Abstract
Objective
Questions remain regarding the optimal use of bone-targeted agents in patients with metastatic bone disease. The purpose of this study was to assess current clinical practice regarding the use and administration of bone-targeted agents by Canadian oncologists in patients with metastatic breast and prostate cancer.
Methods
A survey was designed to explore; bone-targeted agent use in metastatic bone disease, variability in the choice and the frequency of administration of these agents. Opinions were sought on potential outcomes for future trials.
Results
A total of 193 clinicians were contacted and 90 completed our survey (response rate 49% after adjustment for inactivity). Survey respondents were medical oncologists (71.1%), radiation oncologists (21.1%) and urologists (7.8%). The findings suggest that once bone-targeted agents are started they are rarely discontinued. More agents are used in breast cancer than in prostate cancer. There was considerable interest in performing studies of de-escalated therapy in both breast and prostate cancer. Physicians requested (86%) that the primary study endpoint be the occurrence of skeletal related events and not biomarker driven.
Conclusions
Despite clinical practice guidelines and widespread use, significant areas of clinical equipoise with respect to use of bone-targeted agents exist. Findings from this survey suggest that physicians are interested in de-escalated therapy for both breast and prostate patients. However, the use of multiple agents in breast cancer and the desire for skeletal related events to be the primary endpoint means that very large randomized studies will be required.
Keywords: Bone metastasis, Bisphosphonate, Bone targeted agent, Survey
1. Background
Despite the widespread use of bone-targeted therapies such as bisphosphonates (e.g. zoledronate, pamidronate, clodronate) or receptor activator of nuclear factor kappa-B ligand (RANKL) antibodies (i.e. denosumab) in patients with metastatic bone disease, many questions remain about their optimal use [1], [2]. One question in particular pertains to identification of the optimal dosing frequency [3]. Bone-targeted agents are usually given every 3–4 weeks, a dosing interval that is based predominantly on their clinical development as an add-on treatment to standard anti-cancer therapies such as chemotherapy [4], along with data derived from the treatment of hypercalcemia from malignancy [5], [6], [7].
This “one size fits all” approach to the dosing intervals [8] is sub-optimal however, as it ignores the long half-life of these agents in bone [9] and the substantial variability in individual patient risk of skeletal related events (SREs) [10]. Given the modest magnitude of absolute benefit of bone-targeted agents on skeletal related event reductions, [2] it is important to investigate whether to not less frequent administration could affect the efficacy of these agents. This would not only result in reduced financial costs to both the patient and to the health care system, but would also likely reduce drug-associated toxicity. The latter is particularly important as toxicity of these agents has been shown to be related to both the potency and the cumulative dose of the bone-targeted agent [11].
To date, two trials have been presented assessing reduced frequencies of administration of these agents in metastatic breast cancer patients [12], [13] and others are still on going [17]. Despite this, the results of the published trials would suggest that there is still a need for larger definitive studies. In addition, we are not aware of any similar studies planned for prostate cancer where again the benefits of bone-targeted agents in reducing SREs are likely even more modest than that seen in breast cancer patients. We are however aware of considerable variability in clinical practice, not only between cancer centers, but also within centers with respect to a number of questions around optimal use of bone-targeted agents, despite various clinical practice guidelines [3].
Before contemplating a trial to formally assess the feasibility of de-escalated bone-targeted treatment in both prostate and breast cancer patients, we wished to conduct a survey of potential collaborating physicians at Canadian hospitals regarding their current clinical practice in these populations and their views on this matter to assist with design of future trials. In particular, we hoped a survey would help establish current standards of care, the extent of clinical equipoise with respect to de-escalation, physician comfort with entering patients on such a trial, and finally, what the most important outcomes and related effect sizes might be for clinicians in order to establish the comparability of these approaches to treatment.
2. Methods
2.1. Questionnaire design
The survey was designed by the authors through three rounds of question development and refinement, and consists of three components. The first component was devised to collect pertinent demographic information of the population of respondents, as well as to determine what proportion amongst them use bone-targeted agents to treat their patients. The second component was designed to collect information from those respondents who prescribe bone-targeted agents with regard to intended benefits from bone-targeted agent use, scenarios in which they prescribe bone-targeted agents, and choice of agent for their patients in order to gain an understanding of current Canadian clinical practice. In the third component, respondents were presented with a series of questions related to the design of a future clinical trial geared toward studying the clinical benefits of de-escalated therapy, with topics of interest including outcome selection and patient inclusion criteria.
2.2. Survey frame and implementation
A member of the authorship team (MC) has held a national annual meeting related to the study of bone in oncology patients since 2005, and participants from past years' meetings were considered an accessible, representative, and appropriate group to approach as a population for this survey [3]. A list of all participants' email addresses was compiled, and these individuals were then sent a link via electronic mail inviting them to participate in the survey. The survey was designed and implemented using the online tool www.FluidSurveys.com. The survey was initiated at the start of July 2012 and remained open until September 1, 2012. Two reminder notices were sent to participants in July and August of 2012. Local research ethics board approval was received before commencing the study. The survey questions used in this study can be found in the online supplement.
2.3. Data analysis
Electronic invites were sent to a total of 193 clinicians, and a total of 90 invitees responded; 11 of the email invites were associated repeatedly with automatic out of office responses, and were thus excluded from the denominator (survey response rate=49.5%). All measures of respondent characteristics including profession, type of center for clinical practice, province, populations treated (breast cancer, prostate cancer, or both), number of new patients and follow up patients seen annually, and use of bone-targeted agents were compiled and reported as proportions of the total number of respondents. For summary statistics calculated in relation to components two and three of the survey as described above, a denominator of 66 respondents was used as physicians not using bone-targeted agents in their practice were not asked to respond to the questions associated with these components. We tabulated proportions of different responses for each question. Where relevant, we stratified findings according to the type of patients treated by the respondents (i.e. breast or prostate cancer; where respondents treated both populations, they were included within both groups). Data were analyzed using Microsoft Excel 2010 (Microsoft Corporation, Seattle, Washington).
3. Results
3.1. Survey component 1: characteristics of the respondent population
Physician demographics from the population of respondents are shown in Table 1. The distribution of characteristics shows that the majority work in teaching hospitals, consistently manage moderate to large numbers of patients, and that 55.6% (50/90) see at least one new patient per month, suggesting the population is an experienced group of oncologists. Approximately two thirds of the respondents were medical oncologists, with the remainder consisting of radiation oncologists and urologists. Physicians were located predominantly in Ontario (60%), Quebec (13.3%) and Alberta (11.1%). Totals of 43, 26 and 18 respondents treated breast cancer, prostate cancer, or both, respectively; 3 respondents failed to indicate their specialty. Amongst the 90 respondents, 73.3% (43/43 treating breast cancer patients, 12/26 treating prostate cancer patients, and 11/18 treating both) indicated that use of bone-targeted agents is a part of their clinical practice, and thus continued on to complete component two of the survey.
Table 1.
Summary of respondent population.
|
Characteristic |
Summary measure |
|---|---|
| Total # responders/Total # contacted | 90/182 (49.5%) |
| Specialty | |
| Medical oncologist | 64 (71.11%) |
| Radiation oncologist | 19 (21.11%) |
| Urologist | 7 (7.78%) |
| Location of practice | |
| Teaching hospital | 74 (83.15%) |
| Community hospital | 11 (12.36%) |
| Other (cancer center) | 4 (4.43%) |
| Province of practice | |
| Alberta | 10 (11.11%) |
| British Columbia | 5 (5.56%) |
| New Brunswick | 1 (1.11%) |
| Newfoundland and Labrador | 1 (1.11%) |
| Nova Scotia | 7 (7.78%) |
| Ontario | 53 (58.89%) |
| Prince Edward Island | 1 (1.11%) |
| Quebec | 12 (13.33%) |
| Clinical population treated | |
| Breast cancer | 43 (49.43%) |
| Prostate cancer | 26 (29.89%) |
| Both | 18 (20.69%) |
| Missing | 3 |
| # New breast cancer patients | |
| 1 per year | 1 (1.11%) |
| 1 per month | 40 (44.94%) |
| 1 per week | 12 (13.48%) |
| Other | 9 (10.11%) |
| I do not treat breast cancer | 27 (30.34%) |
| How many breast cancer patients under your care at a given time? | |
| <10 | 3 (3.33%) |
| 10–25 | 21 (23.33%) |
| 26–50 | 20 (22.22%) |
| More than 50 | 18 (20.00%) |
| Not applicable | 28 (31.11%) |
| # New prostate cancer patients | |
| 1 per year | 5 (5.75%) |
| 1 per month | 22 (25.29%) |
| 1 per week | 7 (8.05%) |
| Other | 13 (14.94%) |
| I do not treat prostate cancer | 40 (45.98%) |
| How many prostate cancer patients under your care at a given time? | |
| <10 | 9 (10.00%) |
| 10–25 | 18 (20.00%) |
| 26–50 | 12 (13.33%) |
| More than 50 | 7 (7.78%) |
| Not applicable | 44 (48.89%) |
| Do you routinely prescribe bone-targeted agents for patients with bone metastases from breast or prostate cancer? | |
| Yes | 66 (73.3%) |
| No | 24 (26.7%) |
3.2. Survey component 2: bone-targeted agent use in Canadian practice
3.2.1. Rationale for bone-targeted agent use
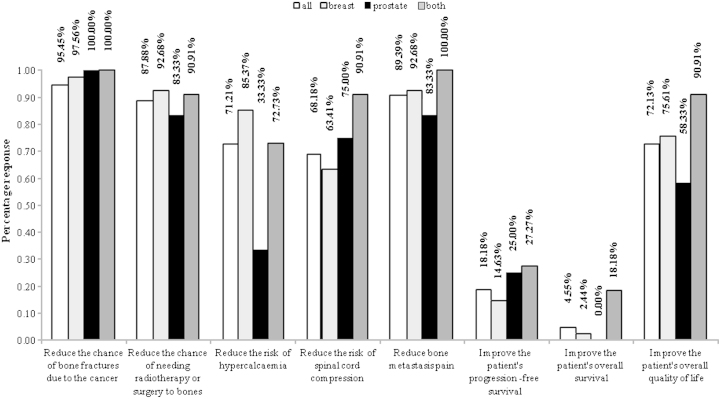
Respondents were first asked to describe the primary reasons they provide bone-targeted agents to their patients, with the ability to select multiple reasons as deemed relevant (see online questionnaire in supplementary data). Fig. 1 provides a quantitative summary of the reasons that were reported. Most respondents primarily provide bone-targeted agents to reduce fracture risk (95.45%), to reduce risk of surgery to bone or radiotherapy (87.88%), to reduce metastasis pain (89.39%), to improve quality of life (72.13%), to reduce hypercalcaemia risk (71.21%), and to reduce risk of spinal compression (68.18%). Few do so based on beliefs that these agents will improve progression-free survival (18.18%) or overall survival (4.55%). Response profiles were generally consistent across sub-populations of those treating breast cancer, prostate cancer, or both diseases (Fig. 1).
Fig. 1.
Reasons for administration of bone targeted agents.
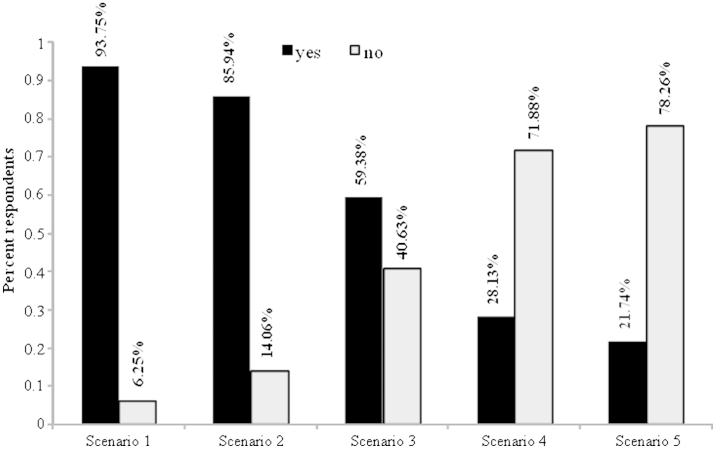
To assess the situations in which bone-targeted treatment might be prescribed, respondents were presented a series of five scenarios and asked whether they would prescribe a bone-targeted agent to the patient in question. Fig. 2 summarizes the related responses. For Scenario 1 the physician was asked, “For a typical patient seen in your clinic with newly diagnosed bone metastases, I would usually recommend a bone-targeted agent even if this patient has little or no pain”. For the responding clinicians 93.75% indicated they would recommend treatment in patients with newly diagnosed bone metastases, even if there is little to no pain present. For Scenario 2 clinicians were asked, “For a typical patient seen in your clinic with newly diagnosed bone metastases, I would usually recommend a bone-targeted agent even if the metastases were blastic”. In this setting, 85.94% of respondents indicated they would recommend treatment. Results from Scenario 3 showed that approximately 60% of respondents would still recommend bone-targeted therapy in patients who have a prognosis of 6 months or less due to visceral metastases. Data from Scenario 4 showed that only 28.13% of responding clinicians would recommend treatment in patients who display poor oral hygiene or who require considerable dental work. Finally, in Scenario 5, amongst the 23 respondents managing prostate cancer patients who use bone-targeted agents, 21.74% indicated that they would only start therapy in conjunction with chemotherapy. Three of the 90 respondents indicated regular monitoring of patients with new bone metastases using biomarkers such as urinary N-telopeptide to assess response to treatment.
Fig. 2.
Summary of respondent answers, scenarios 1–5. Summary of responses to Scenarios 1–5. ‘⁎’ Scenario 5 pertains only to respondents who use bone targeted agents to treat prostate cancer patients (n=23).
3.2.2. Choice of bone-targeted agent and frequency of administration
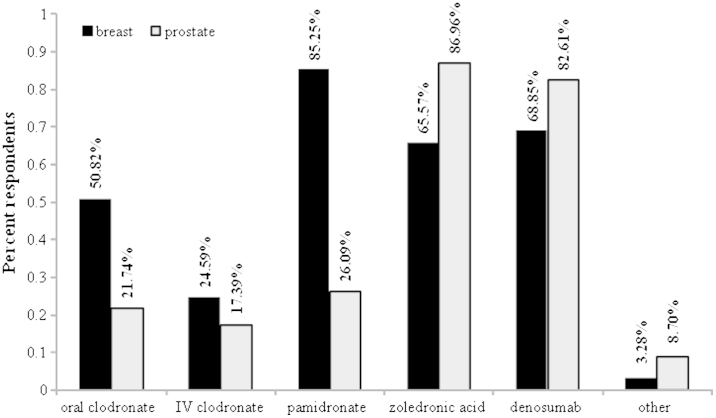
Respondents were asked to comment on their choice of bone-targeted agent. There were some differences in the bone-targeted agents used for breast cancer when compared to prostate cancer (Fig. 3). Amongst respondents who treat breast cancer, pamidronate was the agent most consistently used (52/61=85.25%), while denosumab (42/61=68.85%), zoledronic acid (40/61=65.57%) and oral clodronate (31/61=50.82%) had also been used to various degree by large proportions of clinicians. Amongst respondents who treat prostate cancer, zoledronic acid (20/23=86.96%) and denosumab (19/23=82.61%) were both used.
Fig. 3.
Proportion of respondents indicating use of different bone-targeted agents to treat breast and prostate cancer patients.
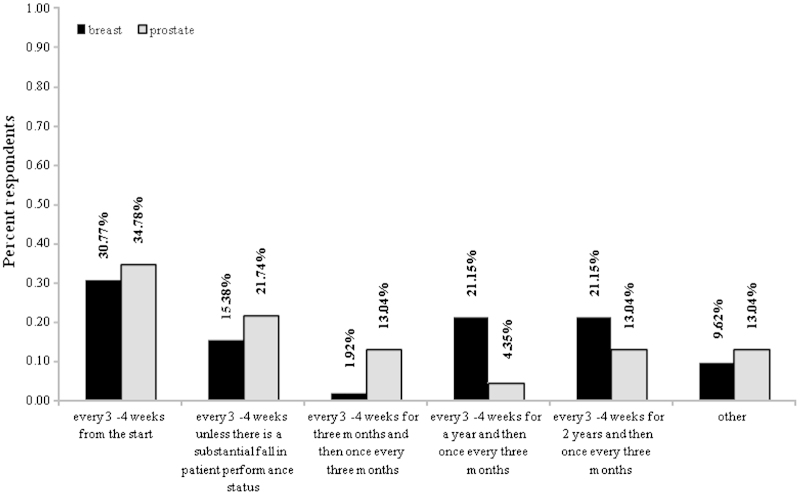
For breast cancer patients, there was a variation in the frequency of administration of bone targeted agents (Fig. 4). After exclusion of two respondents with missing data for this question, a total of 16/52 (30.77%) treat patients every 3–4 weeks from the start, 8/52 (15.38%) responded every 3–4 weeks unless there is a fall in performance status, 1/52 (1.92%) indicated every 3–4 weeks for 3 months before modifying to once every 3 months, 11/52 (21.15%) indicated every 3–4 weeks for a year before modifying to once every 3 months, 11/52 (21.15%) indicated every 3–4 weeks for 2 years before modifying to once every 3 months. Finally, 5/52 (9.61%) respondents noted other scenarios, which relied upon patients' SRE history, extent of disease, and presence of concurrent chemotherapy. For prostate cancer patients, 8/23 (34.78%) treat patients every 3–4 weeks from the start, 5/23 (21.74%) every 3–4 weeks unless there is a fall in performance status, 3/23 (13.04%) indicated every 3–4 weeks for 3 months before modifying to once every 3 months, 1/23 (4.35%) indicated every 3–4 weeks for a year before modifying to once every 3 months. Finally, 3/23 (13.04%) indicated every 3–4 weeks for 2 years before modifying to once every 3 months, and 3/23 (13.04%) noted other frequencies.
Fig. 4.
Distribution of administration frequency of bone targeted agents, by indication. Based on n=52 breast cancer respondents and n=23 prostate cancer respondents. Those respondents who treat both types of patients contributed data for each indication.
3.2.3. Cessation or modification of bone-targeted therapies
Clinicians were asked how frequently they would stop therapy once started. The answers showed that cessation was not common; 28/64 (43.75%) indicated never or <5% of the time, 21/64 (32.80%) between 5% and 25% of the time, 8/64 (12.5%) between 26% and 50% of the time, and 6/64 (9.4%) >50% of the time. This trend was generally consistent amongst the sub-groups of those treating breast cancer, prostate cancer, or both. Clinicians did not feel that worsening of bone pain, the presence of radiographic evidence of progression or the occurrence of an SRE while on a bone-targeted agent was an indication to stop this therapy. Indeed, the most common reasons for treatment cessation were; a marked deterioration of performance status (57.81%), the occurrence of intolerable side effects (18.75%) and progression of disease outside the bone (4.7%). Other assorted reasons (18.75%) included; presence of renal failure, duration of treatment >1–2 years, rising creatinine levels, and proximity to death.
3.3. Survey component 3: potential future trials of de-escalated therapy
In the third survey component respondents were asked to share their views regarding potential patient enrollment criteria, clinical outcomes of interest and minimum clinically important differences for these measures, to explore opinions regarding key parameters for the planning of future trials. When presented with several alternatives regarding potential patient populations that could be studied, physicians were asked to assess whom they would be comfortable enrolling into a randomized controlled trial. The question around duration of prior bone-targeted agent use prior to study randomization (i.e. 3 months vs. 1 year) did not produce a majority decision. However, in a situation of clinical equipoise the finding that 45.5% of respondents were comfortable randomising those who have received 3 months of prior therapy and 59.1% were comfortable with those who have received 1 year treatment tells us that such trials are still very feasible.
Physicians were asked to rank order the potential primary study outcomes for a clinical trial of de-escalated treatment from the most to the least clinically meaningful. SRE was the measure that was most commonly ranked to be most meaningful (57/66=86.4% of respondents), while pain (10.60%) and patient preference (3.03%) were chosen much less frequently. Patient preference was most commonly ranked to be least meaningful (46/66=69.7%). When asked what between-group difference in the Brief Pain Inventory (BPI) score would be an appropriate amount to suggest equivalent effectiveness between groups, 22 (34.4%) suggested 1 point, 30 (46.9%) suggested 2 points, 6 (9.4%) suggested 3 points, and 6 (9.4%) were not comfortable giving a response. When asked what between-group difference in the number of SREs would be an appropriate amount to suggest equivalent effectiveness between groups, 17 (26.6%) suggested a null difference, 24 (37.5%) suggested a difference within 1 SRE, 16 (25%) suggested a difference within 2 SREs, and 3 (4.7%) suggested within 3 SREs (4 (6.3%) were not comfortable providing a response). While designing a clinical trial to compare different frequencies of drug administration should use expected differences in each outcome based on prior trial results, it is important to also obtain feedback on subjective evaluations and feedbacks from physicians who use these agents. This type of feedback also helps ensure that the results of such a trial will likely have a clinical impact on patient care.
4. Discussion
There is considerable interest in optimizing the frequency of administration of bone-targeted agents as the current “one size fits all” approach is likely inefficient, expensive, and there is reason to believe that some patients may achieve equivalent benefit and improved safety with less frequent treatment administration. The results of the two published trials, assessing reduced frequencies of administration of these agents, would suggest there is still a need for larger definitive studies [12], [13]. In addition, we are not aware of any similar studies planned for prostate cancer where again the benefits of bone-targeted agents in reducing SREs are likely even more modest than that seen in breast cancer patients.
We are aware that physician surveys have been previously used to assess; bisphosphonate use for bone pain [14], bisphosphonate use in metastatic breast cancer patients [15] and radiotherapy use for painful bone metastases [16] but are not aware of physician surveys being used to help design trials of bone-targeted agents. Given the need for larger definitive studies this survey was designed to develop a greater understanding of the current clinical practice at Canadian centers in terms of frequency of use of bone-targeted agents and preferred choice of agent and frequency of administration. It was also designed to explore the degree of clinical equipoise which would underlie potential future trials of de-escalated bone-targeted therapy in breast and prostate cancer patients. Input on core methodological considerations for such trials was also explored. The results from this survey tell several important aspects of current clinical practice that can be explored in trials.
The first component was devised to collect pertinent demographic information of the population of respondents, as well as to determine what proportion amongst them that use bone-targeted agents to treat their patients. Firstly, it is evident that bone-targeted agents are commonly used for both breast and prostate patients with metastatic bone disease. It is also clear that the rationale for starting these agents are indeed based on practice guidelines [18], [19].
The second component was designed to gain an understanding of current Canadian clinical practice by collecting information from those respondents who prescribe bone-targeted agents. Specifically we sought to collect information with regard to intended benefits from bone-targeted agent use, scenarios in which they prescribe bone-targeted agents and their choice of agent for their patients. From this part of the study it was clear that the rationale for starting these agents was based on practice guidelines [18], [19]. Respondents primarily provide bone-targeted agents to reduce the risk of skeletal related events and improve pain control. Few felt that these agents had any effect on either progression-free or overall survival. The choice of bone-targeted agent reflected funding and therefore more agents were used in breast cancer patients (pamidronate, clodronate, zoledronic acid or denosumab) than in prostate cancer patients (zoledronic acid or denosumab). Treatments are usually given every 3–4 weeks and once started clinicians rarely stopped therapy. Also of interest was the fact that few clinicians use biomarkers of bone turnover to assess response to therapy. With respect to discontinuation of bone-targeted therapy clinicians did not feel that worsening of bone pain, the presence of radiographic evidence of progression or the occurrence of an SRE while on a bone-targeted agent was an indication to stop this therapy. Indeed, the most common reasons for treatment cessation was a marked deterioration in patient performance status.
In the third component, respondents were presented with a series of questions related to the design of a future clinical trial geared toward studying the clinical benefits of de-escalated therapy, with topics of interest including outcome selection and patient inclusion criteria. With respect to potential future trials, given that once started these agents are rarely stopped, it would appear that initiating a study of effect of drug cessation would be extremely difficult to do from a practical standpoint. It is also evident that there is more variability in the choice of bone-targeted agent in the treatment of breast cancer patients than in prostate cancer, which is likely a reflection of differential funding for these agents. This means that for a de-escalation trial to be performed in breast cancer patients the design would need to incorporate the use of different bone-targeted agents. The results do however confirm that physicians are very interested in de-escalation trials and that they would consider de-escalating therapy from every 3–4 weeks to 3 monthly therapy and entering patients on such a study at anytime from 3 months to 2 years after starting treatment. However, physicians do want the primary study endpoint to be SRE [11] and not biomarker studies using biomarkers of bone turnover as a surrogate for skeletal related event risk [12]. Ultimately the results from our survey confirms that trials exploring de-escalated use of bone targeted agents are wanted for both breast and prostate cancer and that ultimately there will remain a need for further very large randomised trials with skeletal related events as the primary endpoint.
There are limitations to the current study. As with all surveys, there is an inherent bias in those that are contacted and in those that respond. Obtaining 90 responses on this topic is clearly important, however, the study would be strengthened by a greater number of responses. In addition, the majority of respondent were from the two most highly populated Provinces in Canada, however 70% were from Ontario and Quebec, representing around 66% of the total Canadian population. As the respondents tend to have an interest in cancer and bone health and the majority is based in academic centers, there is a bias that the results of our study provide greater insight into practice at an academic center, but potentially less so in a broad scope of practice. However, it is important to appreciate that, in Canada, most cancer care is delivered in large centers and most anti-cancer drugs are funded through central regulatory bodies with specific funding policies, thus reducing variability. Another limitation of this type of survey is that it might not necessarily reflect what physicians actually prescribe. This is important as one study of “real world” bisphosphonate prescribing has suggested that different treatment schedules may result in differences in skeletal morbidity [20]. This study was retrospective and funded by the manufacturer of zoledronate and therefore does not replace the need for prospective randomised studies.
5. Conclusions
We believe that our objectives of developing a sense of current Canadian practice involving bone-targeted agents in patients with metastatic bone disease and exploring methodological considerations for future trials have been achieved. These data will provide valuable information to guide future efforts regarding this area of research.
Conflict of Interest Statement
Mark Clemons has received honoraria for talks from Amgen and Novartis.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jbo.2013.03.001.
Appendix A. Supporting information
Supplementary data
References
- 1.Clemons M., Gelmon K., Pritchard K., Paterson A. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: the state of the art. Current Oncology. 2012;19(5):259–268. doi: 10.3747/co.19.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuchuk I., Clemons M., Addison C. Time to put an end to the one size fits all approach to bisphosphonate use in patients with metastatic breast cancer? Current Oncology. 2012;19(5):e303–e304. doi: 10.3747/co.19.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouganim N., Dranitsaris G., Amir E, Clemons M. Optimising the use of bone-targeted agents in patients with metastatic cancers: a practical guide for medical oncologists. Support Care Center. 2011;19:1687–1698. doi: 10.1007/s00520-011-1230-9. [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi G., Theriault R., Porter L., Blayney D, Lipton A, Sinoff C. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocal 19 Aredia Breast Cancer Study Group. New England Journal of Medicine. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 5.Thiebaud D., Jaeger P., Jacquet A., Burchkardt P. Dose-response in the treatment of hypercalcemis of malignancy by a single infusion of the bisphosphonate AHPrBP. Journal of Clinical Oncology. 1988;6:762–768. doi: 10.1200/JCO.1988.6.5.762. [DOI] [PubMed] [Google Scholar]
- 6.Nussbaum S., Younger J., Vanderpol C., Gagel RF, Zubler MA, Chapman R. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. American Journal of medicine. 1993;95:297–304. doi: 10.1016/0002-9343(93)90282-t. [DOI] [PubMed] [Google Scholar]
- 7.Body J.J., Dumon J. Treatment of tumour-induced hypercalacemia with the bisphosphonate pamidronate: dose-response relationship and influence of tumour type. Annals of Oncology. 1994;5:359–363. doi: 10.1093/oxfordjournals.annonc.a058841. [DOI] [PubMed] [Google Scholar]
- 8.Van Poznak C., Temin S., Yee G., Janjan NA, Barlow WE, Biermann J.S. American Society of Clinical Oncology executive summary of the clinical practise guideline update on the role of bone-modifying agents in metastatic breast cancer. Journal of Clinical Oncology. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel D. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. Journal of Dental Research. 2007;86:1022–1033. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 10.Hortobagyi G., Theriault R., Lipton A., Porter L, Blayney D, Sinoff C. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia breast Cancer Study Group. Journal of Clinical Oncology. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 11.Coleman R. Bone cancer in 2011: prevention and treatment of bone metastases. Nature Reviews Clinical Oncology. 2011;9(2):76–78. doi: 10.1038/nrclinonc.2011.198. [DOI] [PubMed] [Google Scholar]
- 12.Amadori D., Aglietta M., Alessi B., Gianni L, Ibrahim T, Farina G. ZOOM: A prospective, randomized trial of zoledronic acid (ZOL; q 4 wk vs q 12 wk) for long-term treatment in patients with bonemetastatic breast cancer (BC) after 1 yr of standard ZOL treatment. Journal of Clinical Oncology. 2012;30(9005) [Google Scholar]
- 13.Amir E., Freedman O., Carlsson L., Dranitsaris G, Tomlinson G, Laupacis A, et al. Randomized feasibility study of de-escalated (every 12 wk) versus standard (every 3 to 4 wk) intravenous pamidronate in women with low-risk bone metastases from breast cancer. American Journal of Clinical Oncology [published online first] 2012. [DOI] [PubMed]
- 14.Johnson I.S. Use of bisphosphonates for the treatment of metastatic bone pain. A survey of palliative physicians in the UK. Palliative Medicine. 2001;15(2):141–147. doi: 10.1191/026921601671919596. [DOI] [PubMed] [Google Scholar]
- 15.Verma S., Kerr-Cresswell D., Dranitsaris G., Charbonneau F, Trudeau M, Yogendran G. Bisphosphonate use for the management of breast cancer patients with bone metastases: a survey of Canadian Medical Oncologists. Support Care Cancer. 2004;12:852–858. doi: 10.1007/s00520-004-0671-9. [DOI] [PubMed] [Google Scholar]
- 16.Chow E., Danjoux C., Wong R. Palliation of bone metastases: a survey of patterns of practise among Canadian radiation oncologists. Radiotherapy and Oncology. 2000;56(3):305–314. doi: 10.1016/s0167-8140(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 17.Kuchuk I., Addison C., Simos D., Clemons M. A national portfolio of bone oncology trials—the Canadian experience in 2012. Journal of Bone Oncology. 2012 doi: 10.1016/j.jbo.2012.09.001. [published online first] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Poznak C., Von Roenn J., Temin S. American society of clinical oncology clinical practice guideline update: recommendation on the role of bone modifying agents in metastatic breast cancer. Journal of Oncology Practice. 2011;7(2):117–121. doi: 10.1200/JOP.2011.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassinello Espinosa J., Gonzalez Del Alba Baamonde A., Rivera Herrero F., Holgado Martin E. SEOM guidelines for the treatment of bone metastases from solid tumors. Clinical and Translational Oncology. 2012;14(7):505–511. doi: 10.1007/s12094-012-0832-0. [DOI] [PubMed] [Google Scholar]
- 20.Hatoum H.T., Lin S.J., Smith M.R., Guo A., Lipton A. Treatment persistence with monthly zoledronic acid is associated with lower risk and frequency of skeletal complications in patients with breast cancer and bone metastasis. Clinical Breast Cancer. 2011;3:177–183. doi: 10.1016/j.clbc.2011.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data