Abstract
The skeleton is one of the most common sites of metastatic disease, affecting a large number of patients with advanced cancer. Although an increasing number of therapies are available for treatment of bone metastasis, this remains incurable, highlighting the need for better understanding of the underlying biology. Metastatic tumour spread to distant organs is a multistage process, involving not only cancer cells but also those of the surrounding host microenvironment. Tumour associated macrophages are multifunctional cells that contribute both to tumour development and response to treatment by regulating adaptive immunity, remodelling of stroma, mediating basement membrane breakdown and angiogenesis. Although direct evidence for a specific role of macrophages in bone metastasis is limited, their involvement in metastasis in general is well documented. In this review we provide an overview of role of macrophages in tumour progression, with particular emphasis on their potential role in bone metastasis.
Keywords: Macrophages, Bone metastasis, Breast cancer, Tumour-associated macrophages
1. Introduction
The skeleton is a common site of metastatic disease, affecting a large number of patients with advanced cancer [1]. Around 70% of patients who die of prostate and breast cancer concurrently experience bone metastasis and the incidence in kidney, thyroid and bronchus carcinomas reported to be around 30–40%. In contrast, tumours of the gastrointestinal tract rarely metastasise to the skeleton (∼5%) [2].
Metastasis to bone usually signifies an increased morbidity due to skeletal-related events, including bone pain, nerve and spinal cord compression syndrome, hypercalcaemia and pathologic fractures. As a result, the quality of life of the affected individuals may be greatly diminished [1]. Despite significant improvements in the outcome of patients with organ-confined cancer, patients with metastatic disease have not shared the same advances [3]. In an effort to maintain quality of life, improve survival as well as increase therapeutic options available for patients with bone metastasis, the underlying mechanisms should be investigated and understood.
A number of complex steps are involved in the formation of bone metastasis, involving a myriad of interactions between different cell types in conjunction with a large number of soluble factors, extracellular matrix components, hormones, physical properties [3]. Increasing evidence suggest that macrophages contribute both to primary tumour growth and to the subsequent development of metastasis [4]. However, there is very limited evidence for a specific role of macrophages in development and progression of bone metastasis. In this review we will give a brief overview of the current understanding of the contribution of macrophages to cancer metastasis, with particular emphasis on the involvement in tumour spread to the skeleton.
2. Macrophages
2.1. Macrophage classification and subtypes
Macrophages differentiate in tissues from extravasating monocytes and form an important component of the immune system characterised by their multifunctional nature. Monocytes are members of the family of leucocytes originating in the bone marrow, and they share a common progenitor with eosinophils, neutrophils and mast cells among others. Monocytes circulate for several days in the bloodstream where they are first released, followed by their entry into tissues where they replenish the tissue macrophage population [5].
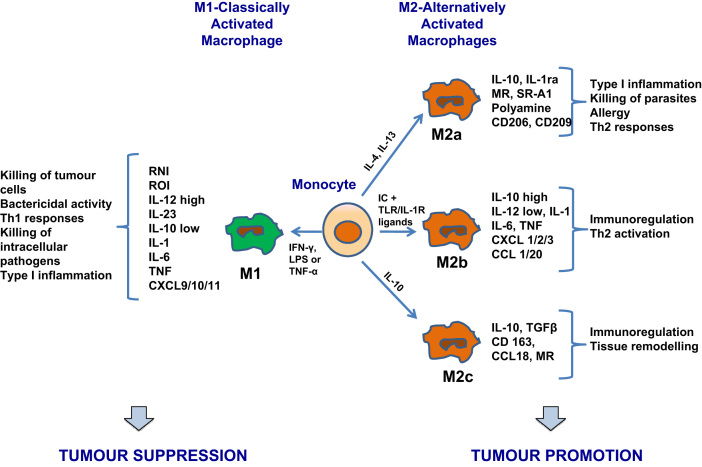
Cells that belong to the monocyte/macrophage lineage are defined by their plasticity and heterogeneity, and their phenotype can be altered and adapted according to their resident microenvironment [6]. Macrophages are classified into M1 and M2 types, reflecting the Th1/Th2 nomenclature (see Fig. 1 and Tables 1). M1, or classically activated macrophages, are promoted by ‘classical activators’, such as interferon γ and lipopolysaccharide [7]. They are characterised by high IL-23 and IL-12 production, high capacity for antigen presentation and eventual activation of polarised Th1 responses [8]. Cytotoxicity against tumour cells, as well as towards cells that have ingested intracellular microorganisms, is a key feature of M1 macrophages, mediated by the release of TNF, nitric oxide and reactive oxygen intermediates [4]. The involvement of M1 macrophages in the adaptive immune system is highlighted by the production of copious amounts of proinflammatory and immunomodulatory cytokines [9].
Fig. 1.
Macrophage polarization. Exposure to different cytokine milieu promotes the differentiation of monocytes into polarised macrophage subsets. When exposed to LPS, IFN-γ or other microbial products, monocytes differentiate into M1 macrophages. When exposed to IL-4, IL-10, IL-13 and immuno-suppressive agents, monocytes differentiate into M2 macrophages. M1 and M2 subsets share different characteristics in their function and phenotype. M1 cells have bactericidal activity, immuno-stimulatory functions and display tumour cytotoxicity. M2 cells promote tissue repair and angiogenesis and have high scavenging ability, favouring tumour progression. Abbreviations are: RNI, reactive nitrogen intermediates; ROI, reactive oxygen intermediates; IL, interleukin; TNF, tumour necrosis factor; CXCL, chemokine (C-X-C motif) ligand; IFN, interferon; LPS, lipopolysaccharide; IC, immune complexes; TLR, toll-like receptor; MR, mannose receptor; SR, scavenger receptor; CCL, CC chemokine ligand; TGF, transforming growth factor.
Table 1.
Characteristics of M1, M2 and TAM. Different macrophage phenotypes share different characteristics in terms of polarising signals, membrane receptors, cytokines and chemokines released and function. The most commonly used markers for each macrophage type is given.
| M1-classically activated macrophages | M2-alternatively activated macrophages | TAM-tumour-associated macrophages | References | |
|---|---|---|---|---|
| Polarising signals | IFN-γ, LPS or TNF-a | IL-4, IL-13 (M2a), IC+TLR/IL-1R Ligands (M2b), IL-10 (M2c) | CSF-1, VEGF, CCL2/3/4/5/8, IL-4, IL-13, IL-10, TGF-β, PGE2 | [4], [9], [10] |
| Membrane receptors | TLR2, TLR4, CD16, CD32, CD64, CD80, CD86 | Scavenge receptor A/B, CD 14, CD 23, CD 163, MR | CD11b, CD45, F4/80 (mice), CXCR4, Gr1,CD68, VEGFR | [4], [9], [10] |
| Cytokines released | High IL-12, IL-23, low IL-10, IL-1, TNF, IL-6, ROI, RNI | High IL-10, low IL-12; TGF-β (M2c); low IL-1, TNF, IL-6 (not M2b); high decoy IL-1RII, IL-1R-antagonist, EGF, FGF, VEGF, TNF-β | FGF, PDGF, EGFR, VEGF, ANG1/2, IL-1/8, TNF-α, TP, MMP-2/9, CSF-1 | [4], [9], [10] |
| Chemokines released | CXCL 8/9/10/11/16 CCL2/3/4/5 | CCL 1/16/17/18/22/24 | CCL-2/3 | [4], [9], [10] |
| Function | Th2 responses, type II inflammation, allergy, killing and encapsulation of parasites | Th2 activation, immunoregulation, matrix deposition and tissue remodelling | Angiogenesis, tumour growth, tumour invasion, intravasation, immunosuppression, metastasis | [4], [9], [10] |
| Markers (mouse) | iNOS, CD197 | Arginase-1, CD163 | F4/80 | [4], [9], [10] |
Abbreviations are: IFN, interferon; LPS, lipopolysaccharide; TNF, tumour necrosis factor; IL, interleukin; IC, immune complexes; TLR, toll-like receptor; CSF, colony stimulating factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; CCL, CC chemokine ligand; TGF, transforming growth factor; PGE, prostaglandin E; ROI, reactive oxygen intermediates; RNI, reactive nitrogen intermediates; EGF, epidermal growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; EGFR, epidermal growth factor receptor; ANG, angiopoietin; MMP, matrix metalloproteinase; CXCL, chemokine (C-X-C motif) ligand.
M2, or alternatively activated macrophages, are induced by IL-4, IL-10 and or IL-13 [7]. M2 is a broad name given to several forms of activated macrophages, excluding classic M1 cells, but including cells exposed to IL-13 or IL-4, IL-10, immune complexes, serocosteroid hormones and glucocorticoid [10]. Their main characteristics are suppression of inflammatory responses, poor antigen capacity and stimulation of Th2 responses [8]. They have been extensively shown to promote angiogenesis, wound healing and tissue remodelling, as well as to scavenge debris [8]. M2 macrophages are further categorised into M2a, M2b and M2c subtypes, depending on the environmental signals that define their activation. M2c is the most immunosuppressive of these phenotypes [9]. However, the different macrophage phenotypes are not considered distinct entities due to the degree of overlap between them, making their separation rather difficult and tentative.
2.2. Tumour-associated macrophages
Rudolf Virschow first proposed an association between inflammation and cancer in 1863, after observing the infiltration of leucocytes in tumours [11], and this view is still supported by today′s epidemiological, clinical and experimental studies [4]. The microenvironment created by inflammation provides the ideal conditions for neoplastic transformation and enhances the growth of tumours [12]. The inflammatory tumour infiltrate comprises a number of different cell types, including granulocytes, lymphoid cells, mast cells, natural killer cells, dendritic cells and macrophages, the most abundant cell type, representing a significant regulator of the association between inflammation and cancer. In breast cancer, macrophages may account for up to 50% of the tumour cell mass [13].
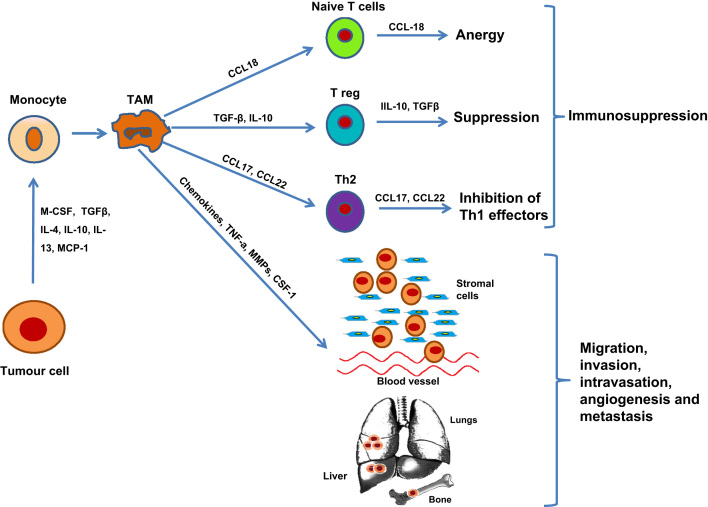
Tumour-derived signals stimulate recruitment of monocytes from the circulation that subsequently develop into tumour-associated macrophages (TAMs) [14], [15] (see Fig. 2). Molecules such as platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), macrophage colony stimulating factor (M-CSF) and transforming growth factor (TGFβ) are chemotactic for macrophages and also stimulate their differentiation and survival [4]. Numerous studies have reported that a large number of TAMs indicates a poorer prognosis of human malignancies [16].
Fig. 2.
The role of tumour-associated macrophages in tumour progression. Chemotactic factors recruit macrophages to tumours, where they develop into tumour-associated macrophages (TAMs) involved in a number of tumour-promoting activities that enhance tumour growth, progression and metastasis. TAMs preferentially migrate into hypoxic areas of tumour where they express and secrete several factors (e.g. VEGF, PDGF, TNF-a, MMPs) that stimulate angiogenesis. Moreover, TAMs promote tumour migration, invasion and intravasation via the production of proteolytic enzymes that break down the basement membrane and remodel the extracellular matrix. TAMs also inhibit host immune responses against the tumour via the secretion of a range of immuno-modulatory growth factors and cytokines. Abbreviations are: M-CSF, macrophage colony-stimulating factor; TGF, transforming growth factor; IL, interleukin; CCL, CC chemokine ligand; TNF, tumour necrosis factor; MMP, matrix metalloproteinase.
The phenotype and the function of differentiated mature TAMs resembles the M2, rather than the M1, phenotype and hence promote tumour growth, invasion and metastasis [4], [9]. The main characteristics of TAMS are poor capacity for antigen presentation, low cytotoxicity for tumour cells, poor NO production and ability to suppress T-cell proliferation [17]. One of the most significant features of TAMs is their capacity to directly promote tumour growth through the initiation and progression of tumour angiogenesis, facilitating the survival and metastatic spread of cancer cells. Their involvement in these processes and how this is related to bone metastasis will be discussed in the following sections.
2.2.1. The role of tumour associated macrophages in angiogenesis
Angiogenesis, an M2-associated function [18], describes the formation of new blood vessels from a pre-existing vascular system [19]. It is a requirement for any tumour to grow to greater than 2 mm in diameter, as it ensures adequate supply of nutrients and oxygen required for tumour cell growth and provides an effective way of removing waste products. Furthermore, angiogenesis provides an exit path for tumour cells into the bloodstream, a fundamental step in the formation of clinically significant metastases [16]. TAMs are considered one of the most important regulators of angiogenesis in tumours as they are involved in the two crucial steps of the process; the development of new blood vessels and their modelling into a comprehensive functional network [14], [19].
Pro- and anti-angiogenic factors secreted by tumour, stroma and inflammatory cells all contribute to the regulation of angiogenesis [20]. Accumulation of TAMs has been linked to the production of these factors, such as VEGF, TGFβ and PDGF, and consequently to angiogenesis, in a number of cancer studies [8]. In human tumours, TAMs have been shown to accumulate mainly in areas of poor vascular supply and necrosis, as well as around blood vessels [16]. An association between the presence of an increased number of macrophages and high vascularity has been described in breast tumours, where lymph node involvement was greater in tumours with higher number of TAMs when compared to tumours with low TAM density [21]. This can provide a partial explanation for the reported correlation between the infiltration of a high number of TAMs and reduced overall survival in patients with primary breast cancer [21]. A similar positive association is identified in several other tumour types [14] including oesophageal squamous cell cancer [22], bladder cancer [23], glioma [24] and prostate cancer [25].
A number of studies have reported that angiogenesis in solid tumours is effectively stimulated by hypoxia, which has been shown to control the release of pro-angiogenic factors by both macrophages and tumour cells in vitro [19]. Different mechanisms lead to hypoxia, with the most important being that the disorganised nature of newly formed tumour blood vessels mean that they are more likely to collapse [26]. If a tumour is growing at a rate higher than that at which the new blood vessels are formed, this may in turn contribute to development of further hypoxic areas [27]. Co-culturing human monocytes with multicellular breast cancer spheroids have indicated that monocytes show a preference to accumulate around the central regions of necrosis, in the inner, hypoxic cell layers [19]. In addition, hypoxia suppresses the migration of macrophages, leading to the immobilisation of TAMs in avascular, hypoxic and necrotic areas of tumours where they appear to collaborate with tumour cells to promote angiogenesis [17]. In breast carcinomas, TAMs express hypoxia-inducible factor-1 (HIF-1) and HIF-2a in response to hypoxia, which in turn promote the release of bFGF, CXCL8 and VEGF, significant stimulators of angiogenesis [17].
Macrophages are considered the main producers of VEGF, a potent pro-angiogenic cytokine that is over-expressed in many tumour types, including endometrial, ovarian, central nervous system, breast and kidney cancers [28]. TAMs also contribute to the production of other important angiogenic factors, such as thymidine phosphorylase (TP), matrix metalloproteinases (MMPs), urokinase plasminogen activator (uPA) and their level of expression correlates with angiogenesis and transition to invasive carcinomas [12], [16], [29], [30], [31]. Moreover, TAMs have been shown to play a key role in the initiation of the ‘angiogenic switch’, a step in tumour development essential for the transition of pre-invasive to invasive, malignant lesions [32]. The expression by both TAMs and tumour cells of MCP-1, a key regulator of macrophage accumulation in tumours, is positively associated with the levels of the angiogenic factors VEGF, TP and tumour necrosis factor (TNF)-a [22].
2.2.2. The role of tumour-associated macrophages in invasion, intravasation and metastasis
The mechanism by which macrophages enhance tumour metastasis is attributed to the paracrine loop that exists between EGF and macrophage colony stimulating factor-1 (M-CSF-1 or CSF-1), and involves interactions between macrophages and tumour cells. The most important source of EGF in tumours is TAMs [12]. In a number of different tumour types, overexpression of EGF receptor is associated with poor prognosis and metastasis [33], [34], [35]. Tumour cells produce CSF-1, which stimulate macrophages to express EGF, which in turn stimulates the expression of CSF-1 by tumour cells thereby creating a positive feedback loop. Disruption of this loop by blocking either CSF-1 receptor signalling, or the EGF receptor, is shown to be sufficient for the inhibition of both macrophages and breast cancer cell invasion and migration, leading to the inhibition of metastasis [36]. Use of in vivo multiphoton microscopy has revealed that metastasis is more frequent when mammary tumour cells are found in close association with TAMs [37]. Intravasation, the entry of tumour cells in blood vessels, is also stimulated by macrophages, potentially through the paracrine loop operating between EGF receptor signalling in macrophages and CSF-1 production by tumour cells [38].
One of the most significant processes associated with invasion and metastasis of tumours is the activation of proteolytic enzymes, leading to destruction of the basement membrane, a natural barrier for the invasion of tumour cells [39]. Macrophages are important sources of proteolytic enzymes (uPA and MMPs, especially MMP-9 and MMP-7), thereby facilitating the escape of cancer cells from the tumour mass and their invasion in the systemic circulation where they may subsequently develop into distant metastases [12].
TAMs have also been shown to directly promote tumour metastasis [6], and early reports suggested that the development of lung tumour nodules is significantly increased by macrophages [40]. Subsequently, a positive correlation has been demonstrated in several tumour types between the macrophage content in the primary tumour and the subsequent establishment of metastases [14], [21], [23]. Systemic depletion of macrophages by silencing of CSF-1 resulted in fewer lung metastases compared with the controls in a transgenic mouse model of PyMT-induced mammary tumours, suggesting that either TAMs or systemic macrophages play a significant role in the formation of distant metastasis [41]. A link between the presence of macrophages in sites of metastasis and the subsequent establishment of metastatic tumours has been identified. Depletion of macrophages in the peritoneum limited the capacity of tumour cells introduced into the portal vein to survive and grow in the liver [42].
In breast cancer, macrophage density appears to be a more effective predictor of survival when compared to nodal status, again supporting the direct role of macrophages in tumour progression [21]. A recent study provides convincing evidence that breast tumour colonisation of lungs is regulated by macrophages. Using three different means of macrophage depletion, a distinct macrophage population was shown to be required in the different steps of metastasis, including extravasation, survival and growth. In addition, the growth of pre-formed metastatic nodules was also inhibited by the depletion of macrophages [43]. Nevertheless, this may vary with tumour type as the efficacy of antibody therapy used for colorectal cancer was significantly reduced following the depletion of liver macrophages [44].
2.2.3. The role of tumour associated macrophages in immunosuppression
Several lines of evidence support that TAMs have an immunosuppressive M2 phenotype [15], and the strategic locations of these cells highlight their importance in regulating anti-tumour immunity [8]. TAMs modulate the host immune response to tumours via the increased expression of enzymes, chemokines and cytokines that modulate the action of antigen-presenting cells as well as the function of T and B cells [6]. TAMs generate factors that prevent the infiltration of cells with anti-tumour activities (e.g. cytotoxic T cells) as well as enhance the recruitment of cells that inhibit immune responses (e.g. regulatory T cells) [6]. Overall, the evidence supports that TAMs are able to down-regulate the normal immune response to tumour cells, thereby supporting tumour growth and aiding metastatic spread.
3. Bone metastasis
Bone metastasis is a multi-step process that takes place in the later stages of tumour progression. Initial steps, all involving macrophages, include the detachment of tumour cells from the primary tumour and their invasion into the circulation. Once in the circulation, tumour cells move towards the preferred target tissues, such as bone, where they adhere to the endosteal surface and form small tumour colonies [45]. The exact mechanism driving the tumour cells to metastasise to bone remains to be identified, but the various cell types (including macrophages), extracellular matrix components and soluble factors present in the bone microenvironment provide the ideal site for tumour cell colonisation and survival [46]. During the development of cancer-induced bone disease, a range of growth factors that enhance tumour cell proliferation are secreted and activated, including insulin-like growth factors (IGF) I and II, transforming growth factor β (TGFβ), platelet-derived growth factors (PDGFs), fibroblast growth factors (FGFs), calcium and bone morphogenic proteins (BPMs) [47]. Cancer cells in turn release factors that that stimulate bone remodelling, therefore promoting the development of osteoblastic or osteolytic metastases [48].
3.1. The potential role of macrophages in bone metastasis
Although many of the studies mentioned so far have implicated macrophages in promoting tumour metastasis to distant sites, there is surprisingly little direct evidence supporting their specific involvement in bone metastasis (see Tables 2). The following sections will summarise our current understanding of macrophages in this process.
Table 2.
Summary of in vivo studies reporting involvement of macrophages in bone metastasis.
| Model | Mechanism | Findings | Ref. |
|---|---|---|---|
| Studies investigating effects of macrophage depletion | |||
| Intracardiac injections of HARA-B human lung cancer cells in BALB/c nude mice | Depletion of macrophages by subcutaneous injections of clodronate-liposome (clodlip) | Administration of clodlip caused reduced numbers of monocytes in peripheral blood and of infiltrating macrophages in tumours compared to control. Significantly reduced incidence of bone metastasis, number of metastatic lesions in bone, as well as number of tumour colonies and tumour area in bone in clodlip treated animals compared to control | [51] |
| Clodlip: 400 μl every 3 days for 6 weeks | |||
| Intratibial injection of human prostate cancer PC-3MM2 cells transfected with lentivirus containing IL-6 small hairpin RNA (shRNA) or non specific RNA in NCL-nu mice | Silencing of IL-6 expression with shRNA in tumour cells combined with depletion of macrophages by clodlip intraperitoneal injections | Both transfection of PC-3MM2 cells with IL-6 shRNA and treatment with clodlip resulted in a significant reduction in the number of TAMs and osteoclasts associated with a significant reduction in bone lysis, tumour size and the incidence of lymph node metastasis compared to control. Combining clodlip treatment and silencing of IL-6 expression in tumour cells resulted in the lowest incidence of lymph node metastasis and bone tumours | [68] |
| Clodlip: 400 μl every 5 days for 5 weeks starting one day prior to tumour cells injection | |||
| Intracardiac injections of PC-3luc human prostate cancer cells in SCID mice | Administration of anti-human and anti-mouse CCL2 antibodies targeting cancer-derived and host-derived CCL2, respectively | Administration of both anti-human and anti-mouse CCL2 antibodies resulted in a significant reduction of tibial tumour burden, 87% and 95% respectively compared with PBS control group | [58] |
| Studies investigating effects of increasing macrophage numbers | |||
| Intracardiac injections of human prostate cancer PC-3lucCCL2 cells over-expressing the monocyte chemotactic protein CCL2 in CB-17 SCID mice | CCL2 was over-expressed in PC-3 cells, tumour growth in bone monitored for up to 7 weeks compared to mock transfected control | CCL2 over-expression increased the accumulation of macrophages in tumours. Statistically significant higher tumour growth rate in bone in PC-3lucCCL2 cell line compared to control | [57] |
3.1.1. Clodronate-liposome depletion of macrophages in models of bone metastasis
In order to establish whether macrophages are involved in the development of bone metastasis, researchers have used pharmacological depletion agents. Administration of clodronate-liposomes in mouse models causes macrophage depletion, resulting in significant inhibition of tumour angiogenesis and tumour growth [49]. The ability of macrophages to phagocytose the liposome encapsulated form enables the release of clodronate, promoting apoptosis. However, macrophages are unable to ingest free clodronate leading to its rapid removal from the circulation [50]. Clodronate-liposomes are shown to reduce survival of macrophages in vitro and the number of monocytes in peripheral blood in vivo, and is therefore a widely used research tool when studying the role of macrophages in biological processes, including tumour progression [51].
Clodronate liposomes have been used to investigate the effect of a reduced number of monocytes in the peripheral blood and macrophages in the tumour stroma in a mouse model of lung cancer-derived bone metastasis [51]. Highly metastatic human lung cancer cells (HARA-b) were injected into the left cardiac ventricle of nude mice on day 0, and animals divided into four treatment groups receiving either PBS (control), 200 μl or 400 μl clodronate-liposome (every 3 days for 6 weeks), or 10 mg/kg reveromycin A (daily for 6 weeks). Treatment with the highest dose of clodronate liposomes or with reveromycin A resulted in a significantly reduced incidence of bone metastasis as well a reduced number of metastatic lesions in the hind limbs. Treatment with reveromycin A resulted in a marked decrease in the number of osteoclasts associated with the bone lesions, compared to control. Clodronate liposomes significantly decreased the number of peripheral monocytes surrounding lung cancer cells and the accumulation of macrophages in tumours, as well as the number of osteoclasts in the trabecular bone of the hind limbs. These results suggest that the inhibitory effect of clodronate-liposome on bone metastasis can be attributed to both the reduction of osteoclasts as reduced infiltration of macrophages in the metastatic lesions.
3.1.2. Macrophage derived chemokines involved in bone metastasis
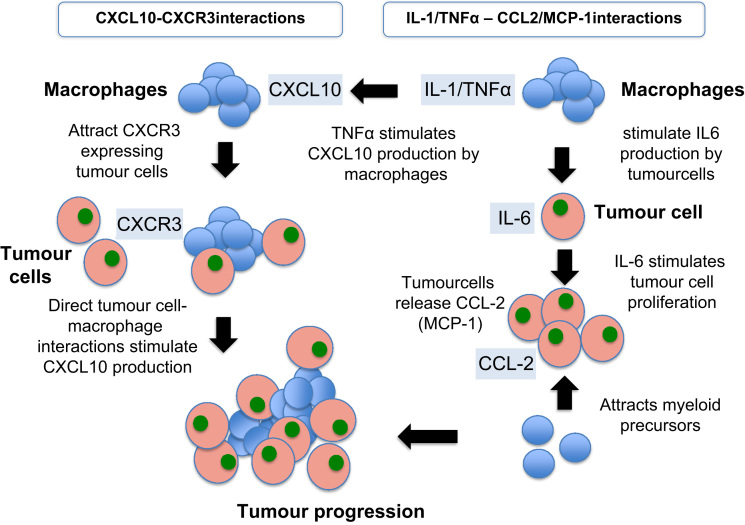
Several lines of evidence indicate an association between chemokines and increased proliferation, enhanced invasiveness, increased angiogenesis and recruitment of TAMs in tumour sites [52]. Fig. 3 shows how such interactions potentially may contribute to development and progression of bone metastasis. Chemokines comprise a group of similarly structured small proteins involved in the inflammatory responses through their ability to recruit leucocytes [53]. The location of the first two cysteine residues classifies chemokines into two main subfamilies, CXC and CC [54]. In tumour progression the expression of specific chemokines, such as CCL-2 and its receptor CCR2, has been identified in different cell types in the tumour microenvironment as well as in tumour cells [53].
Fig. 3.
Molecular interactions between tumour cells and macrophages potentially associated with bone metastasis. Cytokine and chemokine interactions contribute to stimulation of tumour cell proliferation as well as acting as chemoattractants for both macrophage precursors and tumour cells. The figure shows some examples of how key factors may interact to increase tumour growth and hence bone metastasis progression.
3.1.2.1. Monocyte chemoattractant protein 1 (MCP-1; CCL2)
Monocyte chemo-attractant protein 1 (MCP-1; CCL2) belongs to the CC chemokine group and is involved in the recruitment of inflammatory cells (especially monocytes and macrophages) to sites of inflammation, through activation of the CCR2 receptor [55]. Several reports have demonstrated an important role of MCP-1 in the regulation of tumour growth via macrophage infiltration and activation [56], [57], [58]. In a number of tumour types, including breast and prostate cancers [59], [60], [61], elevated serum levels of MCP-1 have been correlated with advanced disease stages, and hence frequently associated with bone metastasis. Recent evidence supports that MCP-1 may play a key role in creating the fertile environment for bone metastasis by increasing the infiltration of TAMs as well as by influencing osteoblasts and osteoclasts activity [52]. Various tumour cells have been shown to secrete increased levels of MCP-1, as well as to express the CCR2 receptor, thereby creating an autocrine/paracrine loop [52].
MCP-1 has been directly implicated in prostate cancer cell proliferation in vitro [62] and has also been identified as one of the main regulators of prostate tumour growth and metastasis [63]. Moreover, inhibition of MCP-1 significantly suppressed prostate tumour growth in vivo and highlighted the involvement of MCP-1 in tumour growth via macrophage infiltration and subsequent angiogenesis [64]. Expression of the MCP-1 receptor, CCR2, is associated with increasing pathological stages of prostate cancer [65], elevated MCP-1 serum levels correlates with lymph node involvement in cervical cancer [59], [66] and tumour cell expression of MCP-1 in squamous cell carcinoma of the oesophagus is linked to lymph node and distant metastasis [22]. In bladder cancer, high urinary MCP-1 levels were significantly correlated with distant metastasis [67].
A recent study found that MCP-1 over-expression in tumour cells resulted in an increased macrophage infiltration and an enhanced growth of PC3 prostate cancer xenografts in vivo. Tumour cells overexpressing MCP-1 lead to a statistically significant higher tumour growth rate in bone compared to control, suggesting that MCP-1 increases tumour growth and bone metastasis via stimulation of macrophage infiltration of the tumour site [57].
MCP-1 has been shown to play a significant role in prostate cancer-induced osteoclastogenesis in vitro and in vivo [57], [61]. In addition, serum MCP-1 levels are shown to be higher in patients with bone metastases compared to patients with localised disease in both prostate and lung cancer [53], [61]. Moreover, knockdown of CCR2 in C4-2B and PC3 prostate cancer cell lines significantly inhibited in vitro cell invasion [61], and knockdown of MCP-1 in PC-3 cells significantly reduced the number of osteoclasts present as well as inhibited prostate cancer cell growth in bone [61]. These findings further support the involvement of MCP-1/CCR2 axis in prostate cancer bone metastasis [61]. A neutralising antibody targeting mouse MCP-1 has been used to inhibit MCP-1 in vivo in an effort to identify its role in the development of bone metastases of prostate cancer. Animals were treated with 2 mg/kg anti-mouse CCL2 (C1142) or isotype control antibody (C1322) prior to intratibial injection of PC-3 or VCaP prostate cancer cells and then twice weekly for the duration of the study. Animals receiving the CCL2 antibody had significantly smaller tumours and reduced bone destruction compared to the mice receiving the isotype control antibody [63]. Taken together there is thus considerable evidence for a role of MCP-1, and hence the macrophages it attracts, in cancer progression.
3.1.2.2. IL-6
The secretion of IL-1 and TNF-α by macrophages stimulates the production of IL-6 by tumour cells. IL-6 is implicated in a number of cancer-related processes including tumour cell proliferation, apoptosis and bone metastasis formation [68]. Clinically, elevated serum IL-6 levels correlate with increasing prostate cancer grade as well as the presence of bone metastases [69]. The role of IL-6 in development of bone metastasis has been studied by injecting the human PC-3MM2 prostate tumour cell line where IL-6 had been silenced by lentivirus shRNA into the tibias of nude mice. Mice were treated intraperitoneally with either clodronate-liposome, liposomes containing PBS, or PBS (every 5 days for 5 weeks). The number of osteoclasts and TAMs in bone tumours was significantly lower in both the IL-6 shRNA transfected cancer cells and clodronate-liposome treated group. These reductions correlated with significant decreases in the incidence of lymph node metastasis, bone lysis and tumour size. The combination of knock-down IL-6 and TAMs reduction by clodronate-liposome led to the lowest incidence of lymph node and bone lesions, further reinforcing the role of TAMs in bone metastasis [68].
3.1.2.3. CCL-2/IL-6 interactions
CCL-2 is one of the most prevalent cytokines in the microenvironment of prostate cancer bone metastases and has a significant impact in tumour progression [52], [57], [58]. Stimulation of myeloma cells by IL-6 results in CCL2 release [52]. Both IL-6 and CCL-2 are found in high concentration in tumour microenvironment and when in concert have been shown to recruit myeloid monocytes and stimulate their differentiation towards M2-type macrophages by inhibition of apoptosis and autophagy [70]. As already discussed, TAMs of the M2 type have been shown to be involved in tumour progression and bone metastasis. The amplification loop between IL-6 and CCL2 is suggested to directly affect malignant cell survival and proliferation in a paracrine or autocrine fashion [70].
3.1.2.4. CXCL10
CXCL10 belongs to the group of IFNγ-inducible CXC chemokines and acts via the CXCR3 receptor. CXCL10/CXCR3 interaction has been well established in chronic Th1-inflammatory diseases [71], [72]. CXCL10 stimulates the chemotactic migration of various tumour cells involved in the formation of bone metastasis, such as mouse B16F10 melanoma cells and human MDA-MB-231 breast cancer cells [71]. However, CXCL10 has contrasting effects on cancer and stromal cells in the tumour microenvironment, illustrating the complexity of the metastatic process and the chemokines involved [71].
A possible explanation of how TAMs promote bone metastasis via CXCL10/CXCR3 interaction has recently been provided by studies in a melanoma model where CXCR3 gene silencing in B16F10 cells inhibited their ability to from bone metastasis following intra-cardiac injection [71]. Although CXCR3 is a common receptor for additional IFNγ-inducible CXC chemokines (e.g. CXCL9 and CXCL11), CXCL10 is highly expressed in bone and hence is considered the key molecule affected by CXCR3 silencing in this model. In addition, a similar suppression of bone metastasis was observed by the use of a neutralising antibody to CXCL10 [71]. Therefore, CXCL10 appears to regulate the formation of bone metastasis, partly by guiding CXCR3-expressing tumour cells to the site of metastasis. In this study, production of CXCL10 by macrophages was stimulated by the direct interaction between macrophages and breast cancer cells in vitro [71]. The authors suggest that tumour cells stimulate CXCL10 production by macrophages through direct cell–cell contact, and that this in turn stimulates the progression of osteolytic bone metastasis.
3.1.2.5. Macrophage-stimulating protein (MSP)
Macrophage-stimulating protein (MSP) is a serum protein involved in macrophage accumulation and activation [73], [74], initially secreted as an inactive single-chain precursor (pro-MSP) and activated after proteolytic cleavage. Pro-MSP is cleaved by membrane-type protease-1 (MT-SP1, matriptase), a protease expressed on various types of epithelial cells, as well as on macrophages. The receptor tyrosine kinase Ron (also known as human MST1R) is the cell surface receptor for MSP [73], demonstrated to be important in tumour progression and metastasis [75]. In a mouse model of breast cancer, investigators have illustrated the role of MSP in promoting tumour growth and metastasis, including osteolytic bone metastasis [72]. Overexpression of MSP in MMTV-PyMT tumours has been correlated to a decreased time to metastasis as well as to an increased number of metastatic foci (especially to bone) in vivo. Moreover, overexpression of MSP, MT-SP1, and MST1R was a strong independent indicator of both metastasis and death in human breast cancer patients [73]. These findings suggest an involvement of MSP in bone metastasis, complementing a recent report that that targeted deletion of the MST1R kinase domain in the MMTV PyMT model of breast cancer results in an inhibition in lung metastasis [76]. MSP may potentially enhance metastasis by interacting with tumour cells and induce their ability migration, invasion and growth in distant tissues [73], processes that all involve tumour-associated macrophages.
4. Summary and conclusions
Studies using both murine models and human tumours have provided unequivocal evidence for a role of TAMs in tumour progression, and potentially in promotion of tumour metastasis, through regulation of adaptive immunity, stroma remodelling, basement membrane breakdown and angiogenesis. Although direct evidence of a specific role for macrophages in bone metastasis remains limited, there is accumulating evidence from in vivo models supporting their involvement. However, it remains to be determined which stages of the process macrophages contribute to, in particular their role in early colonisation of bone compared to the latter stages associated with cancer-induced bone disease. A major complication encountered in the studies that have aimed to determine the role of macrophages in bone metastasis is that the various tools and methods used to modify the number of macrophages also affect osteoclasts, due to the similarity in molecular profile and phagocytic capacity between these closely related cell types. As the osteoclast is established as a key driver of tumour growth and progression in bone, it has therefore been impossible to clearly distinguish between the consequences of macrophage depletion and those mediated by inhibition of bone resorption.
Further studies of the molecular mechanisms that enable macrophages to facilitate bone metastasis will improve our understanding of the complex microenvironment promoting tumour progression, thus providing possible therapeutic targets for the treatment of human bone metastasis.
Macrophages in bone metastasis – main outstanding questions:
-
1.
To what extent can our understanding of the role of macrophages obtained in model systems be transferred the human disease?
-
2.
At what stage(s) of bone metastasis are macrophages critically involved?
-
3.
Is the main role of macrophages to support the development of the metastasis vasculature or are they also involved in other processes?
-
4.
Is there a particular macrophage subtype population and/or distribution in bone metastases?
-
5.
Do macrophages in bone metastasis represent a potential therapeutic target?
-
6.
How are macrophages in bone metastases affected by anti-cancer therapy?
-
7.
Do macrophages in bone metastases modify response to therapies?
-
8.
Are macrophages in bone metastases different from those of other metastatic foci?
-
9.
Is there a specific role for macrophages in bone metastasis or are they involved in metastatic disease in general?
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
IV was supported by a generous grant from Weston Park Hospital Cancer Charity, Sheffield, UK.
References
- 1.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treatment Reviews. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clinical Cancer Research. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R.E., Abrahamsson P.-A., Hadji P. 2nd ed. Bioscientifica; Bristol: 2012. Handbook of cancer-related bone disease. [Google Scholar]
- 4.Allavena P., Sica A., Solinas G., Porta C., Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical Reviews in Oncology/Hematology. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nature Reviews Immunology. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 6.Coffelt S.B., Hughes R., Lewis C.E. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochimica et Biophysica Acta. 2009;1796(1):11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nature Reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 8.Sica A., Schioppa T., Mantovani A., Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. European Journal of Cancer. 2006;42(6):717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. Journal of Translational Medicine 2011;9:177. [DOI] [PMC free article] [PubMed]
- 10.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 12.Pollard J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 13.Mukhtar R.A., Nseyo O., Campbell M.J., Esserman L.J. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Review of Molecular Diagnostics. 2011;11(1):91–100. doi: 10.1586/erm.10.97. [DOI] [PubMed] [Google Scholar]
- 14.Lewis C.E., Pollard J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Research. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 15.Sica A., Larghi P., Mancino A., Rubino L., Porta C., Totaro M.G. Macrophage polarization in tumour progression. Seminars in Cancer Biology. 2008;18(5):349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Bingle L., Brown N.J., Lewis C.E. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. Journal of Pathology. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 18.Leek R.D., Hunt N.C., Landers R.J., Lewis C.E., Royds J.A., Harris A.L. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. Journal of Pathology. 2000;190(4):430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Lewis J.S., Landers R.J., Underwood J.C., Harris A.L., Lewis C.E. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. Journal of Pathology. 2000;192(2):150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Valkovic T., Dobrila F., Melato M., Sasso F., Rizzardi C., Jonjic N. Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Archiv. 2002;440(6):583–588. doi: 10.1007/s004280100458. [DOI] [PubMed] [Google Scholar]
- 21.Leek R.D., Lewis C.E., Whitehouse R., Greenall M., Clarke J., Harris A.L. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Research. 1996;56(20):4625–4629. [PubMed] [Google Scholar]
- 22.Koide N., Nishio A., Sato T., Sugiyama A., Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. American Journal of Gastroenterology. 2004;99(9):1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanada T., Nakagawa M., Emoto A., Nomura T., Nasu N., Nomura Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. International Journal of Urology. 2000;7(7):263–269. doi: 10.1046/j.1442-2042.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishie A., Ono M., Shono T., Fukushi J., Otsubo M., Onoue H. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clinical Cancer Research. 1999;5(5):1107–1113. [PubMed] [Google Scholar]
- 25.Lissbrant I.F., Stattin P., Wikstrom P., Damber J.E., Egevad L., Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. International Journal of Urology. 2000;17(3):445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 26.Bingle L., Lewis C.E., Corke P., Reed M.W., Brown N.J. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. British Journal of Cancer. 2006;94(1):101–107. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murdoch C., Giannoudis A., Lewis C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104(8):2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 28.Leek R.D., Harris A.L., Lewis C.E. Cytokine networks in solid human tumors: regulation of angiogenesis. Journal of Leukocyte Biology. 1994;56(4):423–435. doi: 10.1002/jlb.56.4.423. [DOI] [PubMed] [Google Scholar]
- 29.Hildenbrand R., Dilger I., Horlin A., Stutte H.J. Urokinase and macrophages in tumour angiogenesis. British Journal of Cancer. 1995;72(4):818–823. doi: 10.1038/bjc.1995.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coussens L.M., Tinkle C.L., Hanahan D., Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotchkiss K.A., Ashton A.W., Klein R.S., Lenzi M.L., Zhu G.H., Schwartz E.L. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Research. 2003;63(2):527–533. [PubMed] [Google Scholar]
- 32.Lin E.Y., Li J.F., Gnatovskiy L., Deng Y., Zhu L., Grzesik D.A. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Research. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 33.Sherwood E.R., Lee C. Epidermal growth factor-related peptides and the epidermal growth factor receptor in normal and malignant prostate. World Journal of Urology. 1995;13(5):290–296. doi: 10.1007/BF00185972. [DOI] [PubMed] [Google Scholar]
- 34.Klijn J.G., Look M.P., Portengen H., Alexieva-Figusch J., van Putten W.L., Foekens J.A. The prognostic value of epidermal growth factor receptor (EGF-R) in primary breast cancer: results of a 10 year follow-up study. Breast Cancer Research and Treatment. 1994;29(1):73–83. doi: 10.1007/BF00666183. [DOI] [PubMed] [Google Scholar]
- 35.Wyckoff J., Wang W., Lin E.Y., Wang Y., Pixley F., Stanley E.R. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Research. 2004;64(19):7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 36.Goswami S., Sahai E., Wyckoff J.B., Cammer M., Cox D., Pixley F.J. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Research. 2005;65(15):7031. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 37.Wyckoff J.B., Wang Y., Lin E.Y., Li J.F., Goswami S., Stanley E.R. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Research. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 38.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Hagemann T., Robinson S.C., Schulz M., Trumper L., Balkwill F.R., Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25(8):1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 40.Gorelik E., Wiltrout R.H., Brunda M.J., Holden H.T., Herberman R.B. Augmentation of metastasis formation by thioglycollate-elicited macrophages. International Journal of Cancer. 1982;29(5):575–581. doi: 10.1002/ijc.2910290514. [DOI] [PubMed] [Google Scholar]
- 41.Lin E.Y., Nguyen A.V., Russell R.G., Pollard J.W. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. Journal of Experimental Medicine. 2001;193(6):727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oosterling S.J., van der Bij G.J., Meijer G.A., Tuk C.W., van Garderen E., van Rooijen N. Macrophages direct tumour histology and clinical outcome in a colon cancer model. Journal of Pathology. 2005;207(2):147–155. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 43.Qian B., Deng Y., Im J.H., Muschel R.J., Zou Y., Li J. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4(8):e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otten M.A., van der Bij G.J., Verbeek S.J., Nimmerjahn F., Ravetch J.V., Beelen R.H. Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. Journal of Immunology. 2008;181(10):6829–6836. doi: 10.4049/jimmunol.181.10.6829. [DOI] [PubMed] [Google Scholar]
- 45.Casimiro S., Guise T.A., Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Molecular and Cellular Endocrinology. 2009;310(1–2):71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Yoneda T., Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochemical and Biophysical Research Communications. 2005;328(3):679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Ma B., Fan Q. Mechanisms of breast cancer bone metastasis. Cancer Letters. 2010;292(1):1–7. doi: 10.1016/j.canlet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Blouin S., Basle M.F., Chappard D. Interactions between microenvironment and cancer cells in two animal models of bone metastasis. British Journal of Cancer. 2008;98(4):809–815. doi: 10.1038/sj.bjc.6604238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisberger S.M., Odermatt B., Marty C., Zehnder-Fjallman A.H., Ballmer-Hofer K., Schwendener R.A. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. British Journal of Cancer. 2006;95(3):272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Rooijen N. The liposome-mediated macrophage ‘suicide’ technique. Journal of Immunological Methods. 1989;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 51.Hiraoka K., Zenmyo M., Watari K., Iguchi H., Fotovati A., Kimura Y.N. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Science. 2008;99(8):1595–1602. doi: 10.1111/j.1349-7006.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craig M.J., Loberg R.D. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer and Metastasis Reviews. 2006;25(4):611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 53.Cai Z., Chen Q., Chen J., Lu Y., Xiao G., Wu Z. Monocyte chemotactic protein 1 promotes lung cancer-induced bone resorptive lesions in vivo. Neoplasia. 2009;11(3):228–236. doi: 10.1593/neo.81282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Y., Cai Z., Xiao G., Keller E.T., Mizokami A., Yao Z. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Research. 2007;67(8):3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 55.Charo I.F., Taubman M.B. Chemokines in the pathogenesis of vascular disease. Circulation Research. 2004;95(9):858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 56.Ueno T., Toi M., Saji H., Muta M., Bando H., Kuroi K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clinical Cancer Research. 2000;6(8):3282–3289. [PubMed] [Google Scholar]
- 57.Mizutani K., Sud S., McGregor N.A., Martinovski G., Rice B.T., Craig M.J. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11(11):1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loberg R.D., Ying C., Craig M., Day L.L., Sargent E., Neeley C. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Research. 2007;67(19):9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 59.Hefler L., Tempfer C., Heinze G., Mayerhofer K., Breitenecker G., Leodolter S. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. British Journal of Cancer. 1999;81(5):855–859. doi: 10.1038/sj.bjc.6690776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lebrecht A., Grimm C., Lantzsch T., Ludwig E., Hefler L., Ulbrich E. Monocyte chemoattractant protein-1 serum levels in patients with breast cancer. Tumor Biology. 2004;25(1–2):14–17. doi: 10.1159/000077718. [DOI] [PubMed] [Google Scholar]
- 61.Lu Y., Chen Q., Corey E., Xie W., Fan J., Mizokami A. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clinical & Experimental Metastasis. 2009;26(2):161–169. doi: 10.1007/s10585-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 62.Loberg R.D., Day L.L., Harwood J., Ying C., St John L.N., Giles R. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8(7):578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X., Loberg R., Liao J., Ying C., Snyder L.A., Pienta K.J. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Research. 2009;69(4):1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loberg R.D., Ying C., Craig M., Yan L., Snyder L.A., Pienta K.J. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9(7):556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Y., Cai Z., Xiao G., Liu Y., Keller E.T., Yao Z. CCR2 expression correlates with prostate cancer progression. Journal of Cellular Biochemistry. 2007;101(3):676–685. doi: 10.1002/jcb.21220. [DOI] [PubMed] [Google Scholar]
- 66.Lebrecht A., Hefler L., Tempfer C., Koelbl H. Serum cytokine concentrations in patients with cervical cancer: interleukin-4, interferon-gamma, and monocyte chemoattractant protein-1. Gynecologic Oncology. 2001;83(1):170–171. doi: 10.1006/gyno.2001.6361. [DOI] [PubMed] [Google Scholar]
- 67.Amann B., Perabo F.G., Wirger A., Hugenschmidt H., Schultze-Seemann W. Urinary levels of monocyte chemo-attractant protein-1 correlate with tumour stage and grade in patients with bladder cancer. British Journal of Urology. 1998;82(1):118–121. doi: 10.1046/j.1464-410x.1998.00675.x. [DOI] [PubMed] [Google Scholar]
- 68.Kim S.W., Kim J.S., Papadopoulos J., Choi H.J., He J., Maya M. Consistent interactions between tumor cell IL-6 and macrophage TNF-α enhance the growth of human prostate cancer cells in the bone of nude mouse. International Immunopharmacology. 2011;11(7):862–872. doi: 10.1016/j.intimp.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adler H.L., McCurdy M.A., Kattan M.W., Timme T.L., Scardino P.T., Thompson T.C. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. Journal of Urology. 1999;161(1):182–187. [PubMed] [Google Scholar]
- 70.Roca H., Varsos Z.S., Sud S., Craig M.J., Ying C., Pienta K.J. CCL2 and Interleukin-6 Promote Survival of Human CD11b+ Peripheral Blood Mononuclear Cells and Induce M2-type Macrophage Polarization. Journal of Biological Chemistry. 2009;284(49):34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J.H., Kim H.N., Kim K.O., Jin W.J., Lee S., Kim H.H. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Research. 2012;72(13):3175–3186. doi: 10.1158/0008-5472.CAN-12-0481. [DOI] [PubMed] [Google Scholar]
- 72.Rotondi M., Chiovato L., Romagnani S., Serio M., Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocrine Reviews. 2007;28(5):492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 73.Welm A.L., Sneddon J.B., Taylor C., Nuyten D.S., van de Vijver M.J., Hasegawa B.H. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proceedings of the National Academy of Sciences of United States of America. 2007;104(18):7570–7575. doi: 10.1073/pnas.0702095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M.H., Skeel A., Leonard E.J. Proteolytic cleavage and activation of pro-macrophage-stimulating protein by resident peritoneal macrophage membrane proteases. Journal of Clinical Investigation. 1996;97(3):720–727. doi: 10.1172/JCI118470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camp E.R., Liu W., Fan F., Yang A., Somcio R., RON Ellis L.M. a tyrosine kinase receptor involved in tumor progression and metastasis. Annals of Surgical Oncology. 2005;12(4):273–281. doi: 10.1245/ASO.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 76.Peace B.E., Toney-Earley K., Collins M.H., Waltz S.E. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Research. 2005;65(4):1285–1293. doi: 10.1158/0008-5472.CAN-03-3580. [DOI] [PubMed] [Google Scholar]