Abstract
Multiple myeloma bone disease is marked by severe dysfunction of both bone formation and resorption and serves as a model for understanding the regulation of osteoblasts (OBL) and osteoclasts (OCL) in cancer. Myeloma bone lesions are purely osteolytic and are associated with severe and debilitating bone pain, pathologic fractures, hypercalcemia, and spinal cord compression, as well as increased mortality. Interactions within the bone marrow microenvironment in myeloma are responsible for the abnormal bone remodeling in myeloma bone disease. Myeloma cells drive bone destruction that increases tumor growth, directly stimulates the OCL formation, and induces cells in the marrow microenvironment to produce factors that drive OCL formation and suppress OBL formation. Factors produced by marrow stromal cells and OCL promote tumor growth through direct action on myeloma cells and by increasing angiogenesis. Current therapies targeting MMBD focus on preventing osteoclastic bone destruction; however regulators of OBL inhibition in MMBD have also been identified, and targeted agents with a potential anabolic effect in MMBD are under investigation. This review will discuss the mechanisms responsible for MMBD and therapeutic approaches currently in use and in development for the management of MMBD.
Keywords: Myeloma, Osteoblast, Osteoclast, Myeloma bone disease, Angiogenesis, Bone marrow microenvironment
1. Introduction
Multiple myeloma (MM) is the most frequent cancer to involve the skeleton with 80–90% of patients developing bone lesions during their disease course [1]. Myeloma bone lesions are purely osteolytic and are associated with severe and debilitating bone pain, pathologic fractures, hypercalcemia, and spinal cord compression, as well as increased mortality [2]. It is estimated that 20% of MM patients present with pathologic fractures, 40% develop a fracture in the first year after diagnosis, and up to 60% develop pathologic fractures over the course of their disease [3]. Additionally, patients with pathologic fractures have a 20% increase in mortality when compared to patients without pathologic fractures [4]. The bone destructive lesions can be extensive and severe [5] and bone pain, frequently centered on the chest or back and exacerbated by movement, is present in more than two-thirds of patients at diagnosis [6].
Multiple myeloma bone disease (MMBD) is distinct from the bone disease caused by other types of tumors that metastasize to bone and is marked by dysfunction of both bone formation and bone resorption [5]. While osteolytic metastases from MM and other malignancies induce osteoclastic (OCL) bone resorption, myeloma bone lesions are unique in that osteoblast (OBL) activity is severely decreased or absent [7], [8]. Thus, bone scans in patients with MM frequently underestimate the extent of bone disease [9]. Furthermore, bone lesions in patients with myeloma rarely heal, even when a patient is in prolonged complete remission. MMBD can affect any bone, with predominant areas of involvement occurring in sites of red marrow, such as the vertebral bodies and ribs.
Current therapies targeting MMBD focus on preventing osteoclastic bone destruction. OCL activity is responsible for the bone destruction in myeloma and plays a pivotal role in MMBD through release of growth factors from the bone matrix during the bone resorptive process that enhance tumor growth. Recently, regulators of OBL inhibition in MMBD have also been identified, and targeted agents with a potential anabolic effect in MMBD are under investigation. In this review, mechanisms responsible for MMBD and therapeutic approaches based on these mechanisms will be discussed.
2. Prevalence and presentation of myeloma bone disease
The clinical presentation of myeloma is variable and approximately 11% of patients are initially asymptomatic [10]. (Disease in these patients is generally identified through routine laboratory studies.) Of symptoms reported at presentation, the most common is bone pain, which is present in more than two-thirds of patients [6]. The American Cancer Society estimates that there will be 21,700 new cases of myeloma diagnosed in 2012, including 12,190 in men and 9510 in women, with an estimated 4690 deaths [11]. The majority of myeloma patients are elderly, with a median age at diagnosis of 69 years and a median age at death of 74 years [12]. Treatment of MM has improved markedly over the past 30 years, with an increase in 5-year survival from 25% in 1975 to 41% in 2007, however the disease remains incurable and MMBD remains a major contributor to the morbidity and mortality of myeloma patients.
Up to 90% of MM patients have evidence of osteolysis in the form of generalized osteopenia or discrete lytic lesions over the course of their disease [13], and approximately 80% have radiologic evidence of bone involvement on skeletal survey [14]. Approximately 40% of patients with MM will develop a fracture in the first year after diagnosis, and up to 60% will develop pathologic fractures [3]. The bone destructive lesions in myeloma can be extensive and can affect any bone [5]. The predominant areas of involvement occur in sites of red marrow, such as the vertebral bodies (49%), skull (35%), pelvis (34%) and ribs (33% of patients) [15]. While there is an association between a patient's tumor burden and the number of lytic lesions present [16], and tumor burden, OCL number, and OCL resorptive surface area are correlated in bone marrow biopsies from MM patients [17], [18], an individual's degree of bone disease does not have significant utility in predicting clinical outcomes. Additionally, bone lesions in patients with myeloma rarely heal, even when a patient is in prolonged complete remission.
Approximately 15% of newly diagnosed MM patients are hypercalcemic due to increased bone resorption, decreased bone formation, and impaired renal function, all of which are often exacerbated by immobility. Unlike other malignancies with metastatic bone involvement, parathyroid hormone related protein (PTHrP) is rarely over-produced by myeloma cells. Thus, the severity of hypercalcemia in patients with myeloma is not correlated with serum PTHrP levels and instead reflects tumor burden [6]. Symptomatic hypercalcemia can result in anorexia, nausea, vomiting, confusion, fatigue, constipation, renal stones, depression and polyuria, and is suggestive of a high tumor burden.
Finally, MM patients have accelerated bone loss when compared to age-matched controls. Bone mineral density is decreased in patients with MM as well as in patients with monoclonal gammopathy of undetermined significance (MGUS) [19], [20], a clinically benign condition defined by a low level of monoclonal protein production and the absence of skeletal lesions [19].
3. Mechanisms of myeloma bone disease
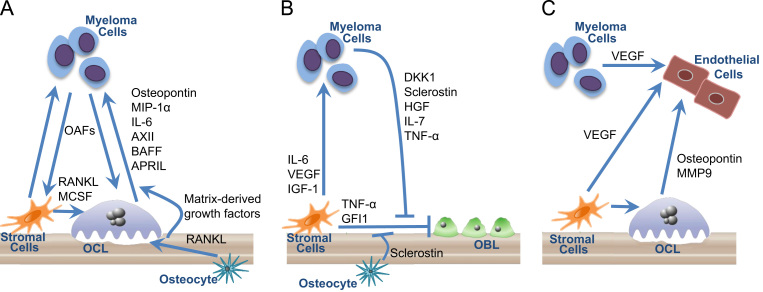
MMBD is characterized by purely osteolytic bone destruction due to increased OCL activity and suppressed or absent OBL activity, and myeloma bone lesions have a characteristic “punched-out” appearance on x-rays. The bone marrow microenvironment in myeloma includes both extracellular and cellular elements, including osteoblasts, osteoclasts, endothelial cells, immune cells and MM cells that contribute to tumor growth and the bone destructive process. Multiple interactions within the bone marrow microenvironment in myeloma are responsible for the abnormal bone remodeling of MMBD (Fig. 1, panels A and B). Myeloma cells drive bone destruction that in turn increases tumor growth; highlighting the critical role that bone disease plays in myeloma. In addition, myeloma cells both directly stimulate OCL formation and induce cells in the marrow microenvironment to produce factors that drive OCL formation and suppress OBL formation. Immune cells contribute to the bone destructive process through production of cytokines and adhesion molecules that increase myeloma cell growth and enhance myeloma cell chemoresistance, increase osteoclastogenesis, suppress osteoblastogenesis, and drive T cell polarization from a predominantly Th1 phenotype to Th17 [21], [22], [23], [24]. Factors produced by marrow stromal cells and OCL promote tumor growth through direct action on myeloma cells [25] and indirectly by increasing angiogenesis (Fig. 1, panel C). [26], [27], [28]. Finally, the bone resorption process itself releases immobilized growth factors such as TGFβ from the bone matrix that also drive tumor growth [29].
Fig. 1.
Cellular interactions in the bone marrow microenvironment in myeloma bone disease. The bone marrow microenvironment in myeloma includes osteoblasts (OBL), osteoclasts (OCL), stromal cells, endothelial cells, and osteocytes. Multiple interactions within the bone marrow microenvironment in myeloma are responsible for the abnormal bone remodeling of multiple myeloma bone disease (MMBD). (A) OCL Activation in MMBD. Myeloma cells directly stimulate OCL formation and induce cells in the marrow microenvironment to produce factors that drive OCL formation. Osteoclast activating factors (OAFs) produced by myeloma cells include RANKL, MIP-1α, IL-3, and TNF-α. Myeloma cells also induce marrow stromal cell production of growth factors that enhance OCL formation including RANKL, MCSF, and (not pictured) IL-6 and TNF-α and decrease production of OCL inhibitory factors, such as OPG. OCLs secrete soluble factors such as osteopontin, MIP-1α, IL-6, AXII, BAFF, and APRIL that stimulate tumor growth. In addition, factors produced by marrow stromal cells and OCL promote tumor growth through direct action on myeloma cells. Osteocytes also regulate osteoclastognesis and bone resorption through expression of RANKL. Finally, the bone destructive process releases bone matrix-derived growth factors such as TGFβ, IGFs, FGF, PDGFs, and BMPs that increase the growth of myeloma cells, further exacerbating the osteolytic process. (B) OBL Suppression in MMBD. MM cell derived OBL-inhibitory factors include DKK1, sclerostin, HGF, IL-7, and TNF-α. MM cells also induce other cells in the marrow microenvironment to increase production of OBL suppressors such as sclerostin (from osteocytes), and TNF-α and GFI1 (from marrow stromal cells). Myeloma cells also induce marrow stromal cells to produce factors that support the myeloma cells, including IL-6, VCAM1, VEGF, and IGF-1. (C) Angiogenesis is enhanced in MMBD. Angiogenesis is enhanced in MM. OCL and endothelial cells are closely apposed in the bone marrow microenvironment, and increased OCL activity appears to contribute to both the increased angiogenesis in MM as well as to tumor growth. Endothelial cell proliferation is enhanced by angiogenic factors such as VEGF produced by myeloma cells and stromal cells. Osteoclasts also secrete angiogenic factors, such as osteopontin and MMP9.
4. Pathogenesis of the increased osteoclast activity in myeloma
Histologic studies of bone biopsies from patients with MM demonstrate that increased OCL activity occurs adjacent to MM cells, suggesting that bone destruction in MM is a local event. This has led to the hypothesis that local cytokines produced or induced by MM cells are responsible for the increased OCL formation and subsequent bone resorptive activity in MM. These osteoclastogenic activating factors, (OAFs), directly increase OCL formation and activity and decrease production of osteoprotegerin (OPG), a soluble decoy receptor for receptor activator of NF-kB ligand (RANKL), a critical differentiation factor for OCLs produced by marrow stromal cells and OBL [30]. OAFs were initially identified in conditioned media from myeloma cell lines and found to stimulate bone resorption in bone organ culture systems [31]. Additional factors identified as OAFs important in MMBD include RANKL, MIP-1α, TNF-α, Interleukin 3 (IL-3), and IL-6. Interestingly, several of these OAFs also suppress OBL formation and/or support myeloma cells directly, indicating that they play multiple roles in MMBD.
Myeloma cells also stimulate cells in the marrow microenvironment, particularly marrow stromal cells and T cells, resulting in increased production of OAFs and decreased production of OCL inhibitory factors. Adhesive interactions between myeloma cells and bone marrow stromal cells via binding of surface VLA-4 (α4β1 integrin) to VCAM-1 on stromal cells results in production of osteoclastogenic cytokines such as RANKL, M-CSF, IL-11, and IL-6 by marrow stromal cells and osteoclastogenic cytokines including macrophage inflammatory protein-1α (MIP-1α) and IL-3 by MM cells [32], [33], [34], [35], [36].
Additionally, OCLs themselves secrete factors that support myeloma cells [37], including IL-6 [38], annexin II [39], osteopontin [40], fibroblast activation protein [41], BAFF, and APRIL [42].
RANK/RANKL: The RANK/RANKL signaling pathway is a critical component of both normal and malignant bone remodeling. RANK is a transmembrane signaling receptor and a member of the tumor necrosis receptor (TNF) superfamily that is found on the surface of OCL precursors [43], [44]. RANK ligand (RANKL) is expressed as a membrane-bound protein on marrow stromal cells and OBL that is secreted by activated lymphocytes. RANKL expression is induced by cytokines that stimulate bone resorption [45] such as PTH, 1,25-OH Vitamin D3, prostaglandins [46], [47], and myeloma cells themselves both produce and induce production of RANKL by marrow stromal cells via adhesive interactions described above, as well as by soluble factors produced by myeloma cells such as Dickkopf1 (DKK1) [48] and TNF-α [49]. In addition, RANKL further increases OCL formation and survival by binding to RANK [50].
OPG, the soluble décor receptor for RANKL, is critical for the regulation of lytic activity in both normal and myelomatous bone [51]. OPG is produced by OBL in the marrow and blocks the interactions of RANKL with RANK, limiting osteoclastogenesis. The RANKL/OPG ratio in the marrow microenvironment in MM is skewed in favor of RANKL [30], and the ratio of RANKL to OPG in the sera of myeloma patients impacts prognosis. Patients with high RANKL:OPG ratios have inferior survival as compared to patients with normal or intermediate RANKL:OPG ratios [52]. Furthermore, preclinical models of myeloma demonstrated that inhibition of RANKL with OPG prevented bone destruction in animal MM models [52], [53], [54]. The clinical impact of denosumab, a human monoclonal antibody to RANKL, on bone metastases in patients with osteoporosis, breast cancer, prostate cancer, and treatment-induced bone disease due to prostate cancer has recently been evaluated, and is discussed later in this review.
TNF-α: TNF-α is elevated in myeloma patients [55] and has multiple functions in MMBD. Myeloma cells induce high levels of TNF-α in the marrow microenvironment [56];,however it has been difficult to clearly demonstrate that myeloma cells themselves produce significant quantities of this cytokine [57]. Myeloma cells and TNF-α increase the transcription factor XBP1 in marrow stromal cells, which contributes to the increased production of VCAM1, RANKL, and IL-6 and enhances stromal cell support of myeloma cell growth and osteoclast formation. In addition, TNF-α is itself a potent inducer of osteoclast formation, and can directly increase osteoclast formation and enhance the effects of RANKL[58]. TNF-α also can block osteoblast differentiation from marrow stromal cells by decreasing expression of critical osteoblast transcription factors such as Runx2, TAZ, and Osx, induce apoptosis of mature osteoblasts, and increase support of myeloma cells by induction of IL-6 [23], [59].
MIP-1α: MIP-1α (CCL3) is a potent chemokine produced by MM cells in 70% of patients that induces OCL formation and has recently also been found to inhibit osteoblast function [60]. MIP-1α acts as a chemotactic factor for OCL precursors and can induce differentiation of OCL progenitors, contributing to OCL formation [61], [62], [63] independent of RANKL. In addition, MIP-1α potentiates both RANKL and IL-6 stimulated OCL formation [64] and plays a role in homing of MM cells to the bone marrow by enhancing myeloma cell adhesion to marrow stromal cells by increasing expression of β1 integrins on MM cells[65]. This enhances marrow stromal cell production of OAFs and the angiogenic factor VEGF. Elevated MIP-1α gene expression and secretion by myeloma cells is highly correlated with bone destruction and decreased patient survival in MM [66]. Translocation 4:14 in MM cells, associated with poor patient prognosis, has been shown to induce constitutive expression of the fibroblast growth factor receptor 3 (FGFR3), resulting in high levels of MIP-1α [67]. In vivo murine models of MM have demonstrated that MIP-1α can induce OCL formation and bone destruction. Blocking MIP-1α expression in MM cells injected into SCID mice or treating the animals with a neutralizing antibody to MIP-1α results in decreased tumor burden and bone destruction [62], [68].
In addition to well-described effects on OCL, MIP-1α inhibits OBL function via downregulation of osterix, an osteogenic transcription factor, and inhibits osteoblast mineralization activity, suggesting that MIP-1α impairs osteoblast function rather than differentiation.
MIP-1α binds to three different receptors: CCR1, CCR5 and CCR9. CCR1 and CCR5 are expressed on MM cells and stromal cells. Inhibitors to CCR1 and CCR5 have been used to delineate the roles of each receptor in the myeloma bone marrow microenvironment[69]. Menu and colleagues demonstrated that MM cell migration to MIP-1α in vitro and homing in vivo were mediated by binding to CCR5 and not CCR1. In vivo inhibition of CCR1 resulted in reduction of osteoclastic bone resorption, suggesting that CCR1 and CCR5 have differential effects on myeloma cell chemotaxis and stimulation of osteolysis. Small molecule antagonists to CCR1 have been studied in models of myeloma and have been shown to block both tumor growth and destruction [69], [70], and to at least partially reverse MIP-1α osteoblast inhibitor effects in vitro and in vivo [60]. These small molecule antagonists are currently in pharmaceutical development [71].
IL-3/Activin A: IL-3 is produced by myeloma cells and T cells in the myeloma microenvironment and approximately 70% of MM patients have elevated IL-3 levels in marrow plasma. IL-3 is a bifunctional cytokine that can both stimulate osteoclastogenesis via an indirect effect [35] and inhibit osteoblast formation [50]. Silbermann and coworkers [72] reported that IL-3 stimulates marrow macrophages in the myeloma microenvironment to produce activin A, a TGF-β family member, and that anti-activin A inhibits the effects of IL-3 on osteoclast formation. Activin A is known to directly induce osteoclast formation and enhance the effects of RANKL on osteoclast formation [73]. Others have reported that levels of activin A are increased in marrow plasma and peripheral blood from patients with myeloma, and that marrow stromal cells and osteoclasts are the major source of activin A in myeloma patients [74]. In addition, elevated circulating activin A levels have been described in newly diagnosed symptomatic myeloma patients as compared with controls and elevated activin A levels correlated with advanced disease stage and were associated with increased bone resorption and extensive bone disease [75]. Interestingly, the mechanism of IL-3 suppression of osteoblast differentiation is also indirect, and requires the participation of CD45+ cells. Osteoclast precursors are also CD45+, suggesting that Activin A is involved as a cross-talk regulatory molecule between osteoclast and osteoblast precursors in both the directions [76].
Activin A signals through the activin A type IIA receptor to increase bone resorption and suppress osteoblast differentiation by inhibiting production of the Dlx5. An activin A receptor antagonist (soluble activin receptor type IIA fusion protein, ActRIIA.muFc; RAP-011) has been shown to block bone destruction, stimulate bone formation, and decrease tumor growth in a murine model of myeloma [77]. A humanized activin A soluble receptor antagonist (ACE-011) has been shown to inhibit bone resorption markers and stimulate bone formation in post menopausal women [78], and a trial of ACE-011 in myeloma patients is ongoing.
Annexin II: Annexin II (AXII) is a recently identified factor produced by stromal cells and osteoclasts that is important in osteoclast formation, hematopoietic stem cell mobilization, and homing of prostate cancer cells to the bone [79]. AXII is upregulated in MM, and myeloma-derived AXII increases proliferation of myeloma cell lines, possibly through an autocrine mechanism [80], [81]. OCL and stromal cell derived AXII enhances the growth of MM cells in the bone marrow by binding to the AXII receptor on MM cells, primarily through a paracrine mechanism [39]. In addition, AXII can induce stromal cell production of RANKL, further stimulating OCL formation.
Ephrin B2/EphB4 bidirectional signaling: Bidirectional signaling between the ligand ephrin B2 from osteoclasts and its receptor EphB4 on bone marrow stromal cells and osteoblasts has been reported to negatively control OCL development from OCL precursors (reverse signaling) and to promote OBL differentiation (forward signaling) [82]. EphrinB2 and EphB4 are decreased in stromal cells from myeloma patients [83]. EphB4-Fc activates ephrinB2 in OCL, but not in stromal cells; and ephrinB2-Fc activates EphB4 in stromal cells. Administration of either peptide to a murine myeloma model stimulated osteoblastogenesis, bone formation, and angiogenesis, but only EphB4-Fc also inhibited osteoclastogenesis and myeloma growth. Thus, enhancing ephrinB2-EphB4 signaling is a possible therapeutic target for MMBD.
Adhesive interactions: Adhesive interactions between myeloma cells and stromal cells play a significant role in both the homing of MM cells to the bone marrow and augmentation of the bone destructive process. These adhesive interactions result in activation of NF-κB and p38 MAP-kinase signaling, which is involved in the induction of RANKL expression by OBL. Blocking p38 MAP-kinase results in inhibition of IL-6 and VEGF production, as well as decreased adhesion of MM cells to marrow stromal cells [84]. Vanderkerken and coworkers reported that inhibition of p38 MAP kinase in the 5T2MM murine model of MM decreased tumor cell burden, prevented development of bone disease and increased overall survival of mice having 5T2 cells [85]. Therefore, this pathway may be a potential therapeutic target for novel therapies for MM disease.
Additionally, adhesive interaction between MM cells and cells in the BM microenvironment increase production of cytokines and chemokines that enhance angiogenesis and contribute to the chemotherapy resistance of tumor cells resistant [86], [87]. Angiogenesis is markedly enhanced in MM, parallels disease progression, and correlates inversely with patient survival [88]. OCL and endothelial cells are closely apposed in MM, and increased OCL activity appears to contribute to both the increased angiogenesis in MM as well as to tumor growth. OCL can support the growth of MM cells through cell-to-cell contact, which results in production of IL-6 and osteopontin [40], [53]. Further, Tanaka and coworkers have shown that OCL enhance angiogenesis in MM through constitutive secretion of pro-angiogenic factors such as osteopontin, which together with vascular endothelial growth factor (VEGF) produced by MM cells, increase angiogenesis [88]. We have reported that OCL are angiogenic cells [27], and that suppression of OCL formation with OPG dose-dependently inhibited angiogenesis and osteoclastogenesis in established bone angiogenesis assays.
5. Osteoblast suppression in myeloma
OBL activity is suppressed in MM, with decreased bone formation and calcification despite increased bone resorption [17], [89]. As a result, serum alkaline phosphatase and osteocalcin are normal or decreased in patients with myeloma bone involvement. Co-culture experiments have demonstrated reduced myeloma cell proliferation in the presence of OBLs as compared with OCL or marrow stromal cells [90], a finding that has been confirmed in murine models of myeloma bone disease [91]. A number of inhibitors of OBL differentiation have been identified in myeloma that are produced by myeloma cells or cells in the myeloma marrow microenvironment. Interestingly, myeloma patient marrow stromal cells retain their aberrant properties, such as increased production of OCL activating factors such as RANKL, IL-6, XBP1, and activin A and suppressed OBL differentiation in long-term tissue culture, suggesting that myeloma cells induce permanent changes in marrow stromal cells.
In addition to TNF-α, MIP-1α, and IL-3/Activin A discussed previously in this review, DKK1, sclerostin, TGFβ, hepatocyte growth factor (HGF) and IL-7 are also potent inhibitors of osteoblast differentiation. Mechanisms responsible for the suppressed osteoblast activity in myeloma are just beginning to be understood, and the basis for the persistent block in osteoblast differentiation in myeloma is unknown.
The formation and differentiation of OBLs from marrow stromal cells requires the activity and function of systemic and local factors, such as parathyroid hormone (PTH), fibroblast growth factor (FGF), and bone morphogenic proteins (BMP). The activity and function of the transcription factor Runx2/Cbfal (Runx2) play a critical role in OBL development and activity. Runx2-deficient mice, which are embryonic lethal, lack OBL and bone formation [92]. Inhibition of Runx2 in OBL precursors has been demonstrated in MMBD [93], and direct cell–cell contact between myeloma cells and OBL progenitor cells as well as soluble factors produced by MM cells downregulate RUNX2 activity [93], however the mechanisms underlying this inhibition are unclear. Myeloma cells also induce marrow stromal cells to produce factors that support myeloma cell growth, survival, and chemoresistance, such as IL-6, annexin II, VCAM1, VEGF, and IGF-1; and mature osteoblasts suppress myeloma cell growth via production of decorin [90], [94]. Thus, suppression of OBL differentiation in myeloma enhances tumor growth due to toxic effects of mature osteoblasts on myeloma cells.
The canonical, or β-catenin dependent, Wnt signaling pathway is critical for the regulation of osteoblast proliferation and survival [95]. Soluble inhibitors of the canonical Wnt pathway such as Dickkopf (DKK1), produced by OBL, and sclerostin, from osteocytes, play important roles in the regulation of bone mass in MM. Additionally, secreted Wnt inhibitors such as the secreted frizzled related proteins (sFRP) [96] can inhibit the canonical as well as the non-canonical Wnt pathways by binding to frizzled.
DKK1: DKK1 is a major inhibitor of OBL differentiation in myeloma by sequestering low-density lipoprotein receptor-related protein (LRP) 5/6 from binding WNT, ultimately downregulating RUNX2 activity [97], [98], [99]. Tian and coworkers reported that primary CD138+ cells from myeloma patients but not MGUS patients produce DKK1, and demonstrated that levels of DKK1 mRNA correlate with the number of focal bone lesions in patients with MM [100]. Others have similarly reported that serum DKK1 levels correlate with the extent of bone disease in MM patients [101], however this finding is controversial as DKK1 expression is lost as MM bone disease progresses [100]. Preclinical studies with antibodies that block DKK1 (BHQ880) have shown that these compounds enhance bone formation and block tumor growth in murine models of myeloma bone disease [102]. However, MM patients with advanced disease do not express DKK1, suggesting that Wnt inhibitors may mediate bone destruction only in the early phases of disease [100].
In addition to inhibiting osteoblastogenesis, elevated DKK1 levels can also enhance osteoclastogenesis. Wnt signaling in OBLs increases expression of OPG [103] and down-regulates the expression of RANKL [104], suggesting a possible mechanism by which inhibition of Wnt signaling in OBL would indirectly increase osteoclastogenesis. Taken together, these studies indicate that DKK1 is a key regulator of bone remodeling in both physiological and pathological conditions and that blocking this factor may contribute to both stimulation of osteoclastogenesis and inhibition of OBL in myelomatous bones.
Sclerostin: Sclerostin is an inhibitor of the canonical downstream Wnt signaling pathway that is produced by osteocytes to inhibit osteoblast differentiation. Several studies have recently demonstrated that myeloma cells may produce sclerostin or induce sclerostin expression in myeloma patients [105], [106], and sclerostin levels correlate with bone destruction in myeloma [107]. In addition, osteocyte apopotosis has been reported in myeloma, and apoptotic osteocytes may release both RANKL and sclerostin [108].
HGF: HGF is a negative regulator of BMP-induced osteoblast differentiation [59]. A myeloma cell line, that produces large amounts of HGF, JJN3, causes 99% loss of osteoblast perimeter when injected into irradiated SCID mice. Myeloma patients with elevated HGF levels in their sera have lower bone specific alkaline phosphatase activity (a marker of osteoblast function) when compared to patients without HGF elevations, and HGF has been shown to inhibit human osteoblast formation [109].
TGFβ: TGFβ is deposited into bone matrix in a latent form by osteocytes and osteoblasts and released in an active form by the enhanced OCL activity in MMBD. TGFβ inhibits osteoblast differentiation in MMBD, and blockade of TGFβ signaling via the TGFβ type I receptor by an inhibitor of the receptor's kinase function, Ki26894, restores osteoblast differentiation suppressed by either TGFβ, myeloma cell line conditioned media, or bone marrow plasma from MM patients [110]. Oral administration of Ki26894 to a SCID-rab model of MMBD injected with INA-6 myeloma cells demonstrated that in vivo targeting of TGFβ signaling decreased MM cell growth in the bone, protected the bone from destruction, and preserved osteoblast differentiation.
TGFβ family members, including BMPs and activin, also modulate OBL differentiation. BMP2 stimulates OBL differentiation via SMAD signaling activation [111], while activin A-mediated upregulation of SMAD2 pathways results in both stimulation of OCL differentiation and function described previously in this review and impairment of osteoblastogenesis [74]. Additionally, activin A levels correlate with advanced ISS stage, bone disease, and decreased survival in patients at diagnosis and relapse [112].
IL-7: IL-7 is another potential inhibitor of OBL differentiation in MM that induces RANKL production by T lymphocytes [113] and mediates MM-induced OBL inhibition by down-regulating RUNX2 transcriptional activity [93]. IL-7 levels are increased in the marrow of MM patients and that IL-7 inhibited both early and late human osteoblast precursor differentiation in a dose-dependent manner, affecting the differentiation of early and late osteoblast precursors by targeting Runx2 activity, though it did not suppress Runx2 activity [114]. IL-7 can also induce GFI1, a transcriptional repressor of the Runx2 gene, and enhance the effects of suboptimal TNF-α on osteoblast suppression, resulting in further suppression of osteoblast differentiation [115].
GFI1: D'Souza and colleagues recently reported that myeloma cells and primary marrow stromal cells from myeloma patients have elevated levels of Gf1 and that myeloma cells induce expression of GFI1 in marrow stromal cells. In addition, they demonstrate that GFI1 is a potent suppressor of osteoblast differentiation [116]. In preliminary studies Galson and colleagues have shown that GFI1 directly interacts with the RUNX2 promoter to block RUNX2 expression, that there are multiple GFI1 sites within the Runx2 promoter, and that mutation of the key GFI1 binding site prevents TNF-α repression of Runx2. GFI1 can also recruit histone deacetylases and other modifying enzymes to the RUNX2 promoter, possibly contributing to the long-term suppression of OBL activity present in MM patients [117].
Adiponectin: Adiponectin is an adipocyte-derived factor that can protect osteoblast differentiation in MM and increase myeloma cell apopotosis [118]. Adiponectin expression is decreased in both murine and human bone marrows permissive for myeloma growth and increasing adiponectin expression with the apolipoprotein peptide mimetic L-4F both reduces tumor burden and MMBD in murine models and, in the absence of myeloma cells, induces an increase in osteoblasts and bone formation without altering osteoclasts. Thus, induction of adiponectin expression is a potential therapeutic target for the osteoblast suppression of MMBD.
6. Treatment of myeloma bone disease
Treatment of myeloma bone disease requires management of both the underlying malignancy and the increased bone destruction and suppressed new bone formation detailed above. Current treatments for myeloma include chemotherapy or novel agents such as proteasome inhibitors or immunomodulatory agents, with or without autologous stem cell transplantation. Bone disease is managed with a combination of bisphosphonate therapy, localized radiation, (for control of bone pain, treatment of impending fractures or solitary plasmacytomas) and kyphoplasty, vertebroplasty, or surgery.
Bisphosphonates remain the standard of care for MM-related bone disease at this time. Bisphosphonates are potent inhibitors of OCL activity, and intravenous bisphosphonates given every 3–4 weeks are the current treatment of choice for the prevention of skeletal-related events. Bisphosphonate therapy slows progression of lytic lesions, prevents development of new pathologic fractures, and improves bone pain through inhibition of osteoclast activity. Oral clodronate treatment has been shown to reduce the development of osteolytic lesions, fractures, hypercalcemia, and bone pain in MM [119]. The Medical Research Council (MRC) IX trial demonstrated that iv zoledronic acid reduced the incidence of skeletal related events (SREs), (hypercalcemia, new bone lesions, and fractures) as compared with oral clodronate in patients with newly diagnosed MM [120]. In addition, patients treated with zoledronic acid had improved disease response rates and overall survival after a median follow-up of 3.7 years, compared with patients treated with clodronate. This suggests that bisphosphonates have a direct anti-myeloma effect, a hypothesis supported by in vitro data [121], [122].
Intravenous pamidronate, 90 mg monthly, or zoledronic acid, 4 mg monthly, are the standard bisphosphonate therapies in myeloma. In the original randomized trial evaluating intravenous pamidronate therapy in myeloma, a significant reduction in the number of skeletal events per patient year was found when compared to placebo (1.3 versus 2.2) when patients were treated for 21 months [9]. When compared with pamidronate in phase III trials, zoledronic acid was found to be as effective as pamidronate in decreasing the number of skeletal complications and the need for radiation therapy [123]. The major benefit of zoledronic acid over pamidronate is that it can be given over a shorter period of time (15 min. versus 2 h).
Current ASCO recommendations suggest initiating bisphosphonate therapy in myeloma when there is evidence of bone involvement [124]. The optimal duration and frequency of bisphosphonate therapy for myeloma are not well understood and are currently being studied. All randomized, placebo-controlled trials of bisphosphonate use in MM to date have given bisphosphonates for a maximum of two years, thus two years is the treatment duration currently recommended by ASCO and the European myeloma network (EMN). Consensus statements recommend treating patients monthly for 2 years and then considering discontinuation of therapy at that time if the patient is in remission or at plateau phase of disease [124], or at physician discretion [125]. A subset of patients in the MRC IX trial did receive zoledronic acid for more than 2 years and had a reduced incidence of SREs and improved overall survival compared with the clodronate treated group [126]. However, it is not known whether this finding is independent of the patients' response to their anti-myeloma therapy. As discussed in a recent evidence-based review of bisphosphonate use in MM, it is not clear whether patients who achieved complete response (CR) in the MRC IX trial continued to have an advantage with continued zoledronic acid use as compared to those who did not achieve a CR [127]. Patients with mild-moderate renal impairment (CrCl: 30–60 mL/min) >3 mg/dl) should receive reduced doses of zoledronic acid. No change in the zoledronic acid infusion time is recommended. In these patients, pamidronate should be administered over an extended infusion time (2–4 h) [127]. DEXA scans are recommended for consideration for patients with monoclonal gammopathy of undetermined significance and low and intermediate risk smoldering MM, given the increased risk of skeletal-related events in the patients compared with age-matched controls [128], [129]. Patients with DEXA scans demonstrating osteoporosis (T score <2) should be treated with bisphosphonates in the same manner as patients with osteoporosis.
An emerging complication of bisphosphonate therapy is osteonecrosis of the jaw (ONJ). ONJ has been reported in association with bisphosphonate use in patients with metastastic bone disease as well as benign osteoporosis, although a cause and effect relationship has not been clearly demonstrated. Patients with myeloma have been reported to have the highest incidence of ONJ (1.6–11%; reviewed in [130]) while patients with postmenopausal osteoporosis who are treated with oral bisphosphonates have an incidence of ONJ of 1/10,000–1/100,000 patient treatment years [131].
Bisphosphonate associated ONJ is defined as the presence of the exposed bone in the mandible or maxilla in patients receiving bisphosphonate therapy that does not heal within 8 weeks of appropriate dental management in the absence of local metastatic disease or previous radiation therapy [130]. Clinical examination usually shows an exposed alveolar ridge with evidence of necrotic bone, often with a purulent discharge. The surrounding gums and mucosal tissue are often inflamed and can be painful to the touch [130]. Patients can have single or multiple lesions with the mandible more frequently involved than the maxilla. Most patients have only exposed bone, although fistulae to the maxillary sinus or the skin rarely occur and pathologic fractures of the mandible have been reported [130]. The overwhelming majority of cases reported are case reports or retrospective studies of patients receiving bisphosphonate therapy. A long-term follow-up study of 97 myeloma patients with ONJ followed for at least 3.2 years identified dental extraction, older age and longer survival as risk factors for ONJ [132]. ONJ resolved in 60 of the 97 patients studied, resolved and recurred in 12 of the patients, and did not heal over a 9-month period in 26% of the patients. Dental extraction preceded development of ONJ in 47% of the patients and was more common in patients with a single episode of ONJ than in patients with recurrent or nonhealing ONJ. ONJ recurrence in these patients was associated with reinitiation of bisphosphonate therapy or dental procedures. Patients developing ONJ following dental procedures were less likely to have a recurrence or nonhealing, and although infrequent, recurrence was linked to re-treatment with bisphosphonates in patients with relapsed myeloma.
The pathophysiology underlying ONJ is unclear. Decreased bone remodeling induced by bisphosphonates or inhibition of osteoclast function, which interferes with healing of the microfractures and trauma occurring after dental extraction, have been hypothesized as potential mechanisms for ONJ, but these have not been confirmed [133]. No specific myeloma treatments have been clearly implicated in the pathogenesis of ONJ, although dexamethasone and thalidomide have been suggested as additional risk factors [134]. Interestingly, patients with ONJ are more frequently diabetic or have impaired glucose tolerance when compared to an age-matched population [135]. Culture of some ONJ lesions has revealed actinomycetes, suggesting that infection may also play a role in the development of ONJ [130].
The decision of whether to stop bisphosphonate therapy in myeloma patients who develop ONJ remains a major clinical question. Bisphosphonates have an extremely long half-life in bone, estimated to be greater than 10 years; so stopping bisphosphonates may or may not have any effect on ONJ. In patients who have progressive bone disease, reinstitution or continuation of bisphosphonate therapy should be considered after the risks and benefits have been discussed with the patient.
6.1. Denosumab
Denosumab, a human monoclonal antibody that binds to RANKL with high affinity and specificity, was approved by the FDA for prevention of SREs in patients with bone metastases from solid tumors in 2010, and is currently under investigation for use in MM bone disease. A recent clinical trial has demonstrated that denosumab inhibits bone resorption and prevents SREs in patients refractory to bisphosphonate therapy [136], [137].
Denosumab inhibits RANKL-RANK interactions, mimicking the endogenous effects of OPG, a soluble RANKL decoy receptor. Recent reports have demonstrated that denosumab treatment prevents bone loss and decreases fractures in patients with osteoporosis or receiving androgen deprivation therapy for prostate cancer [138], [139] while also resulting in a statistically significant improvement in bone mineral density in patients with nonmetastatic prostate or breast cancer [138], [140]. Efficacy advantages for denosumab over zoledronic acid in myeloma have not yet been demonstrated, though myeloma patients were included in a separate clinical trial evaluating the efficacy of denosumab in approximately 1500 patients with solid tumor bone metastasis and 200 patients with myeloma. In this study denosumab reduced skeletal related events and time to next skeletal related event as effectively as zoledronic acid [141].
In clinical trials thus far, denosumab has been well tolerated. Hypocalcemia occurs more frequently in denosumab-treated patients compared with patients treated with zoledronic acid, with an incidence ranging from 5.5 to 13% with denosumab treatment as compared to 3.4–6% with zoledronic acid across the three phase III SRE studies published thus far [142]. Reported rates of ONJ in patients treated with denosumab are similar to those for patients treated with zoledronic acid (1.8%, denosumab; 1.3% zoledronic acid) [143].
6.2. Bortezomib
Bortezomib is a highly active agent for the treatment of MM. Bortezomib is a proteasome antagonist that induces MM cell apoptosis and directly alters OBL and OCL activity by decreasing RANKL and DKK-1 levels in the sera of myeloma patients [144]. Clinical trials with bortezomib indicate that it may also increase OBL activity, induce new bone formation, and potentially repair lytic bone lesions. In human OBL precursor cultures, bortezomib increased markers of OBL differentiation and OBL-specific transcription factors and enhanced bone nodule formation. Bone marrow samples of patients responding to bortezomib had a significantly increased number of osteoblastic cells compared to non-responders. These studies suggest that bortezomib can stimulate OBL in patients whose MM responded to bortezomib [145].
Zangari et al. conducted a retrospective analysis of three trials of bortezomib in patients with relapsed MM [146]. In all three trials, patients who had a partial response to bortezomib therapy had a transient increase in alkaline phosphatase level compared to non-responders. When compared to patients who responded to dexamethasone treatment, the bortezomib-treated group had higher serum levels of alkaline phosphatase than dexamethasone responders, suggesting that the increase in alkaline phosphatase was not merely a result of reduced tumor burden. More recently, a prospective study of bortezomib-associated bone changes [147] has been reported. Bortezomib naïve myeloma patients with relapsed or progressive disease were treated with bortezomib at two dosing levels. Patients achieving stable disease were continued on the regimen and followed until evidence of disease progression. After bortezomib treatment measurements of bone volume/ total volume significantly increased in 6 of 7 patients and trabecular thickness increased from baseline in 5 of 7 patients. Histologic evaluation demonstrated a lack of OBL activity and osteoid formation at baseline compared to bortezomib treatment in patients who responded to therapy. While some have interpreted these findings as evidence that bortezomib directly stimulates OBL and inhibits OCL, Lund et al. [148] have suggested that biochemical markers of bone formation peak after six weeks of bortezomib treatment due to a direct inhibitory effect on bone resorption by OCL that counteracts bortezomib's initial direct OBL stimulatory effect. Alternatively, bortezomib's direct inhibition of myeloma cells in the bone marrow microenvironment might allow for normalization of OBL and OCL function, as these effects are only seen in patients whose disease is bortezomib responsive.
6.3. IMiDs as OCL inhibitors
IMiDs are highly active agents in the treatment of MM [149]. Anderson et al. reported that CC-4047 (pomalidomide), a derivative of thalidomide that has similar actions as lenalidomide [150], inhibited OCL development by affecting the lineage commitment of OCL precursors. CC-4047 down-regulated the expression of PU.1, a critical transcription factor for the development of OCLs. The down-regulation of PU.1 in hematopoietic progenitor cells resulted in a complete shift of lineage development towards granulocytes and away from OCL. This inhibited OCL formation with a concomitant accumulation of immature granulocytes. Similarly, Breitkreutz et al. demonstrated that lenalidomide inhibited OCL formation by targeting PU.1 and down-regulating cathepsin K [151]. These results suggest that like bortezomib, IMiDs may have both bone and anti-MM effects.
6.4. Other anabolic agents for myeloma
Parathyroid hormone has been tested in preclinical models for its capacity to repair bone lesions or inhibit bone destruction in patients with myeloma. Yaccoby and coworkers have shown that PTH can stimulate bone formation in the SCID-RAB model of multiple myeloma [152], both in the implanted bone rudiment and normal mouse bones in this model, and resulted in decreased tumor burden. Teriparatide, recombinant PTH, decreases the risk of vertebral and non-vertebral fractures in post menopausal women with a history of vertebral fractures [153], however no clinical trials have been reported that show that PTH is an effective treatment for myeloma bone disease. Although there has been a concern that PTH may stimulate tumor growth in patients with myeloma, to date PTH receptors have not been detected on myeloma cells.
Another novel anabolic agent that is in clinical trial for patients with myeloma is sotatercept (ACE-011, Acceleron Pharm). Sotatercept is a chimeric fusion protein derived from the extracellular component of the activin A receptor and the Fc domain of human IgG1 that functions as an activin receptor inhibitor, thus blocking osteoblast suppression and osteoclast stimulation by activin. Raje and coworkers reported that activin levels are increased in patients with myeloma, and that OCL and OBL are the primary source of activin in these patients [154]. They further showed that blocking activin inhibits bone destruction in preclinical models of myeloma. A clinical trial of the bone anabolic effects of sotatercept in MM patients with osteolytic lesions is in process.
7. Conclusions
Myeloma bone disease is responsible for some of the most devastating complications of the disease. Patients endure severe bone pain, pathologic fractures, hypercalcemia, and a markedly decreased quality of life. Understanding the pathogenesis of myeloma bone disease has allowed us to identify novel targets for treating the disease. An important feature of myeloma bone disease is that the lytic lesions do not heal even when the patients are in prolonged remission, suggesting that bone repair does not occur at previous sites of bone destruction in patients with myeloma. The development of anabolic agents, which are safe for use in patients with myeloma, may reverse this process and reverse the loss of skeletal integrity in patients with myeloma. With the enhanced median survival in patients with myeloma that has occurred since the introduction of new therapies for treatment of myeloma, managing the bone disease and its complications will be evermore important for myeloma patients. Thus, the future should be bright for patients with myeloma with new agents to block bone destruction as well as potentially build bone at sites of previous bone destruction.
Conflict of Interest Statement
GDR is a consultant to Amgen and develops and presents continuing medical education material for Clinical Care Options. RS presents continuing medical education material for Clinical Care Options.
References
- 1.Roodman GD. Mechanisms of bone metastasis. The New England journal of medicine. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Terpos E, Berenson J, Cook RJ, Lipton A, Coleman RE. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24:1043–1049. doi: 10.1038/leu.2010.62. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research. 2005;20:487–493. doi: 10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 5.Diamond T, Levy S, Day P, Barbagallo S, Manoharan A, Kwan YK. Biochemical, histomorphometric and densitometric changes in patients with multiple myeloma: effects of glucocorticoid therapy and disease activity. British Journal of Haematology. 1997;97:641–648. doi: 10.1046/j.1365-2141.1997.1042920.x. [DOI] [PubMed] [Google Scholar]
- 6.Roodman GD. Diagnosis and treatment of myeloma bone disease. In: Rajkumar SV, Kyle RA, editors. Treatment of multiple myeloma and related disorders. Cambridge University Press; New York: 2009. pp. 64–76. [Google Scholar]
- 7.Nilsson-Ehle H, Holmdahl C, Suurkula M, Westin J. Bone scintigraphy in the diagnosis of skeletal involvement and metastatic calcification in multiple myeloma. Acta Medica Scandinavica. 1982;211:427–432. doi: 10.1111/j.0954-6820.1982.tb01976.x. [DOI] [PubMed] [Google Scholar]
- 8.Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 9.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma aredia study group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 10.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proceedings. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 11.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA: Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 12.Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008 National Cancer Institute; 2011.
- 13.Roodman G. In: GAM, MEA, B LJ, editors. Skeletal imaging and management of bone disease. American Society of Hematology Educational Program Hematology; 2008:313–9. 10.1182/asheducation-2008.1.313 [DOI] [PubMed]
- 14.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23:1545–1556. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clinic Proceedings. 1975;50:29–40. [PubMed] [Google Scholar]
- 16.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Taube T, Beneton MN, McCloskey EV, Rogers S, Greaves M, Kanis JA. Abnormal bone remodelling in patients with myelomatosis and normal biochemical indices of bone resorption. European Journal of Haematology. 1992;49:192–198. doi: 10.1111/j.1600-0609.1992.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 18.Valentin-Opran A, Charhon SA, Meunier PJ, Edouard CM, Arlot ME. Quantitative histology of myeloma-induced bone changes. British Journal of Haematology. 1982;52:601–610. doi: 10.1111/j.1365-2141.1982.tb03936.x. [DOI] [PubMed] [Google Scholar]
- 19.Dhodapkar MV, Weinstein R, Tricot G, Jagannath S, Parfitt AM, Manolagas SC. Biologic and therapeutic determinants of bone mineral density in multiple myeloma. Leukemia and Lymphoma. 1998;32:121–127. doi: 10.3109/10428199809059252. [DOI] [PubMed] [Google Scholar]
- 20.Ng AC, Khosla S, Charatcharoenwitthaya N, Kumar SK, Achenbach SJ, Holets MF. Bone microstructural changes revealed by high-resolution peripheral quantitative computed tomography imaging and elevated DKK1 and MIP-1alpha levels in patients with MGUS. Blood. 2011;118:6529–6534. doi: 10.1182/blood-2011-04-351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: implications for bone disease. Blood. 2009;114:3413–3421. doi: 10.1182/blood-2009-03-211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roodman GD. Pathogenesis of myeloma bone disease. Journal of Cellular Biochemistry. 2010;109:283–291. doi: 10.1002/jcb.22403. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematology/Oncology Clinics of North America. 2007;21:1007–1034. doi: 10.1016/j.hoc.2007.08.007. vii–viii. [DOI] [PubMed] [Google Scholar]
- 26.Bruni-Cardoso A, Johnson LC, Vessella RL, Peterson TE, Lynch CC. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Molecular Cancer Research. 2010;8:459–470. doi: 10.1158/1541-7786.MCR-09-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ. Osteoclasts are important for bone angiogenesis. Blood. 2010;115(1):140–149. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. Journal of Clinical Investigation. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-beta (TGF-beta )-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. Journal of Biological Chemistry. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 30.Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Josse RG, Murray TM, Mundy GR, Jez D, Heersche JN. Observations on the mechanism of bone resorption induced by multiple myeloma marrow culture fluids and partially purified osteoclast-activating factor. The Journal of Clinical Investigation. 1981;67:1472–1481. doi: 10.1172/JCI110177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 33.Giuliani N, Colla S, Rizzoli V. New insight in the mechanism of osteoclast activation and formation in multiple myeloma: focus on the receptor activator of NF-kappaB ligand (RANKL) Experimental Hematology. 2004;32:685–691. doi: 10.1016/j.exphem.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- 35.Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103:2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]
- 36.Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149–2154. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 37.Edwards CM, Zhuang J, Mundy GR. The pathogenesis of the bone disease of multiple myeloma. Bone. 2008;42:1007–1013. doi: 10.1016/j.bone.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hideshima T, Chauhan D, Podar K, Schlossman RL, Richardson P, Anderson KC. Novel therapies targeting the myeloma cell and its bone marrow microenvironment. Seminars in Oncology. 2001;28:607–612. doi: 10.1016/s0093-7754(01)90033-8. [DOI] [PubMed] [Google Scholar]
- 39.D'Souza S, Kurihara N, Shiozawa Y, Joseph J, Taichman R, Galson DL, et al. Annexin II interactions with the annexin II receptor enhance multiple myeloma cell adhesion and growth in the bone marrow microenvironment. Blood. 2012;119(8):1888–96. [DOI] [PMC free article] [PubMed]
- 40.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T. Osteoclasts enhance myeloma cell growth and survival via cell–cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 41.Ge Y, Zhan F, Barlogie B, Epstein J, Shaughnessy J, Jr., Yaccoby S. Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. British Journal of Haematology. 2006;133:83–92. doi: 10.1111/j.1365-2141.2006.05976.x. [DOI] [PubMed] [Google Scholar]
- 42.Abe M, Kido S, Hiasa M, Nakano A, Oda A, Amou H. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006;20:1313–1315. doi: 10.1038/sj.leu.2404228. [DOI] [PubMed] [Google Scholar]
- 43.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochemical and Biophysical Research Communications. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- 45.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 46.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofbauer LC, Heufelder AE. Osteoprotegerin and its cognate ligand: a new paradigm of osteoclastogenesis. European Journal of Endocrinology. 1998;139:152–154. doi: 10.1530/eje.0.1390152. [DOI] [PubMed] [Google Scholar]
- 48.Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 50.Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget's disease and multiple myeloma. Immunological Reviews. 2005;208:252–266. doi: 10.1111/j.0105-2896.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi Y, Udagawa N, Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Critical Reviews in Eukaryotic Gene Expression. 2009;19:61–72. doi: 10.1615/critreveukargeneexpr.v19.i1.30. [DOI] [PubMed] [Google Scholar]
- 52.Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J. Soluble receptor activator of nuclear factor kappaB ligand–osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102:1064–1069. doi: 10.1182/blood-2003-02-0380. [DOI] [PubMed] [Google Scholar]
- 53.Yaccoby S, Pearse RN, Johnson CL, Barlogie B, Choi Y, Epstein J. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. British Journal of Haematology. 2002;116:278–290. doi: 10.1046/j.1365-2141.2002.03257.x. [DOI] [PubMed] [Google Scholar]
- 54.Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–3540. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- 55.Li B, Shi M, Li J, Zhang H, Chen B, Chen L. Elevated tumor necrosis factor-alpha suppresses TAZ expression and impairs osteogenic potential of Flk-1+ mesenchymal stem cells in patients with multiple myeloma. Stem Cells and Development. 2007;16:921–930. doi: 10.1089/scd.2007.0074. [DOI] [PubMed] [Google Scholar]
- 56.Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. European Journal of Cancer. 2006;42:1564–1573. doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto I, Kawano M, Sone T, Iwato K, Tanaka H, Ishikawa H. Production of interleukin 1 beta, a potent bone resorbing cytokine, by cultured human myeloma cells. Cancer Research. 1989;49:4242–4246. [PubMed] [Google Scholar]
- 58.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. The Journal of Clinical Investigation. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roodman GD. Osteoblast function in myeloma. Bone. 2011;48:135–140. doi: 10.1016/j.bone.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P. A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25:1174–1181. doi: 10.1038/leu.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–2202. [PubMed] [Google Scholar]
- 62.Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. The Journal of Clinical Investigation. 2001;108:1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003;102:311–319. doi: 10.1182/blood-2002-12-3905. [DOI] [PubMed] [Google Scholar]
- 64.Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97:3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- 65.Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha ) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101:3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- 66.Magrangeas F, Nasser V, Avet-Loiseau H, Loriod B, Decaux O, Granjeaud S. Gene expression profiling of multiple myeloma reveals molecular portraits in relation to the pathogenesis of the disease. Blood. 2003;101:4998–5006. doi: 10.1182/blood-2002-11-3385. [DOI] [PubMed] [Google Scholar]
- 67.Masih-Khan E, Trudel S, Heise C, Li Z, Paterson J, Nadeem V. MIP-1alpha (CCL3) is a downstream target of FGFR3 and RAS-MAPK signaling in multiple myeloma. Blood. 2006;108:3465–3471. doi: 10.1182/blood-2006-04-017087. [DOI] [PubMed] [Google Scholar]
- 68.Alsina M, Boyce B, Devlin RD, Anderson JL, Craig F, Mundy GR. Development of an in vivo model of human multiple myeloma bone disease. Blood. 1996;87:1495–1501. [PubMed] [Google Scholar]
- 69.Menu E, De Leenheer E, De Raeve H, Coulton L, Imanishi T, Miyashita K. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clinical and Experimental Metastasis. 2006;23:291–300. doi: 10.1007/s10585-006-9038-6. [DOI] [PubMed] [Google Scholar]
- 70.Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood. 2007;110:3744–3752. doi: 10.1182/blood-2007-05-093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gladue RP, Brown MF, Zwillich SH. CCR1 antagonists: what have we learned from clinical trials. Current Topics in Medicinal Chemistry. 2010;10:1268–1277. doi: 10.2174/156802610791561237. [DOI] [PubMed] [Google Scholar]
- 72.Silbermann R, Bolzoni M, Storti P, Palma BD, Bonomini S, Anderson J, et al. Bone marrow monocyte/macrophage derived activin a mediates the osteoclastogenic effects of IL-3 in myeloma. ASH Annual Meeting Abstracts 2011;118:3933.
- 73.Fuller K, Bayley KE, Chambers TJ. Activin A is an essential cofactor for osteoclast induction. Biochemical and Biophysical Research Communications. 2000;268:2–7. doi: 10.1006/bbrc.2000.2075. [DOI] [PubMed] [Google Scholar]
- 74.Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5124–5129. doi: 10.1073/pnas.0911929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Terpos E, Christoulas D, Kastritis E, Gkotzamanidou M, Gavriatopoulou M, Eleutherakis-Papaiakovou E., et al. Elevated levels of circulating activin-A correlate with features of advanced disease, extensive bone involvement and inferior survival in patients with multiple myeloma. ASH Annual Meeting Abstracts 2010;116:2967.
- 76.Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 77.Chantry AD, Heath D, Mulivor AW, Pearsall S, Baud'huin M, Coulton L. Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25:2633–2646. doi: 10.1002/jbmr.142. [DOI] [PubMed] [Google Scholar]
- 78.Ruckle J, Jacobs M, Kramer W, Pearsall AE, Kumar R, Underwood KW. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research. 2009;24:744–752. doi: 10.1359/jbmr.081208. [DOI] [PubMed] [Google Scholar]
- 79.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J. Annexin II/Annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. Journal of Cellular Biochemistry. 2008;370:370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Claudio JO, Masih-Khan E, Tang H, Goncalves J, Voralia M, Li ZH. A molecular compendium of genes expressed in multiple myeloma. Blood. 2002;100:2175–2186. doi: 10.1182/blood-2002-01-0008. [DOI] [PubMed] [Google Scholar]
- 81.Bao H, Jiang M, Zhu M, Sheng F, Ruan J, Ruan C. Overexpression of Annexin II affects the proliferation, apoptosis, invasion and production of proangiogenic factors in multiple myeloma. International Journal of Hematology. 2009;90:177–185. doi: 10.1007/s12185-009-0356-8. [DOI] [PubMed] [Google Scholar]
- 82.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metabolism. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 83.Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD, Jr., Barlogie B. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009;114:1803–1812. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen AN, Stebbins EG, Henson M, O'Young G, Choi SJ, Quon D. Normalizing the bone marrow microenvironment with p38 inhibitor reduces multiple myeloma cell proliferation and adhesion and suppresses osteoclast formation. Experimental Cell Research, 2006;312:1909–1923. doi: 10.1016/j.yexcr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 85.Vanderkerken K, Medicherla S, Coulton L, Van Camp B, Protter A, Higgins L. Inhibition of p38α MAPK reduces tumor burden, prevents the development of myeloma bone disease, and increases survival in the 5T2 and5T33 murine models of myeloma. Blood. 2006;108:981a. doi: 10.1158/0008-5472.CAN-06-4361. [DOI] [PubMed] [Google Scholar]
- 86.Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood. 2004;103:3503–3510. doi: 10.1182/blood-2003-07-2340. [DOI] [PubMed] [Google Scholar]
- 87.Nimmanapalli R, Gerbino E, Dalton WS, Gandhi V, Alsina M. HSP70 inhibition reverses cell adhesion mediated and acquired drug resistance in multiple myeloma. British Journal of Haematology. 2008;142:551–561. doi: 10.1111/j.1365-2141.2008.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka Y, Abe M, Hiasa M, Oda A, Amou H, Nakano A. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clinical Cancer Research. 2007;13:816–823. doi: 10.1158/1078-0432.CCR-06-2258. [DOI] [PubMed] [Google Scholar]
- 89.Bataille R, Chappard D, Marcelli C, Dessauw P, Sany J, Baldet P. Mechanisms of bone destruction in multiple myeloma: the importance of an unbalanced process in determining the severity of lytic bone disease. Journal of Clinical Oncology. 1989;7:1909–1914. doi: 10.1200/JCO.1989.7.12.1909. [DOI] [PubMed] [Google Scholar]
- 90.Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 91.Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kobayashi T, Kronenberg H. Minireview: transcriptional regulation in development of bone. Endocrinology. 2005;146:1012–1017. doi: 10.1210/en.2004-1343. [DOI] [PubMed] [Google Scholar]
- 93.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 94.Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112:159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 96.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 97.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. The Journal of Biological Chemistry. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 98.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nature Cell Biology. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 99.Giuliani N, Mangoni M, Rizzoli V. Osteogenic differentiation of mesenchymal stem cells in multiple myeloma: identification of potential therapeutic targets. Experimental Hematology. 2009;37:879–886. doi: 10.1016/j.exphem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 100.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. The New England Journal of Medicine. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 101.Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. European Journal of Haematology. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 102.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Developmental Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 104.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. Journal of Cell Science. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 105.Brunetti G, Oranger A, Mori G, Specchia G, Rinaldi E, Curci P. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Annals of the New York Academy of Sciences. 2011;1237:19–23. doi: 10.1111/j.1749-6632.2011.06196.x. [DOI] [PubMed] [Google Scholar]
- 106.Costa AG, Bilezikian JP. Sclerostin: therapeutic horizons based upon its actions. Current Osteoporosis Reports. 2012;10:64–72. doi: 10.1007/s11914-011-0089-5. [DOI] [PubMed] [Google Scholar]
- 107.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. International Journal of Cancer Journal International du Cancer. 2012;131:1466–1471. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 108.Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012;26:1391–1401. doi: 10.1038/leu.2011.381. [DOI] [PubMed] [Google Scholar]
- 109.Standal T, Abildgaard N, Fagerli UM, Stordal B, Hjertner O, Borset M. HGF inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma. Blood. 2007;109:3024–3030. doi: 10.1182/blood-2006-07-034884. [DOI] [PubMed] [Google Scholar]
- 110.Takeuchi K, Abe M, Hiasa M, Oda A, Amou H, Kido S. Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PloS one. 2010;5:e9870. doi: 10.1371/journal.pone.0009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–57. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 112.Terpos E, Kastritis E, Christoulas D, Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N, Papatheodorou A, Dimopoulos MA. Elevated levels of circulating activin-a correlate with features of advanced disease, extensive bone involvement and inferior survival in patients with multiple myeloma. Blood. 2010 doi: 10.1093/annonc/mds068. [DOI] [PubMed] [Google Scholar]
- 113.Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100:4615–4621. doi: 10.1182/blood-2002-04-1121. [DOI] [PubMed] [Google Scholar]
- 114.Giuliani N, Rizzoli V. Myeloma cells and bone marrow osteoblast interactions: role in the development of osteolytic lesions in multiple myeloma. Leukemia and Lymphoma. 2007;48:2323–2329. doi: 10.1080/10428190701648281. [DOI] [PubMed] [Google Scholar]
- 115.D'Souza S, Jin S, Sammut B, Velu CS, Yu S, Xiao G., et al. Multiple myeloma cell induction of GFI-1 in stromal cells suppresses osteoblast differentiaion in patients with myeloma. 32nd Annual Meeting of the American Society of Bone and Mineral. Toronto, Canada; 2010. p. 742A.
- 116.D'Souza S, del Prete D, Jin S, Sun Q, Huston AJ, Kostov FE. Gfi1 expressed in bone marrow stromal cells is a novel osteoblast suppressor in patients with multiple myeloma bone disease. Blood. 2011;118:6871–6880. doi: 10.1182/blood-2011-04-346775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jin J, Zeng H, Schmid KW, Toetsch M, Uhlig S, Moroy T. The zinc finger protein Gfi1 acts upstream of TNF to attenuate endotoxin-mediated inflammatory responses in the lung. European Journal of Immunology. 2006;36:421–430. doi: 10.1002/eji.200535155. [DOI] [PubMed] [Google Scholar]
- 118.Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–5882. doi: 10.1182/blood-2011-01-330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McCloskey EV, MacLennan IC, Drayson MT, Chapman C, Dunn J, Kanis JA. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in Adults. British Journal of Haematology. 1998;100:317–325. doi: 10.1046/j.1365-2141.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 120.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia. 1998;12:220–229. doi: 10.1038/sj.leu.2400892. [DOI] [PubMed] [Google Scholar]
- 122.Avcu F, Ural AU, Yilmaz MI, Ozcan A, Ide T, Kurt B. The bisphosphonate zoledronic acid inhibits the development of plasmacytoma induced in BALB/c mice by intraperitoneal injection of pristane. European Journal of Haematology. 2005;74:496–500. doi: 10.1111/j.1600-0609.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 123.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer Journal. 2001;7:377–387. [PubMed] [Google Scholar]
- 124.Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 125.Terpos E, Sezer O, Croucher PI, Garcia-Sanz R, Boccadoro M, San Miguel J. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2009;20:1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 126.Morgan GJ, Davies FE, Gregory WM, Szubert AJ, Bell SE, Drayson MT. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council Myeloma IX Trial. Blood. 2012;119:5374–5383. doi: 10.1182/blood-2011-11-392522. [DOI] [PubMed] [Google Scholar]
- 127.Terpos E, Roodman GD, Dimopoulos MA. Optimal use of bisphosphonates in patients with multiple myeloma. Blood. Feb 2013 [epub ahead of print] doi: 10.1182/blood-2012-10-435750. [DOI] [PubMed] [Google Scholar]
- 128.Berenson JR, Anderson KC, Audell RA, Boccia RV, Coleman M, Dimopoulos MA. Monoclonal gammopathy of undetermined significance: a consensus statement. British Journal of Haematology. 2010;150:28–38. doi: 10.1111/j.1365-2141.2010.08207.x. [DOI] [PubMed] [Google Scholar]
- 129.Minter AR, Simpson H, Weiss BM, Landgren O. Bone disease from monoclonal gammopathy of undetermined significance to multiple myeloma: pathogenesis, interventions, and future opportunities. Seminars in Hematology. 2011;48:55–65. doi: 10.1053/j.seminhematol.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van den Wyngaert T, Huizing MT, Vermorken JB. Osteonecrosis of the jaw related to the use of bisphosphonates. Current Opinion in Oncology. 2007:315–322. doi: 10.1097/CCO.0b013e32819f820b. [DOI] [PubMed] [Google Scholar]
- 131.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 132.Badros A, Evangelos T, Goloubeva O, Meiller T, Kastritis E, Verrou E. Long-Term follow-up of multiple myeloma (MM) patients (pts) with osteonecrosis of the jaw (ONJ) Blood. 2007;110:1030a. [Google Scholar]
- 133.Clarke BM, Boyette J, Vural E, Suen JY, Anaissie EJ, Stack BC., Jr. Bisphosphonates and jaw osteonecrosis: the UAMS experience. Otolaryngol Head and Neck Surgery. 2007;136:396–400. doi: 10.1016/j.otohns.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 134.Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2006;24:945–952. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 135.Khamaisi M, Regev E, Yarom N, Avni B, Leitersdorf E, Raz I. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. Journal of Clinical Endocrinology and Metabolism. 2007;92:1172–1175. doi: 10.1210/jc.2006-2036. [DOI] [PubMed] [Google Scholar]
- 136.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 137.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 138.Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. The New England Journal of Medicine. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. The New England Journal of Medicine. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 140.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 141.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 142.Lipton A, Jacobs I. Denosumab: benefits of RANK ligand inhibition in cancer patients. Current Opinion in Supportive and Palliative Care. 2011;5:258–264. doi: 10.1097/SPC.0b013e328349731c. [DOI] [PubMed] [Google Scholar]
- 143.Limited AM. XGEVA US Prescribing Information. Thousand Oaks, CA; 2013.
- 144.Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. British Journal of Haematology. 2006;135:688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 145.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 146.Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. British Journal of Haematology. 2005;131:71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 147.Zangari M, Yaccoby S, Pappas L, Cavallo F, Kumar NS, Ranganathan S. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica. 2011;96:333–336. doi: 10.3324/haematol.2010.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lund T, Soe K, Abildgaard N, Garnero P, Pedersen PT, Ormstrup T. First-line treatment with bortezomib rapidly stimulates both osteoblast activity and bone matrix deposition in patients with multiple myeloma, and stimulates osteoblast proliferation and differentiation in vitro. European Journal of Haematology. 2010;85:290–299. doi: 10.1111/j.1600-0609.2010.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumar S, Rajkumar SV. Thalidomide and lenalidomide in the treatment of multiple myeloma. European Journal of Cancer. 2006;42:1612–1622. doi: 10.1016/j.ejca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 150.Anderson G, Gries M, Kurihara N, Honjo T, Anderson J, Donnenberg V. Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood. 2006;107:3098–3105. doi: 10.1182/blood-2005-08-3450. [DOI] [PubMed] [Google Scholar]
- 151.Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22:1925–1932. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- 152.Li X, Pennisi A, Yaccoby S. Small leucine-rich proteoglycans (SLRPs) are involved in the anti-myeloma response of osteoblasts. Blood. 2007;110:250a. [Google Scholar]
- 153.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England Journal of Medicine. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 154.Vallet S, Siddhartha M, Vaghela N, Pozzi S, Santo L, Cirstea D. Restoration of bone balance via activin a inhibition results in anti-myeloma activity. Blood. 2008;112:240A. [Google Scholar]