Abstract
Background
Bone-targeted agents are usually administered to breast cancer patients with bone metastases every 3–4 weeks. Less frequent (‘de-escalated’) treatment may provide similar benefits with improved safety and reduced cost.
Methods
To systematically review randomised trials comparing de-escalated treatment with bone-targeted agents (i.e. every 12–16 weeks) to standard treatment (i.e. every 3–4 weeks), a formal systematic review of the literature was performed. Two individuals independently screened citations and full text articles. Random effects meta-analyses of clinically important outcomes were planned provided homogeneous studies were identified.
Results
Five relevant studies (n=1287 patients) were identified. Sample size ranged from 38 to 425. Information on outcomes including occurrence of SREs, bone pain, urinary N-telopeptide concentrations, serum C-telopeptide concentrations, pain medication use and safety outcomes was not consistently available. Two trials were non-inferiority studies, two dose-response evaluations and one was a pilot study. Bone-targeted agents use varied between studies, as did duration of prior therapy. Patient populations were considered heterogeneous in several ways, and thus no meta-analyses were performed. Observations from the included studies suggest there is potential that 3 month de-escalated treatment may provide similar benefits compared to 3–4 weekly treatment and that lower doses of zoledronic acid and denosumab might be equally effective.
Conclusions
Studies comparing standard and de-escalated treatment with bone-targeted agents in breast cancer are rare. The benefits of standard treatment compared to de-escalated therapy on important clinical outcomes remain unclear. Future pragmatic studies must be conducted to determine the merits of this approach.
Keywords: Bone metastasis, Breast cancer, Systematic review, De-escalated treatment
1. Background
Bone is the most common site of breast cancer recurrence [1]. Patients diagnosed with bone metastases are presently treated with bone-targeting agents, such as bisphosphonates and RANK ligand antibodies, every 3–4 weeks for the remainder of their life [2]. Historically, the frequency of dose administration was developed partly on schedules based on hypercalcaemia trials and limited bone marker studies but also for convenience rather than efficacy and safety purposes, as it allowed investigators to deliver bone-targeted agents at the same time patients were also receiving chemotherapy (i.e. every 3 weeks) or every 4 weeks if the patient was receiving endocrine therapies. However, this rationale ignores the pharmacokinetics of BTAs, which may have a half-life in bone of many years [3], [4], and the modest absolute magnitude of benefit of these agents [5].
Despite the widespread use of BTAs, the question around optimal dose and dosing intervals remains unanswered [6], [7], [8]. This is particularly important given that drug induced toxicities are directly related to both the potency of the agent and also the cumulative dose received. Indeed, the incidence of BTA-associated osteonecrosis of the jaw is now approaching 10% in some selected chart reviews and online registries, making this by far the most common and serious side effect of treatment [9]. A number of reported pilot studies [10], [11], [12], [13] have suggested that patients can derive similar palliative benefits from bone-targeted agents when given at less frequent intervals, while others are ongoing [14]. While we have previously used the term de-escalation to imply reduced frequency of administration [11] it can also relate to a reduced dose per unit time. In order to assess the need for further randomized controlled trials of standard 3–4 week treatment with bone-targeted agents in breast cancer patients with metastatic disease compared to de-escalated treatment, we performed a systematic review of the published literature.
2. Methods
2.1. Study question and inclusion criteria
Our systematic review was designed to summarize available information addressing the following research question: “Does de-escalated treatment (i.e. every 3–4 months) with bone-targeted agents in breast cancer patients with metastatic disease provide similar benefit to 3–4 weekly treatment?” The Population–Intervention–Comparator–Outcome–Study Design (PICOS) framework was employed to structure the research question and to design the literature search. The population of interest was breast cancer patients with metastatic disease to bone; the intervention of interest was de-escalated/de-intensified treatment with any bone-targeted agent (denosumab, pamidronate, zoledronate, ibandronate, clodronate), while the comparator was standard 3–4 weekly treatment with any bone-targeted agent. Outcomes of interest included skeletal related events, bone pain, and quality of life, and only randomized controlled trials were considered eligible.
Inclusion criteria used during Stage 1 (i.e. citation review) and Stage 2 (i.e. full text review) screening closely mirrored the above PICOS criteria, with addition details used to determine inclusion status consisting of the following details: (1) studies were required to include patients with radiological or pathological diagnosed bone metastases from breast cancer; (2) any dose of bone-targeted agent being used was considered to be eligible; (3) no specific criteria relating to duration of treatment with a bone-targeted agent prior to study entry was employed. The clinical outcomes of interest, skeletal related events (SREs), were defined to consist of multiple events which included pathologic fractures, radiotherapy/surgery to bone, spinal cord compression and hypercalcaemia of malignancy. Only validated measures of bone pain [e.g. The Brief Pain Inventory (BPI) and Functional Assessment of Cancer Therapy-Bone Pain (FACT-BP)] and quality of life (e.g. FACT-G, EORTC QLQ-BM22, EORTC QLQ-C15-PAL) were accepted. While the above noted outcomes were of primary interest, studies of relevant design, treatment and patients were still retained even if limited to other outcome measures in order to present a complete overview of the literature.
2.2. Literature search
An information specialist (KC) designed and executed an electronic literature search to seek relevant citations for this systematic review from Ovid Medline (1946-present), PubMed (for non-Medline records), the Cochrane Library (search run March 13, 2013), and from the three major annual oncology conferences held worldwide since 2010 (American Society of Clinical Oncology, the European Society for Medical Oncology, and the San Antonio Breast Cancer Symposium). The full literature search is provided as a supplement to this review (Appendix 1, Appendix 2, Appendix 3). As one of the applicants is also an expert in this field (MC), awareness of the area and contact with other experts was also used as an additional means to identify relevant ongoing work. These efforts did not identify any additional publications.
2.3. Study screening, selection, and risk of bias assessment
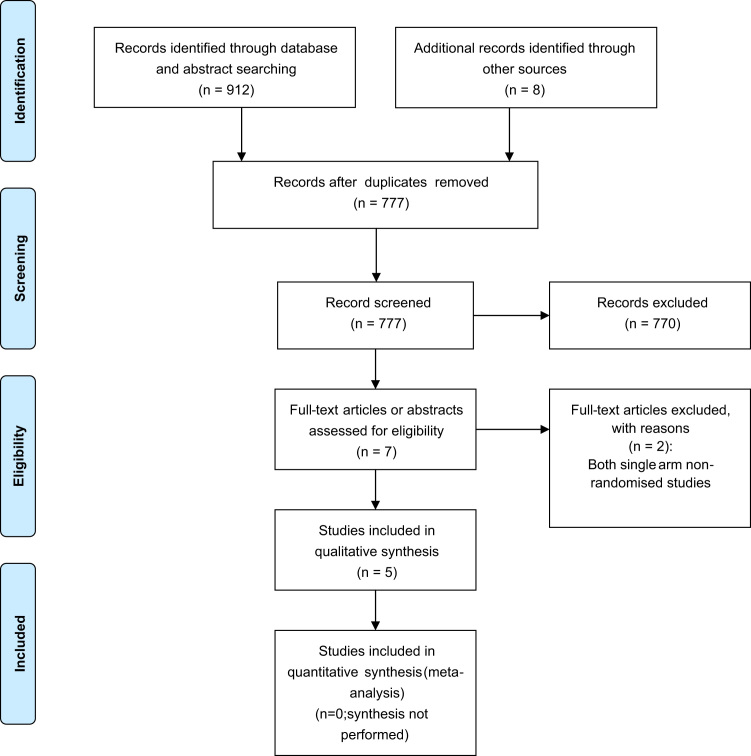
Two reviewers with expertise in oncology (MC, CA) reviewed the citations that were retrieved from the literature search independently. Stage 1 review consisted of screening of titles and abstracts only, while Stage 2 screening consisted of screening of full text articles where available to confirm study selection, or in the case of meeting abstracts, these were limited to their existing text. Following screening at each stage, the reviewers planned to meet to resolve any discrepancies and to consult a third party (BH) if needed. Results from the screening process are presented in a PRISMA flow diagram (Fig. 1) [15]. A list of included and excluded studies is provided in Appendix 4.
Fig. 1.
Systematic review supplement: PRISMA flow diagram.
Risk of bias of all eligible randomized controlled trials was to be assessed using the Jadad scale [16]. However, it was found that only one included study was published in manuscript form, while the remaining studies were published in abstract form only. As a consequence, an assessment of only one eligible study [10] could be performed. Data collection from relevant studies was performed by the two reviewers using a pre-designed extraction form.
2.4. Data analysis
If deemed appropriate following exploration of study and patient characteristics to ensure sufficient clinical and methodological homogeneity across studies, we planned to pursue meta-analyses using random effects models to combine data for outcomes of interest across relevant studies, as described in the Cochrane Handbook [17]. Summary estimates were planned to be reported using appropriate point estimates and corresponding 95% confidence intervals, along with forest plots of all study estimates to provide visualization of variability in findings from study to study for each outcome. Statistical heterogeneity was also to be assessed using both the Cochrane Q statistic and the I2 statistic. Following review of the included studies' characteristics, in particular with regard to patient populations, it was judged by the authors there were important clinical differences that precluded the data from meta-analysis. These differences are discussed in the summary of findings below. Given these differences, a narrative approach to summary of study-specific results was employed.
3. Results
3.1. Eligible studies
Our electronic literature search identified a total of 777 unique citations for review following removal of duplicates. Stage 1 screening identified a total of 7 citations which were considered potentially eligible for inclusion; 4 were meeting abstracts with no further information available which were thus included as is at Stage 2 screening, while 3 were associated with full manuscripts that were retrieved and screened. After Stage 2 screening, a total of 5 studies consisting of 1287 patients were included [10], [11], [13], [18], [19] while 2 were excluded because, while on topic, they were non-randomised, single-arm studies and thus did not fully meet pre-specified inclusion criteria [20], [21]. Fig. 1 provides an overview of the process of study selection.
3.2. Overview of study characteristics and summary of study findings
Two of the five studies selected for inclusion were described in abstract form only, and thus available information regarding these studies was limited. Characteristics of the studies in terms of funding source, patient inclusion criteria, study design and outcomes assessed are described in Table 1. Briefly, 80.0% (4/5) of the studies completed enrollment [10], [11], [18], [19], while the remaining study closed early due to poor patient accrual [13]; three of the abstracts/publications describing these studies were published in 2012, while the remaining two were published in 2011 [19] and 2007 [18]. The number of enrolled patients ranged from a minimum of 38 [11] to a maximum of 425 [10], and 80% (4/5) [10], [13], [18], [19] were funded by industry. Study duration was 10 months in one study [19], 1 year in three studies [10], [11] and 2 years in one study. [13] All trials enrolled patients with metastatic breast cancer, one [11] used additional study entry criteria based on low baseline serum C-telopeptide concentrations (<600 ng/L), and one also enrolled patients with multiple myeloma [19]. Three studies were open label, and involved the comparison of a standard treatment frequency (i.e. q3–4 weeks) compared to a de-escalated therapy (i.e. q12–16 weeks) [11], [13], [10]. One study was a double-blinded comparison of 0.4 mg/2 mg/4 mg zoledronic acid q4 weeks for up to 10 months and 90 mg pamidronate q4 weeks [19], while another compared 5 groups receiving different denosumab regimens against open label IV-bisphosphonate therapy as chosen as physicians' discretion [18]. The bone targeted agents used within studies were either pamidronate [11], [18], [19], zoledronate [10], [13], [18], [19], denosumab [18] or ibandronate [18]. The minimum extent of bisphosphonate use prior to enrollment varied from 0 months [13] to ≥3 months [11], to 9–12 months [10].
Table 1.
Summary of relevant study characteristics.
| Author (year) | Industry funded? | Study Design Information (duration, blinding/AC, etc) | Sample size | Patient inclusion criteria | Relevant patient demographics | Outcomes assessed | Study duration | Study status (complete/when, ongoing/when expected to complete) |
|---|---|---|---|---|---|---|---|---|
| Amir et al. [10] | No | Open label, randomised, Q4 weekly vs q12 weekly pamidronate | 38 | MBC (bone mets) Baseline serum CTx <600 ng/L | ≥3 months of prior BP use | CTx, Pain (FACT-BP, BPI),SREs, pain medication, BAP | 1 yr | Complete—peer-reviewed publication |
| Amadori et al. [9] | Yes | Open label, randomised Q4 weekly vs q12 weekly zoledronic acid | 425 | MBC (bone mets) | 9–12 months of prior zoledronic acid | SMR; number of SREs/pt/yr, time to first SRE, bone pain, bone marker (N-telopeptide of type I collagen; NTX) levels, and safety | 1 yr | Complete—abstract presentation |
| Coleman et al. [12] | Yesa | Open label, randomised S-ZOL 3–4 w or M-ZOL (15–16 w; 8–9 w or 3–4 w) if urine NTX levels were <50, 50–100, >100 nmol/mmol creatinine | 289 | MBC (bone mets) | No prior BP | SREs | 2 yr | Completed 2 year follow-up despite early closure |
| Berenson et al. [19] | Yes | Double-blind, randomised zoledronic acid 0.4, 2 or 4 mg (4 w for up to 10 months) versus pamidronate 90 mg (4 w for up to 10 months) | 280 | MBC or multiple myeloma; radiologic evidence of 1 or more osteolytic lesions; patients with myeloma required history of 1 or more prior SREs or chemotherapy failure | No prior BP | Need for radiation; SREs; bone mineral density; performance status; pain score; analgesic score; safety | 10 months | Complete—peer-reviewed publication |
| Lipton et al. [18] | Yes | Randomised, blinded study of five denosumab regimens (4 w 30 mg, 120 mg or 180 mg; or 12 w 60 mg or 180 mg) and an IV BP regimen (4 w use of physician’s choice amongst zoledronic acid, pamidronate or inbandronate) | 255 | MBC | No prior IV BP | Bone turnover marker urine N-telopeptide corrected for urine creatinine; SREs; safety | 13 weeks for biomarkers | Complete—peer-reviewed publication |
BAP=bone alkaline phosphatase, CTx=serum C-telopeptide; MBC=Metastatic breast cancer; BP=bisphosphonate, NTX= urine N-telopeptide, yr=year.
Part funded by Novartis.
The incidence of skeletal morbidity was measured in all 5 studies (in terms of either skeletal event rate or the skeletal morbidity rate), bone pain was assessed using varied outcome measures in 2 studies [11], [19], use of pain medication in 1 study [11], serum C-telopeptide concentrations in 1 study [11], urine N-telopeptide in 2 studies [13], [18] and quality of life in 1 study [11]. Unfortunately, numeric details of findings for these outcomes were not always reported.
There were important differences between the trials in terms of prior bisphosphonate therapy of the enrolled populations. Three studies enrolled patients with none at all [13], [18], [19], one required a minimum of 3 months [11] treatment with no upper limit of use, and one required specifically 9–12 months [10]. The requirement for 9–12 months of 4-weekly bisphosphonate is suited to a more fit population without kidney damage during that time frame, which is an important difference in study populations. The REFORM study [11] included patients with a longer history of metastatic disease than the other studies. Given that metastatic breast cancer would additionally be characterized by limited survival and reduced performance status with time, it is likely that these patients would have a worse performance status than the patients in other studies where randomization was performed earlier in the patients' disease journey. This information was not available from the study manuscripts. The REFORM [11] study incorporated eligibility screening of patients for the study by markers of bone turnover, while the BISMARK [13] and ZOOM studies did not, thus meaning that the REFORM cohort are likely quite distinct from the other two, as they are effectively screening out patients at higher risk of skeletal morbidity. The Berenson study [19] was a dose-response study involving approximate 2/3 breast cancer and 1/3 multiple myeloma patients with no separation of outcomes for the two tumor types. As noted earlier, as a consequence of the differences noted between study populations, it was decided by the authors that the five included studies were not sufficiently homogeneous for meta-analysis. A summary of study-specific findings was thus prepared. Provided next is a narrative review of the design features and reported findings for each of the included studies.
3.3. The ZOOM study [10]
The ZOOM trial [10] was a phase III prospective, randomized, open-label, multicenter study designed to assess non-inferiority of a standard 3–4 weekly bisphosphonate and de-intensified bisphosphonate treatment. The primary endpoint was the skeletal morbidity rate (SMR; defined as the mean number of SREs per patient per year) based on a sample of 425 patients. Patients had to have received between 9–12 months of standard 3–4 weekly zoledronic acid prior to randomization. The results showed that the SMR was similar between treatment arms: 0.26 (95% confidence interval [CI]=0.15–0.37) in the de-intensified arm vs. 0.22 (95% CI=0.14–0.29) in the q4 weekly arm. The mean difference and corresponding 95% CI were 0.04 (−0.09 to 0.17). The upper limit of the confidence interval (0.17) was less than the re-calculated non-inferiority margin of 0.19, indicating that the efficacy of the q12 week arm was not inferior to the q4 week arm. Safety analyses showed that zoledronic acid was well tolerated. Renal adverse events were reported in similar proportions of patients in both arms, and 7 cases of osteonecrosis of the jaw were reported (1.65% overall; 4 cases in the 3–4 weekly group vs. 3 in the de-intensified group). The authors stated that SMR was similar between the study arms in order to confirm non-inferiority of bone-targeted therapy every 12 weeks, however acknowledged that a larger definitive Phase III trial is required.
3.4. The BISMARK study [13]
The hypothesis of treating patients based on markers of bone resorption was tested in a large National Cancer Research Institute-supported Phase 3 clinical trial in the United Kingdom (BISMARK, EudraCT no. 2005-001376-12). The BISMARK study [13] looked at use of a biomarker of bone turnover to determine whether patients could receive zoledronic acid q4 or as needed on a schedule based on the N-telopeptide (NTX) concentrations at each clinic visit. Under the marker-directed schedule, patients received zoledronic acid 4 mg intravenously every 3–4 weeks (if NTX level was >100 nM/mM creatinine), every 8–9 weeks (if NTX level was 50–100 nM/mM creatinine), or every 15–16 weeks (if NTX level was <50 nM/mM creatinine). The primary endpoint was development of an on-study SRE, while secondary endpoints included quality of life (QLQ-C30 and the QLQ-BR23 breast-specific module), pain, analgesic use, health economics, change in systemic therapy, and survival from the time of randomization. Despite a planned sample size of 1400 patients, the trial was closed after 289 patients due to poor accrual, and is no longer active. Data on these 289 patients were recently presented [13]. Despite the study being underpowered to demonstrate non-inferiority, the results suggested that adjustment of dosing schedule based on NTX may be a sub-optimal strategy, as patients who received the biomarker driven arm did numerically have more SREs. Ultimately, BISMARK was unable to answer the primary study outcome as to whether or not biomarkers of bone turnover could be used to drive treatment with zoledronic acid or not [13].
3.5. The REFORM study [11]
Amir et al. [11] recently published the results of the REFORM study, which was designed to see if an RCT of de-intensification of bone-targeted therapies was feasible. It was a pilot randomised, non-inferiority study in which patients with breast cancer and bone metastases with low-risk disease as determined by serum C-telopeptide (CTx) levels <600 ng/L after ≥3 months of pamidronate treatment were enrolled. Patients were randomly allocated 1:1 into the experimental (q12 weekly pamidronate) and control (q3–4 weekly pamidronate) groups. CTx levels were checked every 12 weeks across a 48-week time period. Those remaining in the low-risk group continued to receive their allocated treatment, while those whose CTx levels rose above 600 ng/L were switched back to treatment every 4 weeks. Number of SREs and pain scores (BPI and FACT-BP questionnaires) were recorded for each enrolled patient. The results showed that of 38 randomised patients, 14 control group participants (73.7%) and 13 de-escalated group participants (68.4%) maintained CTx in the low risk range for the duration of the study (test of 2 proportions, p=0.64). There were no statistically significant differences in pain scores between the groups. Interestingly, despite a time-dependent increase in CTx in the de-escalated group, this was not associated with an increase in SREs over the one year study period. The results of this study showed that it is feasible to perform randomised trials of de-escalated therapy. Of the 54 patients approached about the study, 44 agreed to be consented, confirming significant patient interest in de-escalation of bone-targeted therapies.
3.6. Berenson et al. [19]
Berenson et al. [19] reported findings from a randomized, double-blinded, multi-center study of 280 patients which evaluated the dose-response relationship for zoledronic acid (0.4 mg, 2 mg or 4 mg) when administered as a 5-minute infusion in patients with malignant osteolytic lesions as a result of breast cancer or multiple myeloma. A group of patients was also randomized to pamidronate 90 ms. Patients were not eligible if they had previously been treated with bisphosphonates. The study collected data regarding the need for radiation therapy (the primary outcome), other SREs, observed changes in bone mineral density, performance status, bone markers, and measures of pain and analgesia use. Study findings showed that the pamidronate group (18% vs 24%) and the two highest dose groups of those receiving zoledronic acid (2.0 mg: 19% vs 24%; 4.0 mg: 21% vs 24%) were all found to be associated with a statistically significantly lower frequency of the need for radiation therapy compared to the 0.4 mg zoledronic acid group. Proportions of patients in the 0.4 mg, 2 mg, 4 mg zoledronic acid groups and pamidronate group that had 1 or more SREs were 46%, 35%, 33%, and 30%, respectively; amongst the first 25% of patients in each group who experienced an SRE, no difference in the median time to first SRE was found between zoledronic acid groups, while the difference between the 0.4 mg group and the pamidronate group reached statistical significance (167 days versus 254 days). Differences in pain scores between treatment groups were not clinically important, though more patients receiving 4 mg zoledronic acid (67%) reported a pain decrease during the study than in the pamidronate group (51%) and those receiving 0.4 mg (51%) and 2 mg zoledronic acid (48%). Unfortunately, the interval between imaging tests was not stated in the paper, and the combined data for breast cancer and multiple myeloma data is presented. Essentially however, there was no significant difference between 2 mg and 4 mg zoledronic acid in terms of SREs and the authors concluded that a 5 min infusion of 2–4 mg zoledronic acid was, at minimum, as effective as 90 mg pamidronate administered over two hours.
3.7. Lipton et al. [18]
Lipton et al. [18] reported findings from a randomized, multi-center, partially blinded study which randomized 255 women with metastatic breast cancer to receive either one of five denosumab regimens (30 mg, 120 mg or 180 mg every 4 weeks; 60 mg or 180 mg every 12 weeks) or open label IV-bisphosphonate therapy every 4 weeks as chosen by the consulting physician (using either zoledronic acid, pamidronate or ibandronate). The study’s primary outcome of interest was 13-week change in bone turnover marker urine N-telopeptide corrected for urine creatinine, while secondary outcomes included related bone turnover marker measures, the occurrence of SREs, and safety. Eligible patients could not have received prior IV bisphosphonate treatment, and were followed for 56 weeks. The biomarker studies showed no differences between the doses or dosing intervals of denosumab or between the denosumab and the bisphosphonate arms. Median time to first on-study SRE was found to be comparable across the denosumab and IV bisphosphonate managed groups based on Kaplan-Meier analysis, while totals of 9% of subjects across the denosumab groups and 16% in the IV bisphosphonates group experienced at least one SRE. The authors concluded that denosumab may produce results similar to IV bisphosphonates in terms of both suppression of bone turnover and the risk of skeletal related events.
4. Discussion and conclusions
4.1. Summary of findings
In this systematic review, we performed a systematic search of the literature and recent conference abstracts from major oncology conferences to identify randomized controlled trial evidence evaluating the merits of de-escalated treatment (e.g. every 12 weeks) of breast cancer patients with bone-targeted agents in comparison to current standard practice of every 3–4 weeks. Our search identified a total of only five relevant studies that enrolled a total of just 1287 patients, and one of these studies was closed early as a consequence of slow patient accrual. The five studies had varied inclusion criteria and study endpoints. Sample size across studies ranged from 38 to 425, four of five were industry funded, and three were reported in 2012 (two in abstract form only) while the others were reported in 2001 and 2007. Four studies were of approximately one year in duration while the fifth was planned for two years of follow-up, however it terminated early due to poor patient enrollment. While studies indicated collection of several outcomes including the occurrence of SREs, bone pain, N-telopeptide, serum C-telopeptide, pain medication use and safety outcomes, information on these outcomes was not consistently available, in particular due to limited detail in those studies described as abstracts.
Two trials (the ZOOM [10] and BISMARK [13] studies) were planned as non-inferiority studies, while the REFORM [11] study was designed as a pilot study to assess feasibility. Other differences between the studies included the bisphosphonates used (i.e. zoledronic acid, pamidronate, denosumab and ibandronate) and the duration of bone-targeted therapy prior to study entry, i.e. none (BISMARK [13], Lipton et al. [18], Berenson et al. [19]), any duration ≥3 months (REFORM [11]), and 9–12 months (ZOOM [10]). The trials are therefore quite heterogeneous in design and patient population. The similarities and differences between these studies confirm that there is indeed considerable interest in de-escalated bone-targeted therapy, however there is clearly a need for larger definitive studies to be performed if there is to be a change in practice.
4.2. Implications of existing studies for future research
The ZOOM study is the only study presented to date that used SRE rates to compare standard and de-escalated treatment groups. In recent years, many researchers have used biomarkers of bone resorption as a surrogate of SRE risk to design smaller trials assessing optimal doses [22], frequency of administration [11], and choice of bone-targeted agent [23], [24]. The role of bone turnover markers for predicting SRE risk in longer term studies is increasingly being questioned [13], and they are best only to be used as a surrogate for planning larger definitive studies [25].
As a consequence of the low study accrual observed within the BISMARK trial [13], practice changing results were not generated. Personal communications with the primary investigator of the study suggested that the study was, in retrospect, too complex in its design, and participating physicians found it overly complicated to wait for the NTx result (the sample was supplied when the patient came to clinic, however the result came days later) before booking the next scheduled zoledronic treatment as opposed to simply booking it in the clinic when seeing the patient (PI: R. Coleman, personal communication). Perhaps the most important lesson to be learned from the BISMARK study is that clinical trial design in this setting and patient population must be pragmatic.
In the REFORM trial [11], despite a time-dependent increase in CTx in the de-escalated group, there was no increase in SREs over the one year study period. The results of this study showed that it is feasible to perform randomised trials of de-escalated therapy; of the 54 patients approached about the study, 44 agreed to be consented, confirming significant patient interest in de-escalation of bone-targeted therapies. This is important, as it confirms the results of ZOOM [10] (i.e. an equal number of SREs in both the standard and the de-intensified arms), but also shows the need for a study longer than one year duration to see if the rise in biomarkers is associated with more SREs over the long term, or are not associated with risk of SRE in long-term bisphosphonate treated patients.
The Berenson study [19] is of historical interest as the 2 mg and 4 mg zoledronic acid appeared to show no significant differences in their effects on SREs, and yet it was the higher dose that went on to become the global standard dose. While the Lipton study [18] enrolled 6 blinded cohorts of denosumab with differing doses and dosing-intervals compared with an open label bisphosphonate arm, no difference in biomarkers was seen and it was suggested that denosumab may result in less SREs than the standard bisphosphonate arm. However, the unblended nature of the bisphosphonate therapy makes this difficult to control for.
4.3. Other relevant work of note
Two studies were not included in our systematic review because they were non-randomized, single arm studies; however they also warrant mention. Following the REFORM study, the same research group performed a larger single arm study called the TRIUMPH study, designed to assess the feasibility of using bone turnover markers to identify low-risk patients on ≥3 months of q4 weekly pamidronate and decreasing their treatment frequency to every 12 weeks [21]. As in REFORM, if there was a rise in serum CTx, patients were removed from the study and switched back to q4 weekly treatment. The primary goal was to assess the ability to suppress bone turnover markers in low-risk patients with a 12-week regimen over the one year study period. While analysis is still ongoing, the results thus far have shown that of the 71 patients on study, only 3 have come off due to the occurrence of SREs; this is a lower percentage than observed in the REFORM study. Twelve patients have come off for elevation of CTx, however as in the REFORM study, the SREs were not correlated with rising CTx. Thus, this study to date has three important findings: (1) patients and physicians are highly motivated to enter de-escalation studies, (2) SRE data needs to be extended beyond one year given the long survival of these patients, and (3) markers of bone-turnover are not useful as the primary endpoint of a large trial as they are not acting as a surrogate for SRE risk.
In the single-arm SubDue study, Simmons et al. [20] explored whether the upfront administration (i.e. bone-targeted agent naïve patients) of zoledronic acid every 3–4 weeks is the optimal dosing frequency or due to convention [20]. The purpose of the study was to determine the duration of suppression of bone turnover by zoledronic acid in a population of bisphosphonate naive metastatic breast cancer patients. The study enrolled 26 patients and a single dose of zoledronic acid was given at week 0, with biomarkers of bone turnover (serum CTx) being measured bi-weekly. Pain scores and quality of life data were collected every 4 weeks. Patients remained on study if CTx remained suppressed, but came off study as soon as escape from suppression was noted (defined as a rise>50% of baseline value). At 12 weeks, 73% (95% CI 56%-90%) of patients had continued suppression of bone turnover demonstrated by serum CTx. The patients that escaped CTx suppression did so at a median of 8 weeks after first infusion of zoledronic acid (range 8–10 weeks). Quality of life scores and pain medication use did not change appreciably during this period. Again, this biomarker driven study suggests that bone-targeted agents may not need to be given at conventional intervals in the majority of patients, and may safely be withheld without increased risk to morbidity or decline in quality of life over a 12 week period. While TRIUMPH and SubDue are of interest, they did not fulfill the criteria for this systematic review as they were neither randomised or used SREs as their primary endpoints.
4.4. Study limitations
There are limitations to be noted regarding our systematic review. First, while our search did not include EMBASE, we did perform a rigorous search of multiple databases (Medline, PubMed and the Cochrane Library), and we are not aware of any further work being presented. The topic is also relatively new, and the strength of our literature search was seeking additional information from the past three years from key oncology conferences to seek the availability of timely information. Three of the works were found to be published within the past year. A second limitation is the lack of specific detailed information that was available from some of the included studies given that two were published in abstract form only, which thus precluded the ability to assess the risk of bias in these trials. Third, the extent of available data from the included trials with regard to more clinically important outcomes such as skeletal related events was limited and study duration was often only one year, which may not be adequate for the observation of important differences between treatment regimens. Both design deficiencies contribute to the need for further research to address the current knowledge gap regarding the merits of de-escalated versus standard of care therapy with bone-targeted agents.
5. Conclusions
We identified a clear knowledge gap with regard to establishing the clinical benefits of de-escalated bone targeted agent therapy in breast cancer patients in comparison to current standard practice. Currently available research has been shown to have several deficiencies with regard to comparing these approaches, and a knowledge gap currently remains as to their relative impact on patient outcomes. If future studies can demonstrate comparable benefits and safety of less frequent and/or lower dose treatment, the quality of life benefits to patients and the economic benefits to the health care system represent important changes in the field. Pragmatic studies encompassing lessons learned in terms of study design from past research and involving patient-centered outcomes are needed to explore these issues and address current questions regarding the relative effectiveness and safety of these approaches to treating patients with breast cancer and metastatic bone disease.
Author contributions
MC and CA were responsible for proposal of the clinical research question. BH, CA, KC and MC contributed to authorship of the initial draft of the manuscript. All authors contributed to identification of methods for the systematic review and to critical revision and approval of the final version of the manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Brian Hutton, Email: bhutton@ohri.ca.
Christina L. Addison, Email: caddison@ohri.ca.
Kaitryn Campbell, Email: kaitryn_chris@sympatico.ca.
Dean Fergusson, Email: dafergusson@ohri.ca.
Sasha Mazarello, Email: smazz052@uottawa.ca.
Mark Clemons, Email: mclemons@toh.on.ca.
Appendix 1. Systematic review supplement: SUMMARY of electronic literature search
Ovid MEDLINE(R) In-process and other non-indexed citations and ovid MEDLINE(R) 1946 to present; ovid MEDLINE(R) daily update February 08, 2013.
| # | Searches | Results |
|---|---|---|
| 1 | Breast Neoplasms/ | 197,472 |
| 2 | Carcinoma, Ductal, Breast/ | 10,468 |
| 3 | Inflammatory Breast Neoplasms/ | 113 |
| 4 | ((breast or mamma or mammary) adj3 (cancer* or carcinoma* or neoplasm* or tumor* or tumor*)).ti,ab. | 206,341 |
| 5 | or/1–4 | 256,053 |
| 6 | exp Bone Neoplasms/ | 95 951 |
| 7 | (bone* neoplasm? or (bone* adj2 cancer*)).ti,ab. | 2972 |
| 8 | or/6–7 | 97,043 |
| 9 | exp Neoplasm Metastasis/ | 144,980 |
| 10 | (metastases or metastasis or metastatic or micrometastases or micro-metastases or micrometastasis or micro-metastasis or recurrence* or recrudescence* or recurrent or secondary or spread*).mp. | 1,230,905 |
| 11 | or/9–10 | 1,234,986 |
| 12 | (bone* adj3 (metastases or metastasis or metastatic or micrometastases or micro-metastases or micrometastasis or micro-metastasis)).mp. | 13,770 |
| 13 | 5 and ((8 and 11) or 12) | 6485 |
| 14 | (pamidronate or pamidronic acid or acide pamidronique or acido pamidronico or acidum pamidronicum or amidronate or Aredia).mp. | 2530 |
| 15 | (zoledronate or zoledronic acid or Aclasta or Orazol or Reclast or Zometa).mp. | 2668 |
| 16 | 118,072-93-8.rn. | 1854 |
| 17 | (ibandronate or ibandronic acid or acid ibandronico or Bondronat or Boniva or Bonviva).mp. | 821 |
| 18 | 114,084-78-5.rn. | 531 |
| 19 | (denosumab or Prolia or Xgeva).mp. | 715 |
| 20 | 615,258-40-7.rn. | 428 |
| 21 | or/14–20 | 5730 |
| 22 | (in process or publisher or pubmed-not-medline or in-data-review).st. | 1,472,825 |
| 23 | 13 and 21 | 656 |
| 24 | 22 and 23 | 43 |
| 25 | limit 23 to humans | 596 |
| 26 | 24 or 25 | 639 |
Appendix 2. PubMed search strategy
| Search | Query | Items found |
|---|---|---|
| #23 | Search #13 AND #21 AND #22 | 71 |
| #22 | Search publisher[sb] OR in process[sb] OR pubmednotmedline[sb] | 1,887,893 |
| #21 | Search #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 | 5843 |
| #20 | Search 615,258-40-7[rn] | 425 |
| #19 | Search denosumab[all] OR Prolia[all] OR Xgeva[all] | 735 |
| #18 | Search 114,084-78-5[rn] | 530 |
| #17 | Search ibandronate[all] OR ibandronic acid[all] OR acid ibandronico[all] OR Bondronat[all] OR Boniva[all] OR Bonviva[all] | 824 |
| #16 | Search 118,072-93-8[rn] | 1844 |
| #15 | Search zoledronate[all] OR zoledronic acid[all] OR Aclasta[all] OR Orazol[all] OR Reclast[all] OR Zometa[all] | 2739 |
| #14 | Search pamidronate[all] OR pamidronic acid[all] OR acide pamidronique[all] OR acido pamidronico[all] OR acidum pamidronicum[all] OR amidronate[all] OR Aredia[all] | 2559 |
| #13 | Search #5 AND ((#8 AND #11) OR #12) | 60,823 |
| #12 | Search bone*[all] AND (metastases[all] OR metastasis[all] OR metastatic[all] OR micrometastases[all] OR micro-metastases[all] OR micrometastasis[all] OR micro-metastasis[all] | 775,628 |
| #11 | Search #9 OR #10 | 1,276,344 |
| #10 | Search metastases[all] OR metastasis[all] OR metastatic[all] OR micrometastases[all] OR micro-metastases[all] OR micrometastasis[all] OR micro-metastasis[all] OR recurrence*[all] OR recrudescence*[all] OR recurrent[all] OR secondary[all] OR spread*[all] | 1,276,344 |
| #9 | Search Neoplasm Metastasis[mh] | 144,718 |
| #8 | Search #6 OR #7 | 121,680 |
| #7 | Search bone*[tiab] neoplasm*[tiab] OR (bone*[tiab] AND cancer*[tiab]) | 35,145 |
| #6 | Search Bone Neoplasms[mh] | 96,018 |
| #5 | Search #1 OR #2 OR #3 OR #4 | 272,623 |
| #4 | Search (breast[tiab] OR mamma[tiab] OR mammary[tiab]) AND (cancer*[tiab] OR carcinoma*[tiab] OR neoplasm*[tiab] OR tumor*[tiab] OR tumor*[tiab]) | 228,747 |
| #3 | Search Inflammatory Breast Neoplasms[mh:noexp] | 112 |
| #2 | Search Carcinoma, Ductal, Breast[mh:noexp] | 10,456 |
| #1 | Search Breast Neoplasms[mh:noexp] | 197,708 |
Appendix 3. Cochrane library search and conference abstracts search
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Breast Neoplasms] this term only | 7583 |
| #2 | MeSH descriptor: [Carcinoma, Ductal, Breast] this term only | 192 |
| #3 | MeSH descriptor: [Inflammatory Breast Neoplasms] this term only | 2 |
| #4 | (breast or mamma or mammary) near/3 (cancer* or carcinoma* or neoplasm* or tumor* or tumor*):ti,ab,kw (Word variations have been searched) | 14,333 |
| #5 | #1 or #2 or #3 or #4 | 14,333 |
| #6 | MeSH descriptor: [Bone Neoplasms] explode all trees | 922 |
| #7 | bone* neoplasm? or (bone* near/2 cancer*):ti,ab,kw (Word variations have been searched) | 2282 |
| #8 | #6 or #7 | 2373 |
| #9 | MeSH descriptor: [Neoplasm Metastasis] explode all trees | 3358 |
| #10 | metastases or metastasis or metastatic or micrometastases or micro-metastases or micrometastasis or micro-metastasis or recurrence* or recrudescence* or recurrent or secondary or spread* (Word variations have been searched) | 73,343 |
| #11 | #9 or #10 | 73,381 |
| #12 | bone* near/3 (metastases or metastasis or metastatic or micrometastases or micro-metastases or micrometastasis or micro-metastasis) (Word variations have been searched) | 849 |
| #13 | #5 and ((#8 and #11) or #12) | 598 |
| #14 | pamidronate or pamidronic acid or acide pamidronique or acido pamidronico or acidum pamidronicum or amidronate or Aredia (Word variations have been searched) | 420 |
| #15 | zoledronate or zoledronic acid or Aclasta or Orazol or Reclast or Zometa (Word variations have been searched) | 391 |
| #16 | ibandronate or ibandronic acid or acid ibandronico or Bondronat or Boniva or Bonviva (Word variations have been searched) | 178 |
| #17 | denosumab or Prolia or Xgeva (Word variations have been searched) | 76 |
| #18 | #14 or #15 or #16 or #17 | 933 |
| #19 | #13 and #18 | 170 |
ASCO (2010-2012), 2010–11 searched via EMBASE
2012 searched at: http://www.asco.org/ASCOv2/Meetings/Abstracts.
Abstract: Breast* AND bone* AND (pamidronate OR pamidronic OR amidronate OR Aredia OR zoledronate OR zoledronic OR Aclasta OR Orazol OR Reclast OR Zometa OR ibandronate OR ibandronic OR Bondronat OR Boniva OR Bonviva OR denosumab OR Prolia OR Xgeva) AND (metastases OR metastasis OR metastatic OR micrometastases), 4 relevant results.
ESMO (2010–2012), 2010 searched via EMBASE
2011–12 searched at: http://www.esmo.org/education-research/abstracts-virtual-meetings-and-meeting-reports.html.
2012: Breast* AND bone* AND (pamidronate OR pamidronic OR amidronate OR Aredia OR zoledronate OR zoledronic OR Aclasta OR Orazol OR Reclast OR Zometa OR ibandronate OR ibandronic OR Bondronat OR Boniva OR Bonviva OR denosumab OR Prolia OR Xgeva) AND (metastases OR metastasis OR metastatic OR micrometastases), 8 results, 0 relevant.
2011 (ECCO joint): searched manually, 0 relevant.
San Antonio Breast Cancer Symposium (2010–2012) searched at: http://www.sabcs.org/Resources/index.asp.
Breast* bone* metasta* OR (pamidron* OR zoledron* OR ibandron* OR denosumab), 2 relevant results.
Appendix 4. Systematic Review Supplement: List of Included and Excluded Studies from the Literature Search and Stage 2 Screening
Included studies
-
1.
Amir E, Freedman O, Carlsson L, et al.: Randomized Feasibility Study of De-escalated (Every 12 wk) Versus Standard (Every 3 to 4 wk) Intravenous Pamidronate in Women With Low-risk Bone Metastases From Breast Cancer. Am J Clin Oncol, 2012.
-
2.
Amadori D, Aglietta, M., Alessi, B., Gianni, L., Ibrahim, T., Farina, G., Gaion, F., Bertoldo, F., Santini, D., Rondena, R., Bogani, P., Ripamonti, C.: ZOOM: A prospective, randomized trial of zoledronic acid (ZOL; q 4 wk vs q 12 wk) for long-term treatment in patients with bone-metastatic breast cancer (BC) after 1 yr of standard ZOL treatment. Journal of Clinical Oncology 30:9005, 2012.
-
3.
Coleman RE, Wright, J., Houston, S., Agrawal, R., Purohit, O.P., Hayward, L., Simmonds, P., Waterhouse, A., Marshall, H.: Randomized trial of marker-directed versus standard schedule zoledronic acid for bone metastases from breast cancer., ASCOAnnual Meeting 2012 Chicago, IL, Journal of Clinical Oncology, 2012, pp 511.
-
4.
Lipton A, Steger G, Figueroa J et al. Randomized active controlled phase II study of denosumab efficacy and safety in patients with breast cancer related bone metastases. Journal of Clinical Oncology 2007; 25 (28): 4431–4437.
-
5.
Berenson J, Rosen L, Howell A et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double blind, randomized dose-response study. Cancer 2001; 91: 1191–1200.
Excluded studies (non-randomised, one arm studies)
4. N Bouganim, L Vandermeer, I Kuchuk, S Dent, S Hopkins, X Song, D Robbins, P Spencer, S Mazzarello, JF Hilton, E Amir, G Dranitsaris, C Addison, R Mallick, and MJ Clemons. Evaluating efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal related events. TRIUMPH: A pragmatic multicentre trial. Cancer Res 2012;72(24 Suppl):Abstract nr P3-13-05.
5. Simmons CE, Goodwin, P.J., Hogeveen, S., Clemons, M., Haq, R., Brezden, C.B., Ebrahim, J., Han, D.: Continued suppression of bone turnover following a single dose of zoledronic acid: Time to re-think dosing intervals in the management of bone metastases?, ASCO Annual Meeting 2012. Chicago, IL, Journal of Clinical Oncology, 2012, pp 9111.
References
- 1.Coleman R.E., Smith P., Rubens R.D. Clinical course and prognostic factors following bone recurrence from breast cancer. British Journal of Cancer. 1998;77(2):336–340. doi: 10.1038/bjc.1998.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma S., Kerr-Cresswell D., Dranitsaris G., Charbonneau F., Trudeau M., Yogendran G. Bisphosphonate use for the management of breast cancer patients with bone metastases: a survey of Canadian Medical Oncologists. Supportive Care in Cancer. 2004;12(12):852–858. doi: 10.1007/s00520-004-0671-9. [DOI] [PubMed] [Google Scholar]
- 3.Kimmel D.B. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. Journal of Dental Research. 2007;86:1022–1033. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 4.Doshi S., Sutjandra L., Zeng J., Sohn W., Peterson M., Jang G. Denosumab dose selection for patients with bone metastases from solid tumor. Clinical Cancer Research. 2012;18(9):2648–2657. doi: 10.1158/1078-0432.CCR-11-2944. [DOI] [PubMed] [Google Scholar]
- 5.Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 6.Bouganim N., Dranitsaris G., Amir E., Clemons M. Optimising the use of bone-targeted agents in patients with metastatic cancers: a practical guide for medical oncologists. Supportive Care in Cancer. 2001;19:1687–1696. doi: 10.1007/s00520-011-1230-9. [DOI] [PubMed] [Google Scholar]
- 7.Clemons M., Gelmon K., Pritchard K., Paterson A.H.G. Bone-targeted agents and skeletal- related events in breast cancer patients with bone metastases: the state of the art. Current Oncology. 2012;19(5):259–268. doi: 10.3747/co.19.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuchuk I., Clemons M., Addison C. Time to put an end to the “one size fits all” approach to bisphosphonate use in patients with metastatic breast cancer? Current Oncology. 2012;19(5):e303–e304. doi: 10.3747/co.19.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuchuk I., Mazzarello S., Butterfield K., Appleton A., Addison C.L., Clemons M. Oral care and use of bone-targeted agents in patients with metastatic cancers: a practical guide for dental surgeons and oncologists. Journal of Bone Oncology. 2013;2(1):38–46. doi: 10.1016/j.jbo.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amadori D., Aglietta M., Alessi B., Gianni L., Ibrahim T., Farina G. ZOOM: a prospective, randomized trial of zoledronic acid (ZOL; q 4 wk vs q 12 wk) for long-term treatment in patients with bone metastatic breast cancer (BC) after 1 yr of standard ZOL treatment. Journal of Clinical Oncology. 2012;30(9005) [Google Scholar]
- 11.Amir E., Freedman O., Carlsson L., Dranitsaris G., Tomlinson G., Laupacis A. Randomized feasibility study of de-escalated (every 12 wk) versus standard (every 3 to 4 wk) intravenous pamidronate in women with low-risk bone metastases from breast cancer. American Journal of Clinical Oncology. 2012 doi: 10.1097/COC.0b013e3182568f7a. published online first. [DOI] [PubMed] [Google Scholar]
- 12.Coleman R. Bone cancer in 2011: prevention and treatment of bone metastases. Nature Reviews Clinical Oncology. 2011;9(2):76–78. doi: 10.1038/nrclinonc.2011.198. [DOI] [PubMed] [Google Scholar]
- 13.Coleman R.E., Wright J., Houston S., Agrawal R., Purohit O., Hayward L. Randomized trial of marker-directed versus standard schedule zoledronic acid for bone metastases from breast cancer. Journal of Clinical Oncology. 2012:511. [Google Scholar]
- 14.Kuchuk I., Addison C., Simos D., Clemons M. A national portfolio of bone oncology trials—the Canadian experience in 2012. Journal of Bone Oncology. 2012 doi: 10.1016/j.jbo.2012.09.001. published online first. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):112. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011 [updated March 2011].
- 18.Lipton A., Steger G., Figueroa J. Randomized active controlled phase II study of denosumab efficacy and safety in patients with breast cancer related bone metastases. Journal of Clinical Oncology. 2007;25(28):4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 19.Berenson J., Rosen L., Howell A. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double blind, randomized dose-response study. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Simmons CE, Goodwin PJ, Hogeveen S. Continued suppression of bone turnover following a single dose of zoledronic acid: time to re-think dosing intervals in the management of bone metastases? Journal of Clinical Oncology. 2012:9111. [Google Scholar]
- 21.Bouganim N., Vandermeer L., Kuchuk I., Dent S., Hopkins S., Song X. Evaluating efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal related events. TRIUMPH: a pragmatic multicentre trial. Cancer Research. 2012;72(24 Suppl) doi: 10.1007/s10549-014-2906-x. Abstract nr P3-13-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berenson J.R., Hillner B.E., Kyle R.A., Anderson K., Lipton A., Yee G.C. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. Journal of Clinical Oncology. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 23.Clemons M., Dranitsaris G., Ooi W., Cole D.E.A. Phase II trial evaluating the palliative benefit of second-line oral ibandronate in breast cancer patients with either a skeletal related event (SRE) or progressive bone metastases (BM) despite standard bisphosphonate (BP) therapy. Breast Cancer Research and Treatment. 2008;108:79–85. doi: 10.1007/s10549-007-9583-y. [DOI] [PubMed] [Google Scholar]
- 24.Clemons M.J., Dranitsaris G., Ooi W.S., Yogendran G., Sukovic T., Wong B. Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. Journal of Clinical Oncology. 2006;24:4895–4900. doi: 10.1200/JCO.2006.05.9212. [DOI] [PubMed] [Google Scholar]
- 25.Holen I., Coleman R.E. Bisphosphonates as treatment of bone metastases. Current Pharmaceutical Design. 2010;16:1262–1271. doi: 10.2174/138161210791034003. [DOI] [PubMed] [Google Scholar]