hormone receptor-negative if <10% of cells were ER+ and PgR-positive (PgR+). Stratification was based on nodal status. All results were based on two-sided analyses and quantified with P values. A MaDoS documentation system was used to access patient data on baseline characteristics, clinical interventions, and outcomes. All statistical calculations were performed using WinSTAT.
2.4. Calculations
From the clinical evidence presented thus far, BPs have a theoretical role in adjuvant breast cancer therapy both to prevent SREs during endocrine therapy and to provide anticancer benefits. As this knowledge becomes common among physicians, BP use during adjuvant therapy in breast cancer may increase in clinical practices. Examining the management of breast cancer cases in a clinical practice over the time course of the clinical data presentation would reveal any effects of the clinical evidence on the real-world treatment of breast cancer. Moreover, if adjuvant BP use increased, then clinical benefits from the addition of BPs to adjuvant therapy could be assessed in the patient population treated in clinical practice. Data from such an evaluation could provide realistic expectations of treatment results on which physicians could base their treatment decision. Therefore, we conducted this retrospective analysis of our large and long-term patient database.
3. Results
3.1. Study population
A total of 1646 women were eligible for inclusion in the analysis, of whom 962 received BPs (BP group) and 684 did not (no-BP group). At the date of database lock (November 1, 2012), follow-up data were obtained at 12–24 months after completion of adjuvant therapy in all patients, and thereafter until last follow-up visit or documented death.
Demographic and baseline characteristics were generally balanced between the two treatment groups, with the following exceptions: fewer patients in the BP group compared with the no-BP group had histologic grade 3 tumors (29% vs. 33%, respectively), and more patients in the BP group were <75 years of age (87% vs. 74%, respectively), node positive (43% vs. 34%, respectively), and had received adjuvant chemotherapy (62% vs. 33%, respectively) (Table 1). The majority of patients had tumors that were ≥10% ER+ (83% in the BP group and 81% in the no-BP groups) and ≥70% of women in each group had PgR+ tumors. In the BP group 100% of patients received endocrine therapy, as did 96% of patients in the no-BP group. The majority of patients in the BP group received (at any time) ibandronate (25%), zoledronic acid (23%), or alendronate (16%).
Table 1.
Patient demographics and baseline disease characteristics.
| BP group (N=937) |
No-BP group (N=630) |
P value |
|||
|---|---|---|---|---|---|
| Age, years | |||||
| Mean (SD) | 59.5 (12.8) | 64.9 (13.4) | <0.001 | ||
| <40 | 45 | 5% | 23 | 4% | 0.032 |
| ≥40 | 892 | 95% | 607 | 96% | |
| <75 | 819 | 87% | 464 | 74% | |
| ≥75 | 118 | 13% | 166 | 26% | <0.001 |
| BMI, median (SD) | 26.9 (5.0) | 27.3 (5.4) | 0.28 | ||
| Cancer stage | |||||
| T1 | 620 | 66% | 401 | 64% | 0.88 |
| T2, T3, or T4 | 317 | 34% | 229 | 36% | |
| Nodal status | |||||
| N0 | 537 | 57% | 413 | 66% | |
| N+ | 400 | 43% | 217 | 34% | 0.0003 |
| Histologic grade | |||||
| G1 or G2 | 662 | 71% | 421 | 67% | |
| G3 | 275 | 29% | 209 | 33% | 0.19 |
| ER-positivea | |||||
| ≥10% of cells | 778 | 83% | 509 | 81% | 0.36 |
| <10% of cells | 159 | 17% | 121 | 19% | |
| PgR-positivea | |||||
| ≥10% of cells | 660 | 70% | 453 | 72% | 0.40 |
| <10% of cells | 277 | 30% | 177 | 28% | |
| HER2 status | |||||
| HER2 positive | 125 | 13% | 75 | 12% | 0.29 |
| HER2 negative | 750 | 80% | 517 | 82% | 0.29 |
| Adjuvant therapy | |||||
| Endocrine therapy | 778/778 | 100% | 488/509 | 96% | |
| Chemotherapy | 581 | 62% | 208 | 33% | <0.001 |
| Bisphosphonate therapy | |||||
| Zoledronic acid | 216 | 23% | — | ||
| Ibandronate | 238 | 25% | — | ||
| Clodronate | 87 | 9% | — | ||
| Alendronate | 152 | 16% | — | ||
| Switch to zoledronic acid | 98 | 11% | — | ||
| Switch to other BP | 93 | 10% | — | ||
| Other BP or multiple BP switches | 53 | 6% | — | ||
| Patient subgroups included in Kaplan–Meier analyses | |||||
| N+, ER+ ≥10% | 338 | 36% | 171 | 27% | <0.001 |
| Hormone receptor negativea | 154 | 16% | 114 | 18% | 0.36 |
BP, bisphosphonate; ER, estrogen receptor; HER2, human epidermal growth factor receptor-2; N+, node positive; PgR, progesterone receptor; SD, standard deviation.
Tumors were considered ER/PgR-positive if ≥10% of cells were ER- and PgR-positive, and HR-negative if <10% of cells were ER- and PgR-positive.
3.2. Bisphosphonate use during the study period
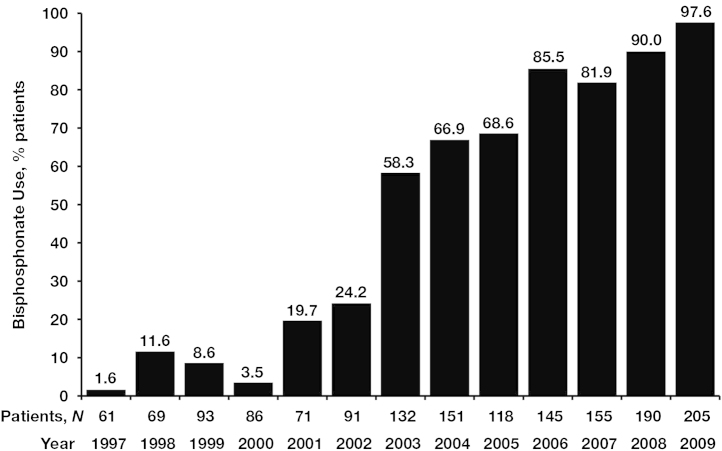
The use of BPs increased during the 12-year study period, from approximately 2% of patients in 1997 to 98% of patients in 2009 (Fig. 1). From 1997 to 2001, 34 of 380 patients (9%) received BPs compared with 346 of 380 patients (91%) who did not, and from 2002 to 2009, 903 of 1187 patients (76%) received BPs compared with 284 of 1187 (24%) who did not.
Fig. 1.
Frequency of bisphosphonate use during the study period (1997–2009).
3.3. Efficacy analysis
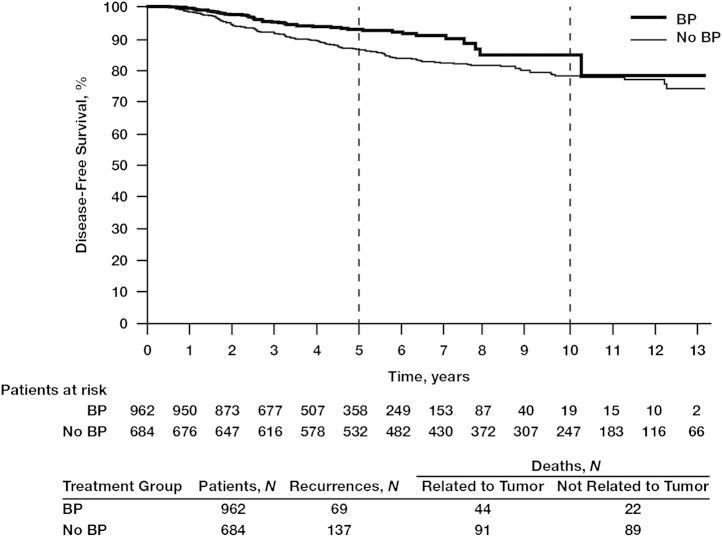
In the overall population at the end of follow-up, the DFS rate was 83.0% in the BP group compared with 79.6% in the no-BP group. Kaplan–Meier estimates of DFS demonstrated a statistically significant improvement in patients who received BPs compared with those who did not (P=0.0017; Fig. 2).
Fig. 2.
Kaplan–Meier estimates for disease-free survival in the overall patient population treated with or without bisphosphonates (BPs).
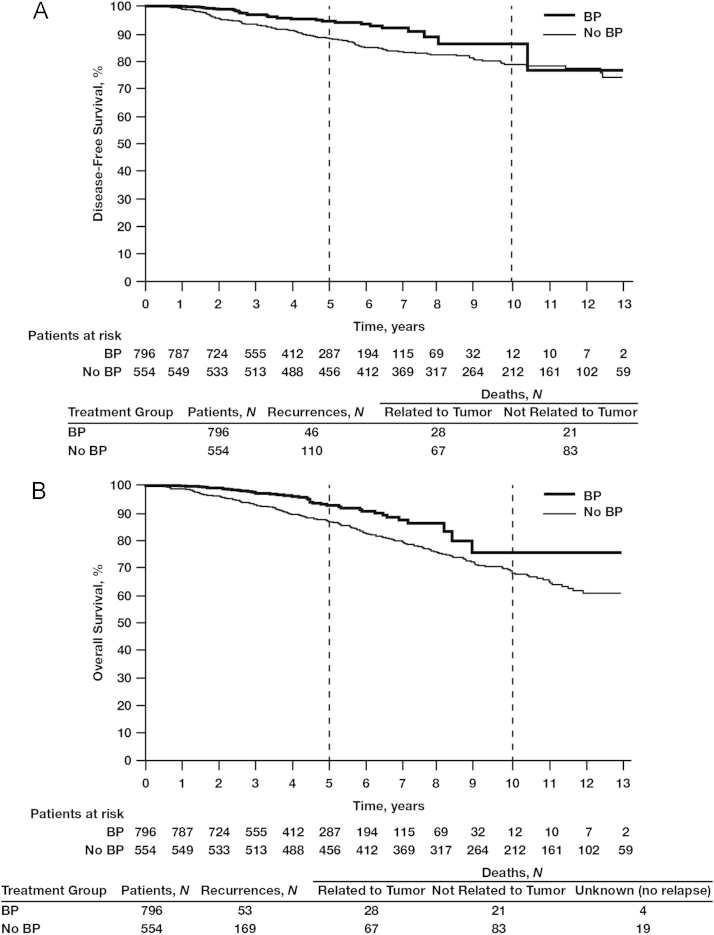
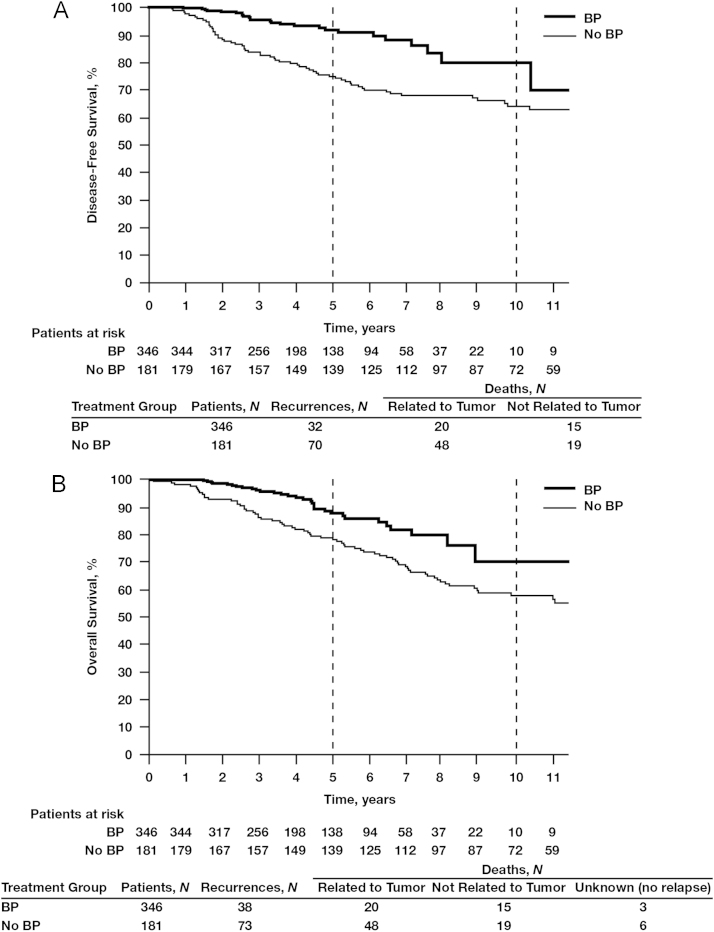
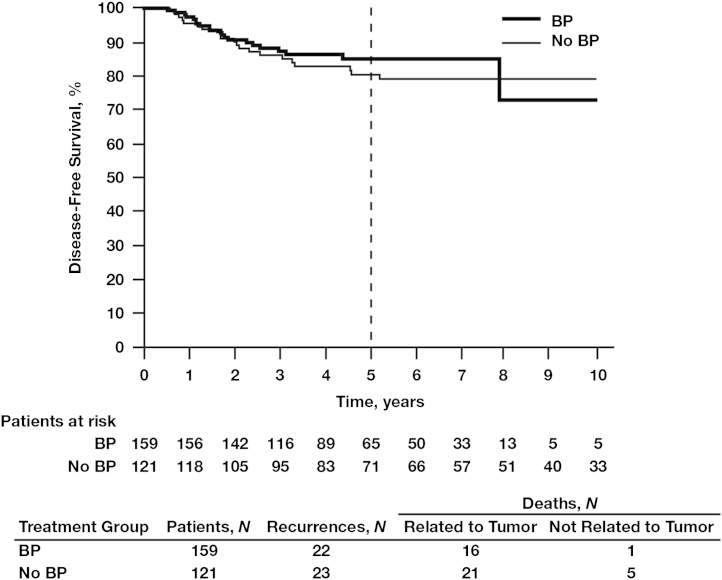
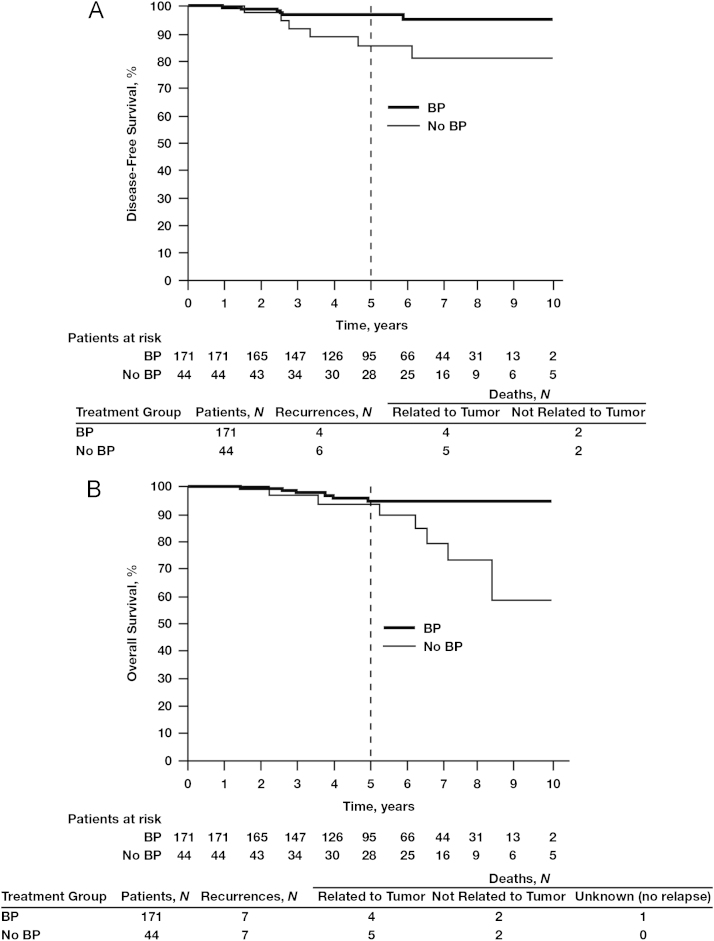
The 5-year DFS benefits of adjuvant BP use compared with no BP use were apparent irrespective of nodal status: 92.5% vs. 87.4%, respectively (Fig. 2). In women with hormone receptor-positive tumors and node-positive status (irrespective of the number of lymph nodes affected), those treated with BPs (N=796) had a significantly better 5-year DFS rate of 93.7% compared with 89.0% for those in the no-BP group (N=554; HR=0.59; 95% CI, 0.41–0.84; P=0.0038; Fig. 3A). In this patient subset, BP treatment was also associated with significantly improved OS rates at 10 years compared with no BP use (78.4% vs. 70.8%, respectively; HR=0.60; 95% CI, 0.43–0.84; P<0.0026; Fig. 3B), possibly influenced by the increased age in the no-BP group vs. the BP group (age >70 years; mean age BP group, 77.1±5.7 vs. no-BP group, 78.6±5.5 years; standard deviation, 12.5 years) (data not shown). The DFS benefit of BP therapy was most profound among women with hormone receptor-positive tumors who were node positive, with 5-year DFS rates of 91.2% in the BP group (N=346) and 76.6% in the no-BP group (N=181; HR=0.38; 95% CI, 0.25–0.59; P=0.000018; Fig. 4A). In this patient subset, BP treatment was also associated with a statistically significant improvement in OS (HR=0.56; 95% CI, 0.37–0.80; P=0.006; Fig. 4B) potentially also related to mean age differences between the groups. Interestingly, in the subset of patients with hormone receptor-negative tumors irrespective of nodal status, there was no statistically significant difference in DFS between the BP group (N=159) and the no-BP group (N=121) at 5 and 10 years (86.5% vs. 81.6% and 78.3% vs. 80.4%, respectively, HR=0.80; 95% CI, 0.45–1.46; P=0.48; Fig. 5).
Fig. 3.
Kaplan–Meier estimates of disease-free survival (A) and overall survival (B) in patients with hormone receptor-positive (≥10% estrogen receptor-positive) breast cancer (irrespective of nodal status) treated with or without bisphosphonates (BPs).
Fig. 4.
Kaplan–Meier estimates of disease-free survival (A) and overall survival (B) in patients with node-positive and hormone receptor-positive breast cancer treated with or without bisphosphonates (BPs). Node-positive refers to N1-N3.
Fig. 5.
Kaplan–Meier estimates of disease-free survival in patients with hormone receptor-negative breast cancer (irrespective of nodal status) treated with or without bisphosphonates (BPs).
Among BP-treated patients with hormone receptor-positive tumors (irrespective of nodal status), estrogen levels influenced survival outcomes regardless of patient age. Disease-free survival was significantly longer in patients with estrogen levels <10 pg/mL (N=171) compared with patients with levels ≥10 pg/mL (N=44) (HR=0.17; 95% CI, 0.05–0.62; P=0.007; Fig. 6A). The between-group difference based on estrogen levels (<10 pg/mL vs. ≥10 pg/mL) was also apparent for OS (HR=0.28; 95% CI, 0.10–0.80; P=0.018; Fig. 6B).
Fig. 6.
Kaplan–Meier estimates of disease-free survival (A) and overall survival (B) in bisphosphonate (BP)-treated patients with hormone receptor-positive breast cancer (irrespective of nodal status), by estrogen levels.
Additional analyses were performed to assess the potential effects of imbalances in BP use during the study period (1997–2001 and 2002–2009) and certain patient characteristics on the outcomes of the study. Among patients with hormone receptor-positive disease in the no-BP group for the periods 1997–2001 (N=320) and 2002–2009 (N=234), there were no statistically significant differences in DFS with regard to nodal status. Among patients <75 years of age with hormone receptor-positive disease (irrespective of nodal status), 5-year DFS was significantly better in the BP group compared with the no-BP group (94.1% vs. 89.8%, respectively; HR=0.62; 95% CI, 0.42–0.94; P=0.023). Similarly, among patients <75 years of age with node-positive and receptor-positive disease, 5-year DFS was significantly better in the BP group (N=295) compared with the no-BP group (N=124) (91.8% vs. 76.0%, respectively; HR=0.39; 95% CI, 0.24–0.63; P=0.00013), but this benefit did not extend to a statistically significant OS benefit (HR=0.68; 95% CI, 0.40–1.17; P=0.17). In patients with hormone receptor-positive, node-negative disease, irrespective of age, no difference in DFS was observed among BP-treated patients who received chemotherapy (N=138) and those who did not receive chemotherapy (N=312) (96.0% vs. 96.1%, respectively). This comparison was not possible for the no-BP group given the small number of patients (N=18) in this subgroup who received chemotherapy.
4. Discussion
This retrospective analysis examined data from patients with early breast cancer treated over a 12-year period (1997–2009) in a real-world setting. The proportion of patients receiving adjuvant BP therapy dramatically increased during the study period, from <2% in 1997 to nearly 100% in 2009 (Fig. 1), presumably because of proactive management of bone health and accumulating evidence of the potential anticancer benefits of BPs [18], [19], [24], [25], [26]. For example, the earliest clinical evidence of such benefits, demonstrated with clodronate, contributed to a modest increase in BP use of approximately 10% in 1998 and 1999. In 2001 and 2002, BP use more than doubled with the introduction of zoledronic acid. Moreover, initial reports from the Austrian Breast and Colorectal Cancer Study Group-12 (ABCSG-12) and Zometa–Femara Adjuvant Synergy Trial (ZO-FAST) trials of the anticancer potential of zoledronic acid increased BP use to 90% or more in 2008 and 2009. Our analysis revealed that BP therapy initiated within 1 year of breast cancer diagnosis and continued for at least 3 months was associated with significantly prolonged DFS compared with no BP therapy in women receiving adjuvant therapy for early breast cancer. The benefits of adjuvant BP therapy appear more pronounced in women with hormone receptor-positive vs. hormone receptor-negative tumors and increased with the extent of lymph node involvement; indeed, even OS benefits could be observed in this patient subgroup. These findings are consistent with those of previous clinical trials evaluating anticancer benefits with BPs in the adjuvant breast cancer setting [18], [19], [27], [28].
To date, the most promising data on the potential anticancer benefits of BPs have been reported with zoledronic acid. Three clinical trials have directly assessed the effects of adjuvant zoledronic acid on disease recurrence and survival in women receiving various adjuvant therapies for breast cancer. In the ABCSG-12 trial, 1803 premenopausal women with hormone receptor-positive early breast cancer received ovarian suppression with the gonadotropin-releasing hormone analogue goserelin and were treated with tamoxifen or anastrozole with or without zoledronic acid for 3 years [19]. At a median follow-up of 48 months, the combination of zoledronic acid plus endocrine therapy significantly reduced the relative risk of disease progression by 36% (HR=0.64; P=0.01) and the risk of recurrence by 35% (HR=0.65; P=0.01) compared with endocrine therapy alone. These zoledronic acid benefits were maintained at the 84-month follow-up, more than 5 years after treatment completion, for both DFS (HR=0.72; P=0.014) and OS (HR=0.63; P=0.049), and were greatest among women >40 years of age at study entry compared with women ≤40 years of age (DFS: HR=0.66, P=0.013; OS: HR=0.57, P=0.042) [29]. The ZO-FAST trial evaluated the effects of immediate versus delayed zoledronic acid on bone mineral density, disease recurrence, and survival in 1065 postmenopausal women with hormone receptor-positive early breast cancer who received adjuvant therapy with letrozole [18]. Compared with delayed zoledronic acid treatment, immediate zoledronic acid was associated with a significant 41% reduction in the risk of DFS events (HR=0.59; P=0.0314) at 36 months [18], and continued to reduce the risk at 60 months (HR=0.66; P=0.0375) [30]. Treatment with immediate zoledronic acid reduced both skeletal and nonskeletal DFS events compared with delayed zoledronic acid [30]. In contrast, in the AZURE trial (N=3360) in women with breast cancer who received standard adjuvant systemic therapy (including endocrine therapy), zoledronic acid therapy did not show a survival benefit at 59 months in the overall population [23]. However, prespecified subgroup analyses in women who had been postmenopausal for more than 5 years at baseline (N=1041) showed that adding zoledronic acid significantly improved invasive DFS (HR=0.75; P=0.02) and OS (HR=0.74; P=0.04) [23], [28]. In contrast to our retrospective analysis, the beneficial effects of zoledronic acid on disease recurrence and survival outcomes in the postmenopausal subset in AZURE were independent of hormone-receptor status and the extent of lymph node involvement (all study participants in AZURE were node positive at study entry) [23]. Data from these trials suggest that estrogen effects on the bone microenvironment may play a role in determining the characteristics of patients most likely to benefit from adjuvant zoledronic acid therapy. Therefore, patients with hormone receptor-negative disease in our analysis likely did not benefit from BP therapy because they did not receive endocrine therapy and consequently would have had higher estrogen levels. Ongoing trials evaluating bone-targeted therapies in the adjuvant setting for anticancer benefits include denosumab in women with high-risk early breast cancer receiving neoadjuvant or adjuvant therapy (D-CARE) [31] and zoledronic acid, clodronate, and ibandronate in women with stage 1, 2, or 3 breast cancer receiving adjuvant therapy after surgery [32].
Results of the current retrospective analysis must be interpreted with several limitations in mind. First, although a retrospective study may provide a window into real-world clinical outcomes, it is not powered to detect differences to the same degree as a prospective, controlled trial. Moreover, the current patient population was heterogeneous with respect to BP and anticancer therapy use and certain baseline characteristics, and statistical adjustment for confounding variables was not included in the analysis. Finally, adverse events were not captured and analyzed, which prevented assessment of the benefit:risk profile associated with adjuvant BPs in clinical practice.
In conclusion, this large, single-center, retrospective study demonstrated significant DFS benefit in patients with early breast cancer who received adjuvant BPs compared with no BPs. The DFS benefit was most notable in BP-treated patients with node-positive and hormone receptor-positive breast cancer; however, the influence of estrogen levels on outcomes was clearly evident and it remains possible that even patients with hormone receptor-negative disease may benefit from BP therapy if their estrogen levels are low. These analyses support the anticancer activity of adjuvant BPs demonstrated in several large phase 3 trials involving more than 6000 patients with early breast cancer. The results of these trials and our retrospective analysis, although promising, indicate a need to further refine our understanding of the effects of BPs on disease outcomes and the patient populations that may benefit most from BP therapy.
Approval statement
Approval was not required for this analysis.
Role of the funding source
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. The sponsor had no other role in the conduct of the research or the preparation of the article.
Conflict of interest
Dr. Hadji has received honoraria, unrestricted educational grants, and research funding from the following companies: Amgen, Novartis, GlaxoSmithKline, Eli Lilly, AstraZeneca, Roche, and Pfizer. All other authors have no conflict of interest to declare.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Marithea Goberville, PhD, ProEd Communications, Inc., for medical editorial assistance with this manuscript.
Footnotes
ABCSG-12, Austrian Breast and Colorectal Cancer Study Group-12; AZURE, Adjuvant Zoledronic acid to redUce REcurrence; BP, bisphosphonate; CI, confidence interval; DFS, disease-free survival; ER+, estrogen receptor-positive; HR, hazard ratio; IV, intravenous; OS, overall survival; PgR+, progesterone receptor-positive; PO, oral administration; RANKL, receptor activator of nuclear factor kappaB ligand; SRE, skeletal-related events; TNM, tumor-node-metastasis; ZO-FAST, Zometa–Femara Adjuvant Synergy Trial.
Contributor Information
Peyman Hadji, Email: hadji@med.uni-marburg.de.
Matthias Frank, Email: matthias.frank@og.ortenau-klinikum.de.
Andreas Jakob, Email: andreas@onkologie-offenburg.de.
Jan Willem Siebers, Email: info@jwsiebers.de.
References
- 1.Aebi S, Davidson T, Gruber G, Castiglione M. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl. 5) doi: 10.1093/annonc/mdq159. : v9-14. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69(1):73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST study results. Cancer. 2008;112(5):1001–1010. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 5.Gnant M, Dubsky P, Fitzal F, Blaha P, Schoppmann S, Steger G. Maintaining bone density in patients undergoing treatment for breast cancer: is there an adjuvant benefit? Clin Breast Cancer. 2009;9(Suppl. 1):S18–S27. doi: 10.3816/CBC.2009.s.002. [DOI] [PubMed] [Google Scholar]
- 6.Amgen Inc. Xgeva® (denosumab) injection [package insert], 〈http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/125320s007lbl.pdf〉; 2010 (accessed 26.07.2012).
- 7.National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology: Breast Cancer, 〈V2.2011. www.nccn.com〉; (accessed 26.06.2012).
- 8.Chlebowski RT, Chen Z, Cauley JA, Anderson G, Rodabough RJ, McTiernan A. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J Clin Oncol. 2010;28(22):3582–3590. doi: 10.1200/JCO.2010.28.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br J Cancer. 2010;102(5):799–802. doi: 10.1038/sj.bjc.6605555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. J Clin Oncol. 2010;28(22):3577–3581. doi: 10.1200/JCO.2010.28.1113. [DOI] [PubMed] [Google Scholar]
- 11.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34(5):453–475. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Neville-Webbe HL, Gnant M, Coleman RE. Potential anticancer properties of bisphosphonates. Semin Oncol. 2010;37(Suppl. 1):S53–S65. doi: 10.1053/j.seminoncol.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Lipton A. Improving progression-free and overall survival in patients with cancer: a potential role for bisphosphonates. Expert Opin Pharmacother. 2011;12(5):749–762. doi: 10.1517/14656566.2011.538384. [DOI] [PubMed] [Google Scholar]
- 14.Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11(5):421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin AY, Park JW, Scott J, Melisko M, Goga A, Moasser MM, et al. Zoledronic acid as adjuvant therapy for women with early stage breast cancer and disseminated tumor cells in bone marrow (abstract 559). In: Poster presented at: 44th Annual Meeting of the American Society of Clinical Oncology. Chicago, IL: May 30–Jun 3; 2008.
- 16.Rack B, Juckstock J, Genss EM, Schoberth A, Schindlbeck C, Strobl B. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010;30(5):1807–1813. [PubMed] [Google Scholar]
- 17.Solomayer E, Gebauer G, Hirnle P, Janni W, Lück HJ, Becker S. Influence of zoledronic acid on disseminated tumor cells (DTC) in primary breast cancer patients (abstract 2048) Cancer Res. 2009;69(Suppl. 1):170s–171s. doi: 10.1093/annonc/mdr612. [DOI] [PubMed] [Google Scholar]
- 18.Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21(11):2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 19.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360(7):679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 20.Diel IJ, Jaschke A, Solomayer EF, Gollan C, Bastert G, Sohn C. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19(12):2007–2011. doi: 10.1093/annonc/mdn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powles T, Paterson A, McCloskey E, Schein P, Scheffler B, Tidy A. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Res. 2006;8(2):R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauri D, Valachis A, Polyzos NP, Tsali L, Mavroudis D, Georgoulias V. Does adjuvant bisphosphonate in early breast cancer modify the natural course of the disease? A meta-analysis of randomized controlled trials. J Natl Compr Canc Netw. 2010;8(3):279–286. doi: 10.6004/jnccn.2010.0020. [DOI] [PubMed] [Google Scholar]
- 23.Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 24.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339(6):357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 25.Powles T, Paterson S, Kanis JA, McCloskey E, Ashley S, Tidy A. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20(15):3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 26.Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol. 2001;19(1):10–17. doi: 10.1200/JCO.2001.19.1.10. [DOI] [PubMed] [Google Scholar]
- 27.Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43(7):650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 28.Coleman R, Marshall H, Gregory W, Bell R, Dodwell D, Keane M. Discordant treatment effects according to menopausal status following adjuvant zoledronic acid in stage II/III breast cancer. The AZURE trial (BIG 01/04) (abstract 5019) Eur J Cancer. 2011;47(1):S336. [Google Scholar]
- 29.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Stoeger H, Dubsky P, Jakesz R, et al. Long-term follow-up in ABCSG-12: significantly improved overall survival with adjuvant zoledronic acid in premenopausal patients with endocrine-receptor-positive early breast cancer (abstract S1-2). In: Presented at: CTRC-AACR San Antonio Breast Cancer Symposium. San Antonio, TX: Dec 6–10; 2011.
- 30.de Boer R, Bundred N, Eidtmann H, Llombart A, von Minckwitz G, Martin N, et al. The effect of zoledronic acid on aromatase-inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: the ZO-FAST study 5-year final follow-up (abstract P5-11-01). In: Poster presented at: CTRC-AACR San Antonio Breast Cancer Symposium. San Antonio, TX: Dec 8–12; 2010.
- 31.Amgen, Daiichi Sankyo. Study of denosumab as adjuvant treatment for women with high risk early breast cancer receiving neoadjuvant or adjuvant therapy (D-CARE). Available at: 〈http://www.clinicaltrials.gov/ct2/show/NCT01077154?term=denosumab+early+breast+cancer&rank=1〉; (accessed 19.12.2012).
- 32.Southwest Oncology Group, National Cancer Institute, North Central Cancer Treatment Group, Eastern Cooperative Oncology Group, National Surgical Adjuvant Breast and Bowel Project, Cancer and Leukemia Group B, et al. Zoledronate, clodronate, or ibandronate in treating women who have undergone surgery for Stage I, Stage II, or Stage III breast cancer. Available at: 〈http://www.clinicaltrials.gov/ct2/show/NCT00127205?term=SWOG+0307&rank=2〉; (accessed 20.12.2012).