Abstract
The genus Vigna (Fabaceae) consists of five subgenera, and includes more than 100 wild species. In Vigna, 10 crops have been domesticated from three subgenera, Vigna, Plectrotropis, and Ceratotropis. The habitats of wild Vigna species are so diverse that their genomes could harbor various genes responsible for environmental stress adaptation, which could lead to innovations in agriculture. Since some of the gene bank Vigna accessions were unidentified and they seemed to be novel genetic resources, these accessions were identified based on morphological traits. The phylogenetic positions were estimated based on the DNA sequences of nuclear rDNA-ITS and chloroplast atpB-rbcL spacer regions. Based on the results, the potential usefulness of the recently described species V. indica and V. sahyadriana, and some wild Vigna species, i.e., V. aconitifolia, V. dalzelliana, V. khandalensis, V. marina var. oblonga, and V. vexillata, was discussed.
Introduction
The genus Vigna, in the family Fabaceae, comprises more than 100 wild species [1]. It is an agriculturally important taxon, which includes 10 domesticated species (crops) such as cowpea (Vigna unguiculata (L.) Walpers), mung bean (Vigna radiata (L.) Wilczek) and azuki bean (Vigna angularis (Willd.) Ohwi & Ohashi). Since some of their wild relatives inhabit extreme environments such as arid land, sandy beaches, and limestone karsts [2], they are expected to harbor adaptive genes, which could be used for developing stress-resistant crops for agriculturally unsuitable lands. Moreover, since they have evolved a symbiotic relationship with root-nodulating bacteria, which is also adapted to these extreme environments and contributes toward nitrogen fixation, these legumes have a high potential to contribute toward low-input sustainable agriculture [3, 4].
To introduce useful traits of wild relatives to related crops, interspecific hybridization is the most efficient and reliable strategy. Sequence-based phylogenetic relationships among species play a fundamental role as indicators to predict interspecific cross-compatibility. To increase the genetic diversity of a wild Vigna collection for environmental stress screening, Vigna accessions were introduced from several gene banks. Since some of the gene bank accessions were unidentified and seemed to be novel genetic resources that have not been analyzed at the molecular level, these accessions were identified based on morphological traits, and were included in the phylogenetic analysis.
Although Maréchal et al. [5] described seven subgenera in the genus Vigna, two of them, Macrorhynchus and Sigmoidotropis, have been proposed to be distinct genera, i.e., Wajira and Sigmoidotropis, respectively, based on morphological and molecular phylogenetic analyses [6, 7]. Among the five subgenera presently recognized (Ceratotropis, Haydonia, Lasiospron, Plectrotropis, and Vigna), crop species have been developed only from three subgenera (Ceratotropis, Plectrotropis, and Vigna). Therefore, we have focused on the species belonging to these subgenera in the present study.
The subgenus Ceratotropis, also known as the Asian Vigna, is agronomically the most important taxonomic group, from which seven crops have been domesticated, i.e., moth bean (Vigna aconitifolia (Jacq.) Maréchal), minni payaru (Vigna stipulacea Kuntze), mung bean, black gram (Vigna mungo (L.) Hepper), creole bean (Vigna reflexo-pilosa Hayata), rice bean (Vigna umbellata (Thunb.) Ohwi & Ohashi), and adzuki bean (Vigna angularis (Willd.) Ohwi & Ohashi). Tomooka et al. [8] described 21 species, which were divided into three sections: five species in section Aconitifoliae N. Tomooka & Maxted, 12 species in section Angulares N. Tomooka & Maxted, and four species in section Ceratotropis N. Tomooka & Maxted. Although four new species were recently described in the subgenus Ceratotropis [9–12], their molecular phylogenetic positions have not been studied. In the present study, two newly described species (V. indica and V. sahyadriana) and four wild species (wild V. aconitifolia (Jacq.) Maréchal, Vigna dalzelliana (O. Kuntze) Verdcourt, Vigna khandalensis (Santapau) Raghavan & Wadhwa, V. subramaniana (Babu ex Raizada) Raizada) of the subgenus Ceratotropis, which had not been used in previous molecular phylogenetic studies, were analyzed.
Maréchal et al. [5] described seven species, consisting of two sections in the subgenus Plectrotropis (four species in section Plectrotropis and three species in section Pseudoliebrechtsia). The subgenus Plectrotropis contains a lesser known but potentially important food legume called ‘tuber cowpea’ (Vigna vexillata (L.) A. Rich.) [13]. This fully domesticated form is still cultivated in Bali and Timor, Indonesia. Maréchal et al. [5] recognized six botanical varieties (var. vexillata, angustifolia, doichonema, macrosperma, pluriflora, and yunnanensis) in V. vexillata. Among these varieties, var. macrosperma was reported as a domesticated taxa but its origin was unknown. Later, considering some proposals for new treatments [14, 15], Maxted et al. [16] accepted seven taxonomic varieties in V. vexillata (var. vexillata, angustifolia, davyi, dolichonema, lobatifolia, macrosperma, and ovata). V. vexillata var. davyi and V. vexillata var. lobatifolia were treated as distinct species (Vigna davyi H. Bol., Vigna lobatifolia Baker) in the subgenus Plectrotropis in Maréchal et al. [5] V. vexillata var. ovata was formerly treated as Strophostyles capensis (Thunb.) E. Mey. As such, the taxonomic treatments of the species in the subgenus Plectrotropis are still considered immature, and phylogenetic discussions based on molecular sequence information are necessary. In the present study, accessions of V. vexillata var. vexillata, var. angustifolia, var. lobatifolia, var. macrosperma, var. ovata, and Bali domesticated accessions were analyzed.
In the subgenus Vigna, from which cowpea (Vigna unguiculata) and bambara groundnut (Vigna subterranea) have been domesticated, Maréchal et al. [5] described 36 species in six sections (two species in section Catiang, two in Comosae, one in Liebrechtsia, two in Macrodontae, nine in Reticulatae, and 20 in Vigna). Cowpea is classified under Catiang, and bambara groundnut is in the section Vigna. For Vigna, we are currently focusing on Vigna marina (Burm.) Merrill, which inhabits sandy beaches, as a genetic resource for salinity tolerance, and Vigna luteola (Jacq.) Bentham, which inhabits riversides, as a flood-tolerant genetic resource [17, 18]. These two species are closely related, and Padulosi and Ng [19] described V. marina ssp. oblonga Padulosi as being distributed in coastal areas of West Africa. Sonnante et al. [20] confirmed the genetic independence of V. luteola, V. marina ssp. marina, and V. marina ssp. oblonga based on isozymes and RAPD. In addition, they showed that V. marina ssp. oblonga was more closely related to V. luteola than to V. marina ssp. marina. However, V. marina ssp. oblonga was not included in subsequent phylogenetic analysis based on DNA sequences, although Pasquet et al. [15] described V. marina ssp. oblonga as being a synonym of V. luteola.
We therefore performed a phylogenetic characterization of the aforementioned taxa. To our knowledge, a phylogenetic study using DNA sequences had not been conducted on these taxa based on the DNA sequences of the internal transcribed spacer region of the ribosomal DNA on the nuclear genome (hereafter rDNA-ITS), and the atpB-rbcL intergenic spacer on the chloroplast genome (hereafter atpB-rbcL).
Materials and Methods
Plant materials
Seventy-one accessions of the genus Vigna, consisting of 28 species and three subgenera (Ceratotropis, Plectrotropis, and Vigna) conserved at the National Institute of Agrobiological Sciences, Japan, were used (Table 1). Originally, nine accessions were either unidentified, or seemed to be misidentified, as shown by the bold texts in Table 1. For the morphological analysis and DNA extraction, all the accessions were planted in six 0.3-L plastic pots (one seed/pot), and a 5-L plastic pot (six seeds/pot), and kept in a greenhouse where the temperature was maintained above 20°C with 12 hours of day length. The morphology of each plant was evaluated. For V. aconitifolia, weight of a hundred grains, pod shattering, and water absorbency of the seed were evaluated as domesticated traits. When evaluating pod shattering, 20 pods were dried overnight in a circulating incubator at 40°C. Twenty seeds were submerged in a Petri dish at 25°C for two days, and the number of seeds that absorbed water was recorded. We used common bean (Phaseolus vulgaris cv. Taisho-kintoki) as an outgroup for molecular phylogenetic analysis.
Table 1. Plant materials used for phylogenetic analysis, and the sequence length and accession No. of rDNA-ITS and atpB-rbcL. deposited at DDBJ.
| ID | Section | Species Name | Status | Origin | JP No. | Original Conservation Site | Original ID and Species Identification | rDNA-ITS Sequence Length (bp) | rDNA-ITS DDBJ Accession No. | atpB-rbcL Sequence Length (bp) | atpB-rbcL DDBJ Accession No. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgenus Ceratotropis | |||||||||||
| 1 | Aconitifoliae | V. aconitifolia | Domesticated | India | 245857 | TNAU GB | 2009TN58 | 562 | LC082015 | 700 | LC082267 |
| 2 | Aconitifoliae | V. aconitifolia | Domesticated | India | 245897 | TNAU GB | 2009TN99 | 562 | LC082017 | 699 | LC082269 |
| 3 | Aconitifoliae | V. aconitifolia | Domesticated | Pakistan | 104332 | NIAS GB | 2752(5) | 562 | LC082016 | 699 | LC082268 |
| 4 | Aconitifoliae | V. aconitifolia | Wild | India | 235416 | Australian GB | AUSTRCF106324, Vigna sp. | 562 | LC082014 | 699 | LC082266 |
| 5 | Aconitifoliae | V. aconitifolia | Wild | India | 245864 | TNAU GB | 2009TN66, Vigna sp. | 562 | LC082012 | 699 | LC082264 |
| 6 | Aconitifoliae | V. aconitifolia | Wild | India | 245865 | TNAU GB | 2009TN67, Vigna sp. | 562 | LC082013 | 700 | LC082265 |
| 7 | Aconitifoliae | V. aridicola | Wild | Sri Lanka | 205894 | NIAS GB | 2000S-11 | 561 | LC082018 | 689 | LC082270 |
| 8 | Aconitifoliae | V. aridicola | Wild | Sri Lanka | 205896 | NIAS GB | 2000S-2 | 561 | LC082019 | 689 | LC082271 |
| 9 | Aconitifoliae | V. aridicola | Wild | Sri Lanka | 207977 | NIAS GB | 2001SL-28 | 561 | LC082020 | 690 | LC082272 |
| 10 | Aconitifoliae | V. indica | Wild | India | 235417 | ILRI GB | IL-25019, V. trilobata | 562 | LC082011 | 697 | LC082263 |
| 11 | Aconitifoliae | V. khandalensis | Wild | India | 253828 | TNAU GB | VC76 | 561 | LC082005 | 687 | LC082257 |
| 12 | Aconitifoliae | V. stipulacea | Domesticated | India | 245503 | TNAU GB | 2008TN29 | 561 | LC082007 | 690 | LC082259 |
| 13 | Aconitifoliae | V. stipulacea | Wild | Sri Lanka | 205892 | NIAS GB | 2000S-6 | 562 | LC082006 | 690 | LC082258 |
| 14 | Aconitifoliae | V. subramaniana | Wild | India | 229278 | Australian GB | AUSTRCF106193, Vigna sp. | 562 | LC064351 | 696 | LC064361 |
| 15 | Aconitifoliae | V. subramaniana | Wild | India | 229284 | Australian GB | AUSTRCF85155, V. radiata var. sublobata | 562 | LC064350 | 697 | LC064360 |
| 16 | Aconitifoliae | V. trilobata | Wild | India | 245881 | TNAU GB | 2009TN83 | 562 | LC082010 | 690 | LC082262 |
| 17 | Aconitifoliae | V. trilobata | Wild | Sri Lanka | 210605 | NIAS GB | 2000S-5-1 | 562 | LC082009 | 690 | LC082261 |
| 18 | Aconitifoliae | V. trilobata | Wild | Sri Lanka | 205895 | NIAS GB | 2000S-13 | 562 | LC082008 | 690 | LC082260 |
| 19 | Angulares | V. angularis var. angularis | Domesticated | Japan | 37752 | NIAS GB | ERIMOSHOUZU | 557 | LC081992 | 688 | LC082244 |
| 20 | Angulares | V. angularis var. nipponensis | Wild | Japan | 87910 | NIAS GB | CED96101602 | 557 | LC081993 | 688 | LC082245 |
| 21 | Angulares | V. angularis var. nipponensis | Wild | Laos | 226665 | NIAS GB | 2005L34 | 557 | LC081995 | 688 | LC082247 |
| 22 | Angulares | V. dalzelliana | Wild | India | 235419 | Australian GB | AUSTRCF85146 | 557 | LC081997 | 689 | LC082249 |
| 23 | Angulares | V. dalzelliana | Wild | Myanmar | 210811 | NIAS GB | 2001M24, Vigna sp. | 557 | LC081996 | 696 | LC082248 |
| 24 | Angulares | V. exilis | Wild | Thailand | 205884 | NIAS GB | 99T-10-1 | 557 | LC081985 | 690 | LC082237 |
| 25 | Angulares | V. hirtella | Wild | Sri Lanka | 218935 | NIAS GB | 9902–48 | 557 | LC081984 | 690 | LC082236 |
| 26 | Angulares | V. hirtella | Wild | Thailand | 109681 | NIAS GB | CED891122-(9) | 557 | LC081983 | 691 | LC082235 |
| 27 | Angulares | V. hirtella | Wild | Laos | 220137 | NIAS GB | 2003L-14 | 558 | LC081988 | 689 | LC082240 |
| 28 | Angulares | V. hirtella | Wild | Thailand | 108562 | NIAS GB | 96120305 | 563 | LC081989 | 689 | LC082241 |
| 29 | Angulares | V. minima | Wild | Thailand | 107869 | NIAS GB | CED891125-(10) | 556 | LC081998 | 690 | LC082250 |
| 30 | Angulares | V. minima | Wild | Indonesia | 218938 | Belgian GB | NI1363 | 556 | LC082000 | 690 | LC082252 |
| 31 | Angulares | V. minima | Wild | Papua N.G. | 226877 | NIAS GB | 2005PNG15 | 556 | LC081999 | 692 | LC082251 |
| 32 | Angulares | V. nakashimae | Wild | Japan | 107879 | NIAS GB | Ukushima | 556 | LC082002 | 693 | LC082254 |
| 33 | Angulares | V. nepalensis | Wild | Nepal | 107881 | NIAS GB | Nepalen | 557 | LC081994 | 689 | LC082246 |
| 34 | Angulares | V. reflexo-pilosa var. glabra | Domesticated | Philippines | 109684 | AVRDC GB | V1160 | 557 | LC081986 | 698 | LC082238 |
| 35 | Angulares | V. reflexo-pilosa var. reflexo-pilosa | Wild | Malaysia | 108867 | NIAS GB | 510–1 | 557 | LC081987 | 698 | LC082239 |
| 36 | Angulares | V. riukiuensis | Wild | Japan | 108810 | NIAS GB | Y-4-1 | 556 | LC082001 | 692 | LC082253 |
| 37 | Angulares | V. tenuicaulis | Wild | Myanmar | 227438 | NIAS GB | KYONKADON | 557 | LC081991 | 688 | LC082243 |
| 38 | Angulares | V. tenuicaulis | Wild | Thailand | 109682 | NIAS GB | CED891122-(8) | 557 | LC081990 | 688 | LC082242 |
| 39 | Angulares | V. trinervia | Wild | Malaysia | 108840 | NIAS GB | 503–4 | 561 | LC064352 | 698 | LC064362 |
| 40 | Angulares | V. umbellata | Domesticated | Japan | 99485 | NIAS GB | Menaga | 557 | LC081982 | 689 | LC082234 |
| 41 | Angulares | V. umbellata | Wild | Thailand | 210639 | NIAS GB | 99T-2 | 557 | LC064307 | 689 | LC064328 |
| 42 | Angulares | V. umbellata | Wild | Thailand | 109675 | NIAS GB | (6)-1-1 | 557 | LC081981 | 689 | LC082233 |
| 43 | Angulares | Vigna sp. | Wild | Thailand | 210644 | NIAS GB | 99T-9 | 557 | LC064303 | 689 | LC064324 |
| 44 | Ceratotropis | V. grandiflora | Wild | Thailand | 107862 | NIAS GB | CED891119-(1) | 562 | LC064345 | 694 | LC064355 |
| 45 | Ceratotropis | V. mungo var. mungo | Domesticated | Thailand | 109668 | NIAS GB | Subsomotod | 562 | LC064346 | 689 | LC064356 |
| 46 | Ceratotropis | V. mungo var. silvestris | Wild | India | 107874 | NBPGR | TC2211 | 562 | LC064347 | 690 | LC064357 |
| 47 | Ceratotropis | V. radiata var. radiata | Domesticated | Thailand | 110830 | NIAS GB | CN60 | 595 | LC064348 | 688 | LC064358 |
| 48 | Ceratotropis | V. radiata var. sublobata | Wild | Madagascar | 107877 | AVRDC GB | TC1966 | 587 | LC064349 | 688 | LC064359 |
| 49 | Ceratotropis | V. radiata var. sublobata | Wild | Papua N.G. | 226874 | NIAS GB | 2005PNG08 | 597 | LC082004 | 688 | LC082256 |
| 50 | Ceratotropis | V. sahyadriana | Wild | India | 235420 | Australian GB | AusTRCF104896, Vigna sp. | 568 | LC082003 | 689 | LC082255 |
| 51 | Ceratotropis | Vigna sp. | Wild | India | 110836 | Belgian GB | NI 1135, V. radiata var. setulosa | 564 | LC064353 | 688 | LC064363 |
| 52 | Ceratotropis | Vigna sp. | Wild | India | 245506 | TNAU GB | 2008TN32, V. hainiana | 559 | LC064354 | 688 | LC064364 |
| Subgenus Plectrotropis | |||||||||||
| 53 | Plectrotropis | V. vexillata | Domesticated | Indonesia | 235863 | Belgian GB | NI 1858 | 560 | LC082032 | 683 | LC082284 |
| 54 | Plectrotropis | V. vexillata | Wild | Brazil | 202337 | USDA GB | PI 406391 | 562 | LC082035 | 684 | LC082287 |
| 55 | Plectrotropis | V. vexillata | Wild | Papua N.G. | 230747 | NIAS GB | 2006PNG-37 | 563 | LC082037 | 683 | LC082289 |
| 56 | Plectrotropis | V. vexillata | Wild | Suriname | 202334 | USDA GB | PI 406383 | 563 | LC082036 | 684 | LC082288 |
| 57 | Plectrotropis | V. vexillata var. angustifolia | Wild | Columbia | 235869 | Belgian GB | NI 936 | 563 | LC082038 | 684 | LC082290 |
| 58 | Plectrotropis | V. vexillata var. lobatifolia | Wild | Namibia | 235903 | Belgian GB | NI 546 | 557 | LC082031 | 686 | LC082283 |
| 59 | Plectrotropis | V. vexillata var. macrosperma | Domesticated | Sudan | 235905 | Belgian GB | NI 111 | 559 | LC082034 | 684 | LC082286 |
| 60 | Plectrotropis | V. vexillata var. ovata | Wild | South Africa | 235908 | Belgian GB | NI 1869 | 562 | LC082033 | 684 | LC082285 |
| 61 | Plectrotropis | V. vexillata var. vexillata | Wild | Congo | 235912 | Belgian GB | NI 245 | 563 | LC082039 | 684 | LC082291 |
| Subgenus Vigna | |||||||||||
| 62 | Catiang | V. unguiculata | Domesticated | Nigeria | 86801 | IITA GB | IT 84S 2246 | 581 | LC082027 | 686 | LC082279 |
| 63 | Catiang | V. unguiculata | Domesticated | Sudan | 86877 | IITA GB | TVU 11979 | 581 | LC082026 | 686 | LC082278 |
| 64 | Catiang | V. unguiculata | Domesticated | Sudan | 86879 | IITA GB | TVU 11986 | 581 | LC082028 | 686 | LC082280 |
| 65 | Catiang | V. unguiculata ssp. dekindtiana | Wild | Mali | 89083 | IITA GB | TVNU 457 | 575 | LC082030 | 684 | LC082282 |
| 66 | Catiang | V. unguiculata ssp. sesquipedalis | Domesticated | Sri Lanka | 81610 | NIAS GB | MA | 581 | LC082029 | 686 | LC082281 |
| 67 | Vigna | V. luteola | Wild | Australia | 236246 | Australian GB | AUSTRCF 320527 | 566 | LC082021 | 689 | LC082273 |
| 68 | Vigna | V. luteola | Wild | Brazil | 235855 | Belgian GB | NI 858 | 566 | LC082023 | 689 | LC082275 |
| 69 | Vigna | V. marina ssp. marina | Wild | Japan | 235813 | NIAS GB | 2009IRIO-1 | 569 | LC082022 | 690 | LC082274 |
| 70 | Vigna | V. marina ssp. oblonga | Wild | Benin | 233389 | NIAS GB | 2006BENIN29 | 567 | LC082024 | 690 | LC082276 |
| 71 | Vigna | V. subterranea | Domesticated | unknown | 79992 | NIAS GB | L15-20-2 | 575 | LC082025 | 690 | LC082277 |
| 72 | - | Phaseolus vulgaris | Domesticated | Japan | 219310 | NIAS GB | TAISHOU KINTOKI | 554 | LC082303 | 679 | LC082302 |
Nine accessions which were originally either unidentified, or seemed to be misidentified are shown by bold texts.
DNA Sequencing
We sequenced the rDNA-ITS and atpB-rbcL of 72 accessions. DNA was extracted from young leaves using a modified CTAB method [21]. PCR primers were designed according to the previous study [22]; C2 (5’-TCCTCCGCTTATTGATATGC-3’) and G1 (5’-GGAAGGAGAAGTCGTAACAAGG-3’) for rDNA-ITS, and AT1 (5’-AGAACCAGAAGTAGTAGGAT-3’) and RB (5’-ACACCAGCTTTGAATCCAAC-3’) for atpB-rbcL. The PCR mixture, containing KOD-Plus-Neo one unit (TOYOBO), 1 x PCR Buffer supplied by the manufacturer, 200 μM dNTPs, 1.5 mM MgSO4, 10 ng of the DNA template, and 0.2 μM of each primer pair, was prepared in a total volume of 50 μL. The PCR cycle was as follows: 94°C for 2 min, 35 cycles of 98°C for 10 sec and 68°C for 1 min. The amplified PCR product was mixed with 2 μL of ExoSAP-IT, which had been diluted 20-fold, and incubated at 37°C for 30 min, and 80°C for 15 min. The sequencing reaction was conducted according to the protocol of BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The reactant was precipitated using ethanol, dried, and dissolved in 10 μL Hi-Di Formamid. The mixture was treated at 95°C for 5 min, and the DNA sequence was determined using a ABI PRISM 3130xl DNA Analyzer (Applied Biosystems). Sequencing was repeated until the depth of each base was greater than five, and the nucleotide sequence was determined according to majority rule in cases where a single nucleotide polymorphism was present. The accession numbers of the sequence information deposited in the DNA Data Bank of Japan (www.ddbj.nig.ac.jp/) are shown in Table 1.
Multiple alignment was conducted for each rDNA-ITS and atpB-rbcL using Clustal W [23]. The sequence frame was determined according to the previous study [22], and the trimmed sequence was used to construct a phylogenetic tree by the maximum likelihood estimation using MEGA6 [24]. Bootstrap analysis was conducted with 1000 replications.
Results
Morphology-based species identification
Among the nine unidentified or misidentified accessions, six accessions were identified as the following four species (V. aconitifolia, V. dalzelliana, V. indica, and V. sahyadriana) based on morphological observation.
Accessions ID-4, ID-5, and ID-6, which were collected in India, were identified as the wild forms of moth bean (V. aconitifolia). Seedling, stipule, and seed morphologies of the domesticated and newly identified wild forms of V. aconitifolia are shown in Fig 1. Both domesticated and wild forms showed similar variations in leaflet shape, ranging from entire to deeply lobed. Only seeds of the wild forms were covered with a semi-transparent seed coat covering. While the domesticated forms were characterized by larger seeds with water-permeable seed coat and non-shattering pods, the wild forms were found to have smaller seeds, with a water-proof seed coat and high shattering pods (Table 2).
Fig 1. Domesticated form and wild ancestral form of moth bean (V. aconitifolia).
Scale bars are 1 mm.
Table 2. Comparison of domestication related traits in domesticated and wild V. aconitifolia.
| ID | Status | Seed weight ± SD (g/100 grains)1 | Shattering pods (%) | Germination (%) |
|---|---|---|---|---|
| 1 | Domesticated | 3.39 ± 0.42 a | 0 | 100 |
| 2 | Domesticated | 2.03 ± 0.38 b | 0 | 100 |
| 3 | Domesticated | 2.20 ± 0.11 b | 0 | 100 |
| 4 | Wild | 0.86 ± 0.14 c | 100 | 0 |
| 5 | Wild | 1.15 ± 0.08 c | 100 | 0 |
| 6 | Wild | 1.26 ± 0.11 c | 100 | 0 |
1 Averages of 3 replications. Different letters indicate that seed weights are significantly different, by Tukey—Kramer’s HSD test (P < 0.05).
Morphologies of the seedling, style beak, and seed of the remaining accessions newly identified as V. dalzelliana, V. indica, and V. sahyadriana are shown in Fig 2. Accession ID-23, collected in southern Myanmar, showed hypogeal germination with petiolate primary leaves, glabrous pods, seeds without seed coat coverings (smooth seed coat), small yellow flowers, left curved keel petal with protuberance on left keel (keel pocket), indicating that this accession belonged to the section Angulares in the subgenus Ceratotropis. Additionally, it had a flat style beak (spoon-like shape), which is a key characteristic of V. dalzelliana. Therefore, we have identified this accession as V. dalzelliana.
Fig 2. Morphological characteristics of V. dalzelliana, V. sahyadriana, and V. indica.

Scale bars are 0.3 mm with style beak, and 1 mm with seeds.
Accession ID-50, collected in India, was introduced from the gene bank of Australia (AusTRCF104896), where it was treated as Vigna sp. (Table 1). It showed epigeal germination with sessile primary leaves, seeds with seed coat covering, hairy pods, yellow flower, and left curved keel petal with a prominent protuberance on the left keel petal (keel pocket), indicating that this accession belongs to the section Ceratotropis in the subgenus Ceratotropis. Seed morphology and very long style beak matched the characteristics of V. mungo, whereas the direction of laterally attaching pods to the peduncle matched that of V. radiata. These characteristics matched the key characters of V. sahyadriana well, which was described as a new species by Aitawade et al. [10].
Accession ID-10, collected in India, was introduced from the ILRI (International Livestock Research Institute) gene bank (IL-25019), where it was conserved as V. trilobata. It showed epigeal germination with sessile primary leaves, seeds with seed coat covering, hairy pods, small yellow flowers, left curved keel petal with a small protuberance on left keel petal (keel pocket), and a protruding growth habit with deeply lobed leaflets, indicating this accession belongs to the section Aconitifoliae in the subgenus Ceratotropis. At a glance, it had a very similar overall morphology to V. trilobata. However, its stipule was lanceolate, and its seed was rectangular with a very short, non-protruding hilum, which did not match the key characters of V. trilobata. These characteristics matched those of V. indica, which was described as a new species by Dixit et al. [9].
Accession ID-43, collected in Thailand, was originally identified as V. umbellata. However, it showed some features that did not match the key characteristics of V. umbellata. Accession ID-51, collected in northern India, was introduced from a Belgian gene bank (NI 1135) as V. radiata var. setulosa. Accession ID-52, collected in southern India, was introduced from the Tamil Nadu Agricultural University (TN32) as V. hainiana. Both of these accessions had a similar morphology to that of V. radiata in general. However, they showed some features that did not match the key characteristics of V. radiata. Therefore, we could not determine the taxonomic identification for these three accessions based on the morphological analysis in the present study.
Molecular phylogenetic analysis
DNA sequences of rDNA-ITS and atpB-rbcL were determined for 71 accessions of the genus Vigna. For rDNA-ITS, the total length ranged from 556–597 bp; V. minima, V. riukiuensis, and V. nakashimae had the shortest (556 bp), and V. radiata had the longest rDNA-ITS (587–597 bp). The total lengths of atpB-rbcL ranged from 683 to 700 bp; V. unguiculata and V. vexillata had the shortest (683–686 bp), whereas V. aconitifolia had the longest atpB-rbcL (699–700 bp) (Table 1). The numbers of polymorphic sites in rDNA-ITS and atpB-rbcL were 211 and 80, respectively.
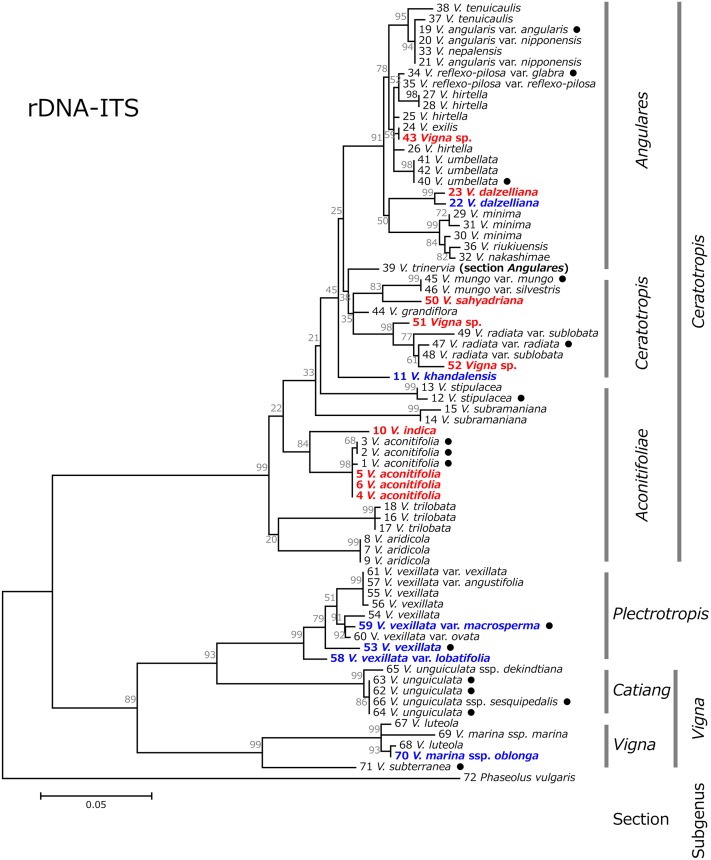
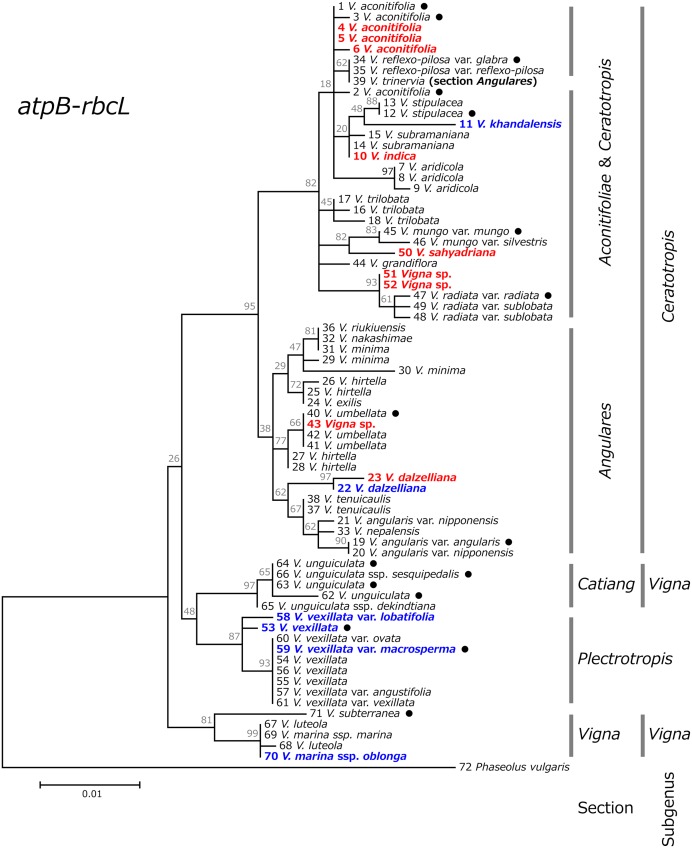
Based on these sequences of rDNA-ITS and atpB-rbcL, phylogenetic trees for respective regions were constructed (Figs 3 and 4). In both phylogenetic trees, the subgenus Ceratotropis formed a single cluster, distinct from the subgenera Vigna and Plectrotropis. The section Catiang in the subgenus Vigna allied with the subgenus Plectrotropis forming a single cluster, while the section Vigna in the subgenus Vigna was distantly allied.
Fig 3. Maximum likelihood tree based on nuclear rDNA-ITS region for the genus Vigna, with Phaseolus vulgaris as an outgroup.
Numbers beside branches represent bootstrap values (%) based on 1000 replications. Scale indicates genetic distance. Domesticated accessions are indicated with black circles, accessions which have been introduced as unidentified or misidentified accessions are indicated with red text, and taxa in which phylogenetic discussion using DNA sequences had not been conducted are indicated with blue text.
Fig 4. Maximum likelihood tree based on chloroplast atpB-rbcL spacer region for the genus Vigna, with Phaseolus vulgaris as an outgroup.
Numbers beside branches represent bootstrap values (%) based on 1000 replications. Scale indicates genetic distance. Domesticated accessions are indicated with black circles, accessions which have been introduced as unidentified or misidentified accessions are indicated with red text, and taxa of which phylogenetic discussion using DNA sequences had not been conducted are indicated with blue text.
The phylogenetic tree based on rDNA-ITS divided the section Aconitifoliae into multiple branches, and clustered the section Ceratotropis and Angulares independently (Fig 3). Alternatively, the phylogenetic tree based on atpB-rbcL divided the subgenus Ceratotropis into two groups, i.e., a blended group comprising the sections Aconitifoliae and Ceratotropis, and the section Angulares (Fig 4). While the section Angulares clustered distinctly from other groups, the interspecific genetic distances within the Angulares cluster were small.
The phylogenetic analysis revealed the species most closely related to the accessions that were newly identified in this study. Accession ID-4, ID-5, and ID-6, identified as a wild form of moth bean, were most closely related to moth bean (V. aconitifolia). Accession ID-23 (Myanmar), identified as V. dalzelliana, was most closely related to the V. dalzelliana collected in India. Accession ID-50, identified as V. sahyadriana, was most closely related to V. mungo. Accession ID-10, identified as V. indica, was most closely related to V. aconitifolia in the phylogenetic tree based on rDNA-ITS, and to V. subramaniana in the phylogenetic tree based on atpB-rbcL. Accession ID-43 (Vigna sp.) was closely related to V. exilis in the rDNA-ITS tree, whereas it was allied with V. umbellata in the atpB-rbcL tree. Accessions ID-51 and ID-52 were most closely related to V. radiata in both trees.
V. khandalensis (accession ID-11) was differentiated substantially from other species, but was relatively close to V. stipulacea. Accessions within V. vexillata showed considerable levels of genetic variation. The accession ID-58 (V. vexillata var. lobatifolia), and the Indonesian domesticated form (accession ID-53) noticeably differentiated from other V. vexillata accessions. V. marina ssp. oblonga (accession ID-70), which was found on the coast of West Africa, was more closely related to V. luteola than to V. marina ssp. marina.
Discussion
Genetic differentiation within the genus Vigna
The subgenus Ceratotropis is thought to have emerged from the subgenus Vigna via the subgenus Plectrotropis [16, 25, 26]. The theoretical basis of this hypothesis is that, while the subgenus Vigna has a symmetric keel without pocket, the subgenus Plectrotropis has a curved keel with a pocket, and the subgenus Ceratotropis has a more prominently twisted keel with a more prolonged pocket. However, the phylogenetic tree using rDNA-ITS in this study suggested the following genetic differentiation patterns. The common ancestor of the genus Vigna first diverged into the common ancestor of the subgenera Vigna plus Plectrotropis, and the common ancestor of the subgenus Ceratotropis. Then, the common ancestor of the subgenera Vigna plus Plectrotropis diverged into the common ancestor of the section Vigna (subgenus Vigna) and the common ancestor of the section Catiang (subgenus Vigna) plus subgenus Plectrotropis. This is supported by the fact that the species in the section Catiang (subgenus Vigna) and the subgenus Plectrotropis have purple flowers, while those in the section Vigna (subgenus Vigna) have yellow flowers. Similar species relationships to our phylogenetic tree were obtained in previous studies using other molecular markers [7, 20, 27]. Therefore, it seems more appropriate to raise the rank of the section Catiang as a subgenus level. However, we leave this taxonomic revision for future work, since we used the limited number of species in the section Catiang, Vigna, and the subgenus Plectrotropis.
“Plectrotropis”, which represents the subgenus, and the section including V. vexillata, has been misspelled as “Plectotropis” in Maréchal et al. [5], and in many subsequent publications such as Tomooka et al. [8] and Maxted et al. [16], but the former should be the correct spelling, as it appeared in Schumach [28] and Baker [29] as a genus name and a subgenus name, respectively.
After cowpea and V. vexillata were shown to be relatively close to each other by molecular analysis [30], an interspecific hybrid between the two species was obtained [31]. Moreover, an interspecific hybrid was obtained between cowpea and V. luteola, which are more distantly related species [32]. In the present study, we propose that V. marina is worth trying for producing interspecific hybrids with bambara groundnut (V. subterranea), based on their relatively close phylogenetic positions. V. marina is highly tolerant to salinity and alkaline soil [17, 33], while bambara groundnut is a crop that is adapted to arid lands [34]. Drought, saline, and alkaline soils are the most important environmental stresses to be addressed in agriculture.
Novel genetic resources in the genus Vigna
Vigna indica T.M. Dixit, K.V. Bhat & S.R. Yadav
Accession ID-10 is revealed to be the only germplasm of V. indica currently available at the gene bank. Although a holotype (Rothe 6229a) of this species was described as V. trilobata (L.) Verdcourt var. pusilla Naik et Pokle [35], results of the phylogenetic analysis supported Dixit et al. [9], in that this taxon is an independent species in the section Aconitifoliae. Whereas V. indica was reported to be morphologically most similar to V. aridicola by Dixit et al. [9], it was also similar to the wild form of V. aconitifolia in its stipule and flower morphology.
In this study, V. indica showed the closest relationship with V. aconitifolia in the rDNA-ITS tree. Conversely, it showed almost the same atpB-rbcL sequence as that of V. subramaniana. These facts suggest the possibility that V. indica is derived from an interspecific hybrid between V. subramaniana and V. aconitifolia. Further studies are necessary to confirm the origin of this species. Additionally, useful traits screening and interspecific cross-compatibility of V. indica should be conducted to determine its usefulness as a genetic resource, especially for moth bean (V. aconitifolia), the most closely related crop.
Vigna sahyadriana Aitawade, K.V. Bhat et S.R. Yadav
Accession ID-50 is the only germplasm of V. sahyadriana available from the gene bank at present. This species was recently described as a new species distributed in Maharashtra, India [10]. Since accession ID-50 was collected in Madhya Pradesh, India, the distribution range of this species seems to have expanded toward the inland of India.
Accession ID-50 was most closely related to, but clearly distinguishable from, black gram (V. mungo) in both phylogenetic trees (Figs 3 and 4). This suggests that the useful traits and interspecific cross-compatibility of V. sahyadriana should be investigated to determine if it can be used as genetic resources for black gram.
Vigna aconitifolia (Jacq.) Maréchal: Wild ancestor of moth bean
Although the wild form of moth bean was documented to be distributed in India [36], living samples have not been identified in the gene bank [27], and therefore its identity and useful traits have not been studied. In this study, we found the wild ancestor of moth bean in a gene bank collection. Accessions ID-5 and ID-6 were collected in Tamil Nadu, and accession ID-4 was collected in Andhra Pradesh, India. The collection sites of these three accessions suggest that the primary habitat of the wild form of moth bean is southeastern India.
Moth beans have been cultivated mainly in arid lands from India to Pakistan, and also in some other counties including Bangladesh, Myanmar, and China [37]. Since moth bean is reported as a crop most tolerant to drought and heat in the subgenus Ceratotropis [38, 39], it is generally thought to be suitable as a crop in tropical arid lands.
Recently, we have found that the wild ancestor of moth bean showed higher drought tolerance than the domesticated forms, and we successfully obtained the F2 lines among the two forms (data not shown). Moreover, since the interspecific hybrid between mung bean and moth bean has been reported [40], wild moth bean would be useful to develop moth bean and mung bean varieties with higher drought tolerance.
Vigna dalzelliana (O. Kuntze) Verdcourt
The geographical distribution of this species was thought to be limited to India and Sri Lanka [8]. Although Thuan [41] reported V. dalzelliata in the Indo-China region (Vietnam, Laos, and Cambodia), it was the result of a misidentification of V. minima specimens [39]. More recently, John et al. [42] reported that they found V. dalzelliana in the Andaman Islands. Identification of accession ID-23 as V. dalzelliana in this study revealed an additional range of geographical distribution for this species, southern Myanmar.
The dissemination pathway of V. dalzelliana from India to southern Myanmar is unknown. Further explorations in the broad areas along the Bengal Gulf (Bangladesh and Myanmar) are necessary. However, since V. dalzelliana also inhabits Sri Lanka and the Andaman Islands [8, 42], researchers must consider the possibility that the distribution range expanded from India to Myanmar via these Islands.
Based on the rDNA-ITS tree, V. dalzelliana is located at the basal position with a V. minima species complex (V. minima, V. nakashimae, V. riukiuensis) [43], and both of these species are well differentiated within the section Angulares (Fig 3). Since V. dalzelliana is the only species known to be distributed in south India, where species of the other two sections are rich, it could be the ancestral species of the section Angulares. Investigating the process of the species emergence and expansion will provide important insights to understand the evolution of this section.
Vigna khandalensis (Santapau) Raghavan & Wadhwa
Vigna khandalensis was reported to inhabit a rainforest climate area in the Western Ghats and the Deccan Plateau in India [44]. It is the only wild species to have an erect plant type in the subgenus Ceratotropis in Vigna. Its seeds were collected as a food during famines [45]. While Tomooka et al. [8] classified this species in the section Aconitifoliae based on the short keel pocket and style beak; Bisht et al. [46] reported that this species is morphologically similar to species in the section Ceratotoropis. The phylogenetic trees in this study suggested that V. khandalensis is a species in the section Aconitifolia, and located at the basal position to the species in the section Ceratotoropis. V. khandalensis was most closely related to V. stipulacea in the section Aconitifoliae, and the two species were similar in that they had large stipules. Since V. stipulacea is a creeping plant cultivated as food, fodder, and green manure in Tamil Nadu, India [2], V. khandalensis might be used to improve V. stipulacea growth. V. khandalensis may also be useful as a genetic resource for other section Ceratotoropis crops, since the interspecific hybrid between this species and mung bean was obtained [47].
Vigna marina (Burm.) Merrill ssp. oblonga Padulosi
V. marina ssp. oblonga was proposed for the plants growing on the coastal zones of West Africa [19]. The phylogenetic tree using rDNA-ITS in this study confirmed that V. marina ssp. oblonga was more closely related to V. luteola than to V. marina ssp. marina (Fig 3), which was suggested by isozyme and RAPD analyses [20]. Additionally, phylogenetic trees suggest that there is a large intraspecific variation in V. luteola.
To address the evolution of V. luteola and V. marina, we need to consider V. oblongifolia A. Rich., a species closely related to these, although it was not included in this study. In V. oblongifolia, two botanical varieties have been described [25]. Phylogenetic trees in the previous studies have shown that V. oblongifolia var. parviflora is more closely related to V. luteola than to V. marina, and V. oblongifolia var. oblongifolia is more distant from these [48, 49]. This suggests that V. marina ssp. oblonga may be more closely related to V. oblongifolia var. parviflora than to V. marina ssp. marina. Therefore, the taxonomic treatment of V. marina ssp. oblonga, and V. oblongifolia var. parviflora should be reconsidered based on intra and inter-specific variations in V. marina, V. luteola, and V. oblongifolia.
Since there are no interspecific crossing barriers among V. marina ssp. marina, V. marina ssp. oblonga, and V. luteola [17, 50], and interspecific hybrid plants between V. oblongifolia and V. luteola were obtained [51], these are thought to form a primary gene pool. Therefore, to introduce the salinity and alkaline tolerance of V. marina into bambara groundnut, interspecific cross-compatibility should be investigated, taking into consideration the use of bridging species in the section Vigna. In Maxted et al. [16], there are 18 species listed in the section Vigna.
Vigna vexillata (L.) A. Rich
The wild forms of this species are widely distributed in pan-tropical regions, including Africa, Asia, Oceania, and America, and its swollen roots have been collected as food [52–54]. This species includes two domesticated forms that are morphologically distinct from each other. One is a twining plant without any taxonomic rank at an intraspecific level, which is cultivated in Bali, Indonesia [13]. Another is an erect plant named V. vexillata var. macrosperma, which is collected in Africa, Central America, and Australia. For both, the domestication origins are unknown.
In this study, the Indonesian domesticated form (accession ID-53) was found to be genetically differentiated from other species. This suggests that the Indonesian domesticated form, and V. vexillata var. macrosperma (accession ID-59), have been domesticated independently from different wild forms. This notion was also supported by the fact that a hybrid among the two domesticated forms was not obtained [55]. Moreover, there is an intraspecific crossing barrier between the Indonesian domesticated form and some wild forms [55]. Therefore, the ancestor of the Indonesian domesticated form is unknown.
Similarly, V. vexillata var. lobatifolia was found to be genetically differentiated from other species. This taxon was described originally as V. lobatifolia Baker [56], then classified as an independent species in the section Pseudoliebrechtsia [25], or the section Plectrotropis [5] in the subgenus Plectrotropis, and then given the current rank as botanical variety of V. vexillata based on isozyme polymorphisms [15, 16, 57]. However, since lobatifolia has a unique habitat (Namib Desert), and is morphologically distinct, we do not reject the taxonomic systems of Verdcourt [25] and Maréchal et al. [5], in which it was treated as an independent species. However, only nine accessions in five varieties of V. vexillata were analyzed for the subgenus Plectrotropis in this study, and thus further studies are required to systematize the taxonomy of this subgenus, and clarify the rank of the Indonesian domesticated forms and V. vexillata var. lobatifolia.
The natural habitat of V. vexillata was very diverse, including arid lands, coastal areas, acidic soil, and alkaline soil [16, 58, 59]. Some accessions have been reported to harbor flood resistance and pest resistance [60–63]. It is therefore believed that this species contains highly useful genetic resources to breed crops for agriculturally unfavorable lands.
Future perspectives
In recent years, research on the use of wild relatives has been actively pursued. In addition to interspecific cross-breeding, new concepts have been proposed such as ‘Reverse Breeding’ [64], which involves regaining the crop stress tolerance, which has been lost in the breeding or domestication process, by backcrossing with the wild form. Another strategy is ‘Neo-Domestication’ [18], or the domestication of the stress-tolerant wild species that cannot be crossed with crop species. This process could be achieved by using mutation breeding, and mutant screening could be accelerated by TILLING, a screening method using the sequence information of domesticated genes. To advance these wild species breeding strategies, more information concerning the correct taxonomic placement, and genetic relationships among species, should be acquired to predict interspecific cross-compatibility, and to select an appropriate breeding strategy.
Acknowledgments
This work was partly supported by JSPS KAKENHI Grant Number 13J09808 and 26850006.
Data Availability
All relevant data are within the manuscript and uploaded to DNA Databank of Japan (DDBJ). Accession numbers are available in Table 1.
Funding Statement
This work was supported by JSPS KAKENHI Grant Number 13J09808 and 26850006 to YT. Role of funders: data collection and analysis.
References
- 1.Schrire BD. Tribe Phaseoleae In: Lewis G, Schrire B, Mackinder B. Lock M. editors. Legumes of the world. Royal Botanic Gardens, Kew; 2005. pp. 393–431. [Google Scholar]
- 2.Tomooka N, Kaga A, Isemura T, Vaughan DA. Vigna In: Kole Chittaranjan. editor. Wild Crop Relatives: Genomic and Breeding Resources Legume Crops and Forages. NY, Springer; 2011. pp. 291–311. [Google Scholar]
- 3.Yokoyama T, Tomooka N, Okabayashi M, Kaga A, Boonkerd N, Vaughan DA. Variation in the nod gene RFLPs, nucleotide sequence of 165 rENA genes, Nod factors, and nodulation abilities of Bradyrhizobium strains isolated from Thai Vigna plants. Canadian Journal of Microbiology. 2006; 52: 31–46. [DOI] [PubMed] [Google Scholar]
- 4.Tomooka N, Kaga A, Isemura T, Vaughan DA, Srinives P, Somta P, et al. Vigna Genetic Resources. Proceedings of the 14th NIAS International Workshop on Genetic Resources, Genetics and Comparative Genomics of Legumes (Glycine and Vigna); 2010. pp. 11–21.
- 5.Maréchal R, Mascherpa JM, Stainer F. Etude taxonomique d’un groupe complexe d’speces des genres Phaseolus et Vigna (Papilionaceae) sur la base de donnees morphologiques et polliniques, traitees par l’analyse informatique. Boissiera. 1978; 28: 1–273. [Google Scholar]
- 6.Thulin M, Lavin M, Pasquet R, Delgado-Salinas A. Phylogeny and biogeography of Wajira (Leguminosae): A monophyletic segregate of Vigna centered in the horn of Africa region. Systematic Botany. 2004; 29: 903–920. [Google Scholar]
- 7.Delgado-Salinas A, Thulin M, Pasquet R, Weeden N, Lavin M. Vigna (Leguminosae) sensu lato: the names and identities of the American segregate genera. American Journal of Botany. 2011; 98: 1694–1715. 10.3732/ajb.1100069 [DOI] [PubMed] [Google Scholar]
- 8.Tomooka N, Vaughan DA, Moss H, Maxted N. The Asian Vigna: Genus Vigna subgenus Ceratotropis genetic resources. Kluwer Academic Publishers; 2002. [Google Scholar]
- 9.Dixit TM, Sutar SP, Yadav SR, Bhat KV, Rao SR. Vigna indica, a new name for Vigna trilobata var. pusilla and a note on section Aconitifoliae in India. Rheedea. 2011; 21: 1–7. [Google Scholar]
- 10.Aitawade MM, Sutar SP, Rao SR, Malik SK, Yadav SR, Bhat KV. Section Ceratotropis of subgenus Ceratotropis of Vigna (Leguminosae-Papilionoideae) in India with a new species from Northern Western Ghats. Rheedea. 2012; 22: 20–27. [Google Scholar]
- 11.Latha M, Sheen S, Krishnaraj MV, Presannakumari KT, Bhat KV, Bisht IS et al. Vigna konkanensis (Fabaceae: Papilionoideae) a new species from the west coast of India. Webbia: Journal of Plant Taxonomy and Geography. 2014; 69: 49–52. [Google Scholar]
- 12.Gaikwad SP, Gore RD, Randive SD, Garad KU. Vigna yadavii (Leguminosae: Papilionoideae), a new species from Western Ghats, India. Biodiversity Data Jornal. 2014; 2: e4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karuniawan A, Iswandi PRK, Heinzemann J, Grüneberg WJ. Vigna vexillata (L.) A. Rich. cultivated as a root crop in Bali and Timor. Genetic Resources and Crop Evolution. 2006; 53: 213–217. [Google Scholar]
- 14.Pienaar BJ, Kok PDF. The Vigna vexillata complex (Fabaceae) in southern Africa. South African Journal of Botany. 1991; 57: 236–245. [Google Scholar]
- 15.Pasquet RS. Notes on the genus Vigna (Leguminosae-Papilionoideae). Kew Bull. 2001; 56: 223–227. [Google Scholar]
- 16.Maxted N, Mabuza Dlamini P, Moss H, Padulosi S, Jarvis A, Guarino L. African Vigna: an ecogeographic study. International Plant Genetic Resources Institute; 2004. [Google Scholar]
- 17.Chankaew S, Isemura T, Naito K, Ogiso-Tanaka E, Tomooka N, Somta P, et al. QTL mapping for salt tolerance and domestication—related traits in Vigna marina subsp. oblonga, a halophytic species. Theoretical and Applied Genetics. 2014; 127: 691–702. 10.1007/s00122-013-2251-1 [DOI] [PubMed] [Google Scholar]
- 18.Tomooka N, Naito K, Kaga A, Sakai H, Isemura T, Ogiso-Tanaka E, et al. Evolution, domestication and neo-domestication of the genus Vigna. Plant Genetic Resources. 2014; 12(S1): S168–S171. [Google Scholar]
- 19.Padulosi S, Ng NQ. A useful and unexploited herb, Vigna marina (Leguminosae-Papilionideae) and the taxonomic revision of its genetic diversity. Bulletin du Jardin Botanique National de Belgique. 1993; 62: 119–126. [Google Scholar]
- 20.Sonnante G, Spinosa A, Marangi A, Pignone D. Isozyme and RAPD analysis of the genetic diversity within and between Vigna luteola and V. marina. Annals of Botany. 1997; 80: 741–746. [Google Scholar]
- 21.Lodhi MA, Ye GN, Weeden NF, Reisch BI. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Molecular Biology Reporter. 1994; 12: 6–13. [Google Scholar]
- 22.Doi K, Kaga A, Tomooka N, Vaughan DA. Molecular phylogeny of genus Vigna subgenus Ceratotropis based on rDNA ITS and atpB–rbcL intergenic spacer region of cpDNA sequences. Genetica. 2002; 114: 129–145. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994; 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdcourt B. Studies in the Leguminosae-Papilionoideae for the ‘Flora of Tropical East Africa’ IV. Kew Bulletin. 1970; 24: 507–569. [Google Scholar]
- 26.Tateishi Y, Ohashi H. Systematics of the azuki bean group in the genus Vigna In: Fujii K, Gatehouse AMR, Jhonson CD, Mitchel R, Yoshida T, editors. Bruchids and Legumes: Economics, Ecology and Coevolution. Kluwer Academic Publishers; 1990. pp. 189–199. [Google Scholar]
- 27.Goel S, Raina SN, Ogihara Y. Molecular evolution and phylogenetic implications of internal transcribed spacer sequences of nuclear ribosomal DNA in the Phaseolus–Vigna complex. Molecular Phylogenetics and Evolution. 2002; 22: 1–19. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher CF. Beskrivelse af Guineiske planter. Kjöbenhavn; 1827. pp. 338–340. [Google Scholar]
- 29.Baker JG. Leguminosae In: Hooker JD, editor. The flora of British India II. Reeve; 1879. pp. 204–207. [Google Scholar]
- 30.Sonnante G, Piergiovanni AR, Ng QN, Perrino P. Relationships of Vigna unguiculata (L.) Walp., V. vexillata (L.) A. Rich. and species of section Vigna based on isozyme variation. Genetic Resources and Crop Evolution. 1996; 43: 157–165. [Google Scholar]
- 31.Gomathinayagam P, Ganesh ram S, Rathnaswamy R, Ramaswamy NM. Interspecific hybridization between Vigna unguiculata (L.) Walp. and V. vexillata (L.) A. Rich. through in vitro embryo culture. Euphytica. 1998; 102: 203–209. [Google Scholar]
- 32.Sen NK, Bhowal JG. Cytotaxonomic studies on Vigna. Cytologia. 1960; 25: 195–207. [Google Scholar]
- 33.Takahashi Y, Naito K, Ogiso-Tanaka E, Inoue J, Hirashima S, Tomooka N. Collection and Conservation of Wild Leguminous Crop Relatives on the Yaeyama archipelago, Okinawa Prefecture, Japan, 2013. Annual report on exploration and introduction of plant genetic resources. 2014; 30: 29–51.
- 34.Smartt J. Grain Legumes. Cambridge University Press, Cambridge; 1990. pp. 160–165. [Google Scholar]
- 35.Naik VN, Pokle DS. Novelties in the flora of Marathwada. 1985; 7: 670–675. [Google Scholar]
- 36.Arora RK, Nayar ER. Wild relatives of crop plants in India, NBPGR Sci. Monograph No. 7. National Bureau of Plant Genetic Resources; 1984.
- 37.Zeven AC, De Wet JMJ. Dictionary of cultivated plants and their regions of diversity excluding most ornamentals, forest trees and lower plants. Pudoc; 1982. [Google Scholar]
- 38.Jain HK, Mehra KL. Evolution, Adaptation, Relationships, and Uses of the Species of Vigna cultivated in India. In: Summerfield RJ, Bunting AH. editors. Advances in Legume Science, Kew Royal Botanic Gardens; 1980. pp. 459–468. [Google Scholar]
- 39.Tomooka N, Kaga A, Vaughan DA. The Asian Vigna (Vigna subgenus Ceratotropis) Biodiversity and evolution In: Sharma AK, Sharma A, editors. Plant Genome: Biodiversity and evolution 1, Part C Phanerogams (Angiosperms- Dicotyledons). Science Publishers; 2006. pp. 87–126. [Google Scholar]
- 40.Pandiyan M, Senthil N, Ramamoorthi N, Muthiah AR, Tomooka N, Duncan V, et al. Interspecific hybridization of Vigna radiata x 13 wild Vigna species for developing MYMV donor. Electronic Journal of Plant Breeding. 2010; 1: 600–610. [Google Scholar]
- 41.Thuan NV. Flore du Cambodge, du Laos et du Vietnam, Volume 17, Leguminosae—Papilionoideae, Phaseoleae. Museum national D'Histoire Naturelle; 1979.
- 42.John KJ, Latha M, Senthil KR, Asokan NR, Abraham Z, Mishra SK. Vigna dalzelliana (O. Kuntz) Verdc.: A new distributional record from Andaman Islands, India. Indian Journal of Plant Genetic Resources. 2009; 22: 138–140. [Google Scholar]
- 43.Yoon MS, Doi K, Kaa A, Tomooka N, Vaughan DA. Analysis of the genetic diversity in the Vigna minima complex and related species in East Asia. Journal Of Plant Research. 2000; 113: 375–386. [Google Scholar]
- 44.Raghavan SR, Wadhwa BM. On the Nomenclature of Vigna grandis (Dalz. & Gibs.) Verdc. Current Science. 1972; 41: 429. [Google Scholar]
- 45.Babu CR, Sharma SK, Johri BM. Leguminosae-papilionoideae: Tribe phaseoleae. The Bulletin of the Botanical Survey of India. 1985; 27: 1–28. [Google Scholar]
- 46.Bisht IS, Bhat KV, Lakhanpaul S, Latha M, Jayan PK, Biswas BK, et al. Diversity and genetic resources of wild Vigna species in India. Genetic Resources and Crop Evolution. 2005; 52: 53–68. [Google Scholar]
- 47.Chavan VM, Patil GD, Bhapkar DG. Improvement of cultivated Phaseolus species—need for interspecific hybridization. Indian Journal of Genetics and Plant Breeding. 1966; 26: 152–154. [Google Scholar]
- 48.Vijaykumar A, Saini A, Jawali N. Phylogenetic analysis of subgenus Vigna species using nuclear ribosomal RNA ITS: evidence of hybridization among Vigna unguiculata subspecies. Journal of Heredity. 2010; 101: 177–188. 10.1093/jhered/esp084 [DOI] [PubMed] [Google Scholar]
- 49.Raveendar S, Lee JR, Park JW, Lee GA, Jeon YA, Lee YJ, et al. Identification of Genus Vigna using ITS2 and matK as a Two-Locus DNA Barcode. Plant Breeding and Biotechnology. 2015; 3: 153–159. [Google Scholar]
- 50.Palmer JL, Lawn RJ, Adkins SW. An embryo-rescue protocol for Vigna interspecific hybrids. Australian Journal of Botany. 2002; 50: 331–338. [Google Scholar]
- 51.Fatokun CA, Perrino P, Ng NQ. Wide crossing in African Vigna species In: Singh BB, Mohan Raj DR, Dashiell KE, Jackai LEN. editors. Advances in cowpea research. Copublication of IITA and JIRCAS; 1996. pp. 50–57. [Google Scholar]
- 52.Irvine FR. Supplementary and emergency food plants of West Africa. Economic Botany. 1952; 6: 23–40. [Google Scholar]
- 53.Bhattacharyya PK, Ghosh AK, Sanyal B, Deb Ray G. Grow Vigna vexillata for protein-rich tuber-cum-pulse crop in North-eastern Hill Region. Seeds and Farms. 1984; 10: 33–36. [Google Scholar]
- 54.Lawn RJ, Cottrell A. Wild mungbean and its relatives in Australia. Biologist. 1988; 35: 267–273. [Google Scholar]
- 55.Damayanti F, Lawn RJ, Bielig M. Genetic compatibility among domesticated and wild accessions of the tropical tuberous legume Vigna vexillata (L.) A. Rich. Crop and Pasture Science. 2010; 61: 786–797. [Google Scholar]
- 56.Baker JG. Papilionaceae In: Oliver D. editor. Flora of tropical Africa II. Reeve; 1871. pp. 1–257. [Google Scholar]
- 57.Garba M, Pasquet RS. The Vigna vexillata (L.) A. Rich. gene pool In: Sorensen M, Estrella JE, Hamann EOJ, Ruiz SAR, editors. Proceedings of the 2nd International Symposium on Tuberous Legumes; 1998. pp. 61–71. [Google Scholar]
- 58.Wong KC. Vigna vexillata (L.) A. Richard In: PROSEA, Plant Resources of South-East Asia No.11: Auxiliary plants. Prosea Foundation; 1997. pp. 261–263. [Google Scholar]
- 59.Lawn RJ, Watkinson AR. Habitat, morphological diversity and distribution of the genus Vigna Savi in Australia. Australian Journal of Agricultural Research. 2002; 53: 1305–1316. [Google Scholar]
- 60.Miller IL, Williams WT. Tolerance of some tropical legumes to six months of simulated waterlogging. Tropical Grasslands. 1981; 15: 39–43. [Google Scholar]
- 61.Birch ANE, Fellows LE, Evans SV, Dhoerty K. Para-aminophenylalanine in Vigna. Possible taxonomic and ecological significance as a seed defence against bruchids. Phytochemistry. 1986; 25: 2745–2749. [Google Scholar]
- 62.IITA. Annual Report and Research Highlights 1987/1988. International Institute of Tropical Agriculture. 1988.
- 63.Ogundiwin EA, Thottappilly G, Aken’Ova ME, Ekpo EJA, Fatokun CA. Resistance to cowpea mottle carmovirus in Vigna vexillata. Plant Breeding. 2002; 121: 517–520. [Google Scholar]
- 64.Palmgren MG, Edenbrandt AK, Vedel SE, Andersen MM, Landes X, Østerberg JT, et al. Are we ready for back-to-nature crop breeding? Trends Plant Science. 2014; 20: 155–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and uploaded to DNA Databank of Japan (DDBJ). Accession numbers are available in Table 1.