Abstract
Aminobisphosphonates are used for the treatment of benign and malignant bone disorders. As inhibitors of the mevalonate pathway they exert direct anti-tumor effects in vitro and in preclinical models of bone metastases. Bisphosphonates are thought to have an anti-angiogenic activity as decreased levels of VEGF have been reported in some, although not all patients, following treatment with bisphosphonates. Direct effects of bisphosphonates on tumor derived VEGF have not been examined in detail. We therefore investigated VEGF expression in breast cancer cell lines following mevalonate pathway inhibition. Treatment of cell lines with increasing doses of zoledronic acid and atorvastatin resulted in increased levels of VEGF production. Similar results were seen with the geranylgeranyltransferase I inhibitor GGTI-298. The induction of VEGF was reversed by the supplementation of geranylgeranyl pyrophosphate but not by farnesyl pyrophosphate indicating that this effect is mediated by inhibited geranylgeranylation. Previous reports have reported decreased VEGF levels in patients following BP treatment in vivo. We assessed VEGF levels in patients with non-metastatic breast cancer following repeated treatment with zoledronic acid. In contrast to our in vitro findings, VEGF serum levels decreased in all patients after 6–9 months of treatment (by an average of 41%) as assessed in a small pilot trial. These results indicate that tissues other than breast tumors contribute to the serum pool of circulating VEGF and may be responsible for the observed VEGF decreases. The increases of VEGF in the cancer cells may provide a rationale for the combined treatment with VEGF inhibitors.
Abbreviations: BP, amino-bisphosphonates; GGPP, geranylgeranyl pyrophosphate; FPP, farnesyl pyrophosphate (FPP); GGPP, geranylgeranyl pyrophosphate; VEGF, vascular endothelial growth factor
Keywords: VEGF, Zoledronic acid, Atorvastatin, Mevalonate pathway, Breast cancer
1. Introduction
The occurrence of osteolytic lesions remains a feared long term complication in patients with breast cancer. Amino-bisphosphonates (BP) are a class of antiresorptive agents approved for the treatment of metastatic bone disease [1], [2]. BP act via inhibition of the FPP-synthase, which is a key enzyme of the mevalonate pathway [3]. Aside from their potent antiresorptive properties, BP have been associated with a direct antitumor potential. In vitro, BP induce apoptosis and decrease proliferation and invasion of tumor cells [4]. While these results were confirmed in a number of preclinical in vivo models, results from two large clinical trials have yielded varying results regarding the adjuvant use of zoledronic acid in breast cancer patients [5], [6]. One trial showed a significant reduction in the risk of disease progression in patients receiving zoledronic acid in addition to endocrine therapy compared to endocrine therapy alone in hormone-responsive breast cancer [5]. The other trial assessed the response of breast cancer patients with different hormone receptor expression and menopausal status to zoledronic acid in addition to their standard adjuvant treatment. This study failed to provide evidence to support the routine use of zoledronic acid in the adjuvant management of breast cancer [6]. One of the proposed antitumor effects of BP is their anti-angiogenic potential. A number of studies have shown that serum levels of VEGF decrease in tumor patients following treatment with bisphosphonates [7], [8]. In one case, serum concentrations of VEGF were shown to be suppressed as early as 7 days after the first infusion of zoledronic acid and remained suppressed for the duration of the study, 84 days after the first infusion [7]. The finding of anti-angiogenic effects of these agents was further supported by the finding that bisphosphonates inhibited tumor vascularization in murine models of prostate carcinoma, melanoma and myeloma [9], [10], [11]. However, these results could not be confirmed in all studies and in some studies VEGF levels remained unaffected [12]. The molecular mechanisms responsible for these effects have not been looked at in detail. It remains unclear, if the observed regulation on VEGF is mediated via direct effects on cancer cells or by effects on other cells such as macrophages or endothelial cells.
This study is aimed at investigating the direct effects on mevalonate pathway inhibition using zoledronic acid, atorvastatin and specific inhibitors of farnesylation and geranylgeranylation in breast cancer cell lines with different metastatic properties and hormone receptor status.
2. Materials and methods
2.1. Cells and reagents
Human breast cancer cells were purchased from ATCC (Manassas, VA), except for the bone seeking MDA-MET cells (subclones of MDA-231 cells) which were a gift of Prof. L. Suva (Arkansas, USA). All cell lines were cultured in DMEM/Ham׳s F-12 (PAA, Pasching, Austria) with 10% fetal calf serum supreme (Lonza, Cologne, Germany) and 1% penicillin/streptomycin (PAA, Pasching, Austria). Cell line authenticity was determined by short tandem repeat profiling and by matching with the known profiles at DSMZ (German Collection of Microorganisms and Cell Culturs). Zoledronic acid, atorvastatin, mevalonate, geranylgeranyl-pyrophosphate (GGPP), farnesyl pyrophosphate (FPP), GGTI-298 and FTI-277 were obtained from Sigma-Aldrich (Munich, Germany). Zoledronic acid was solved in PBS. Mevalonate, FPP and GGPP were dissolved in methanol:NH4OH solution and atorvastatin was dissolved in DMSO. Appropriate controls were added to untreated control cells.
2.2. Cell culture and treatment
Breast cancer cell lines were treated with zoledronic acid (100 µM), atorvastatin (10 µM), FTI-277 (0.01, 0.1, 1 µM) and GGTI-298 (1, 5, 10 µM) for 24 h (unless otherwise indicated). Mevalonate substrates (GGPP, FPP and mevalonate) were supplemented together with atorvastatin and zoledronic acid at concentrations shown to reverse specific pathway inhibition.
2.3. RNA isolation, RT and real-time PCR
RNA from the cell lines was isolated using the HighPure RNA extraction kit from Roche according to the manufacturer׳s protocol. 500 ng RNA were reverse transcribed using Superscript II (Invitrogen, Darmstadt, Germany) and used for SYBR green-based real-time PCR reactions using a standard protocol (Applied Biosystems). Primer sequences for VEGFA were sense: GTGATGATTCTGCCCTCCTC and anti-sense: CCTTGCTGCTCTACCTCCAC; for GAPDH sense: CATCACCATCTTCCAGGAGCG and anti-sense: TGACCTTGCCCACAGCCTTG. PCR conditions were 50 °C for 2 min and 95 °C for 10 min followed by 40 cycles with 95 °C for 15 s and 60 °C for 1 min. The melting curve as assessed in the following program: 95 °C for 15 s, 60 °C for 1 min and 95 °C for 30 s. The results were calculated applying the ΔΔCT method and are presented as relative expression to the house keeping gene (GAPDH) or as a percentage of control.
2.4. VEGF ELISA
VEGF ELISA was purchased from Thermo scientific (Waltham, MA) and conducted as proposed by the manufacturer. Briefly, cell culture supernatants were diluted 1:5 (as predefined by testing), serum samples were used undiluted. Samples were assayed in duplicates. 50 µl standard diluent was given to all wells and 50 µl of samples and diluted standards were pipetted to the appropriate wells and incubated for 2 h. Plates were washed and 100 ml of biotinylated Antibody Reagent was added to all wells, after which wells were incubated for another hour. After washing 100 ml of prepared Streptavidin–HRP Solution was given to all wells, incubated for an hour and washed thrice. Then 100 ml of TMB substrate solution was pipetted into all wells and after 30 min of incubation the reaction was stopped and wells were measured and values were calculated as proposed.
2.5. Western blot
Western blot analyses were performed as previously described [13]. Briefly, cells were washed and scraped in a lysis buffer and quantified. 20 µg of protein were loaded on a SDS–PAGE and transferred onto a 0.2 µm nitrocellulose membrane. After blocking for 1 h with 5% non-fat dry milk in Tris-buffered saline with 1% tween-20 (TBS-T), membranes were incubated with a primary antibody overnight. After washing, the membrane was incubated for 1 h with the HRP-conjugated secondary antibody. Membranes were washed 3 times with TBS-T again, and proteins were visualized with Super Signal (Pierce, Bonn, Germany) enhanced chemiluminescence. Antibody for RAP1A (sc-1482) was from Santa Cruz (Heidelberg, Germany) and the RAS (610001) antibody was from BD Biosciences (Heidelberg, Germany).
2.6. Serum samples
Serum samples of patients were obtained after informed consent and IRB approval. Patients with hormone receptor-negative, non-metastatic breast cancer were treated with infusions of 4 mg zoledronic acid every three months. All patients received 1000 mg Calcium and 1000 IE Vitamin D per day for the duration of the study. None of the patients receives any additional concomitant drugs known to influence bone turnover. None of the patients sustained a fracture during the study period nor were any other anti-resorptives given. Control serum was taken before the first administration of zoledronic acid and before each further administration. All serum samples were collected after an overnight fasting at the same time in the morning. Blood samples were immediately worked up and stored at −80 °C until the analyses were performed.
2.7. Statistical analyses
Results are presented as means±standard deviation (SD). All experiments were repeated at least three times. Statistical evaluations were performed using a one-way ANOVA or a Student´s T-test. P values<0.05 were considered statistically significant.
3. Results
3.1. Zoledronic acid and atorvastatin increase VEGF expression in breast cancer
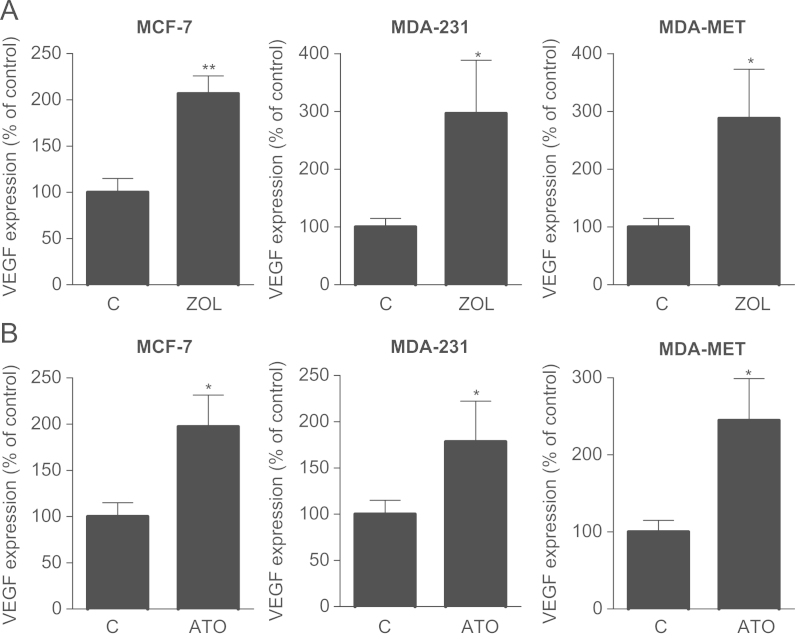
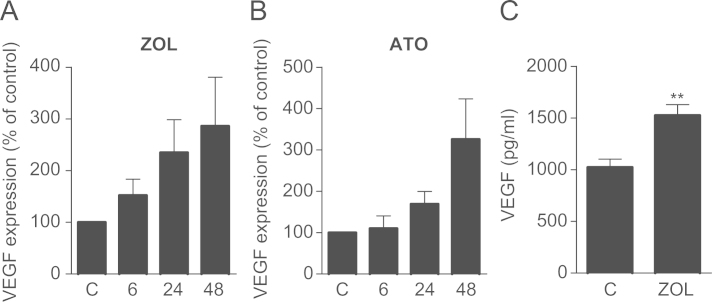
Three different breast cancer cell lines were treated with 100 µM zoledronic acid for 24 h and assessed for VEGF expression. Base line expression of VEGF varied greatly between the different cell lines. The hormone receptor positive MCF-7 cells had considerably lower levels of VEGF compared to MDA-231 and their metastatic subclones (data not shown). This is line with their lower aggressiveness and lower metastatic properties. Following exposure to zoledronic acid VEGF expression was significantly up-regulated in all three tested cell lines (MCF-7, MDA-231 and MDA-MET) to 207%, 296% and 288% compared to PBS-treated cells, respectively (Fig. 1A). To test whether this effect was mediated via mevalonate pathway inhibition, the same cell lines were also treated with 10 µM of atorvastatin. Atorvastatin increased VEGF levels in all cell lines compared to control although the induction was smaller in MDA-231 and MDA-MET cells than previously seen with zoledronic acid (Fig. 1B). When treating MDA-231 cells for up to 48 h, VEGF increases were seen as early as 6 h after treatment with zoledronic acid and increased further after 24 h and 48 h (Fig. 2A). In atorvastatin treated cells, increases of VEGF expression were first noted after 24 h (Fig. 2B). Increased VEGF expression also translated into higher levels of protein secretion as seen by an increase from 1023.1±64.4 pg/ml in control-treated cells to 1524.7±85.8 pg/ml (+50%; P<0.01) in zoledronic acid treated MDA-231 cells (Fig. 2C).
Fig. 1.
Zoledronic acid and atorvastatin increase VEGF expression in breast cancer cell lines. (A+B) Breast cancer cell lines (MCF-7, MDA-231 and MDA-MET) were treated with zoledronic acid (100 µM) and atorvastatin (10 µM) for 24 h. Data are presented as the percentage expression relative to control treated (PBS, DMSO) cells, and are mean±SD of 3 independent experiments. *P<0.05; **P<0.01.
Fig. 2.
Zoledronic acid and atorvastatin increase VEGF expression in breast cancer cell lines. (A+B) Treatment with zoledronic acid (100 µM) or atorvastatin (10 µM) for 6, 24, and 48 h results in a time-dependent increase of VEGF expression in MDA-231 cells. Data are presented as the percentage expression relative to control treated (PBS, DMSO) cells, and are mean±SD of 3 independent experiments. (C) Treatment of MDA-231 cells with zoledronic acid (100 µM) for 24 h increases VEGF protein in the cell supernatant. Values are mean±SD of 3 independent experiments. **P<0.01.
3.2. Induction of VEGF is mediated via inhibition of geranylgeranylation
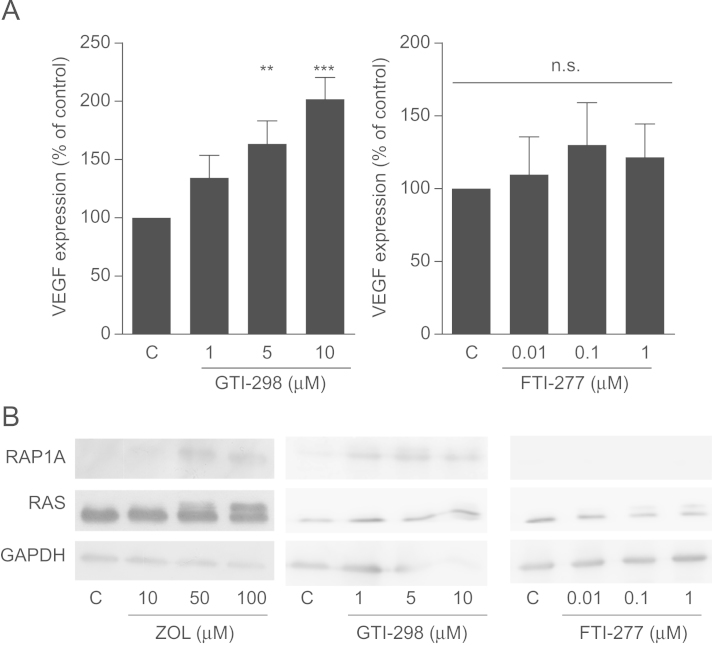
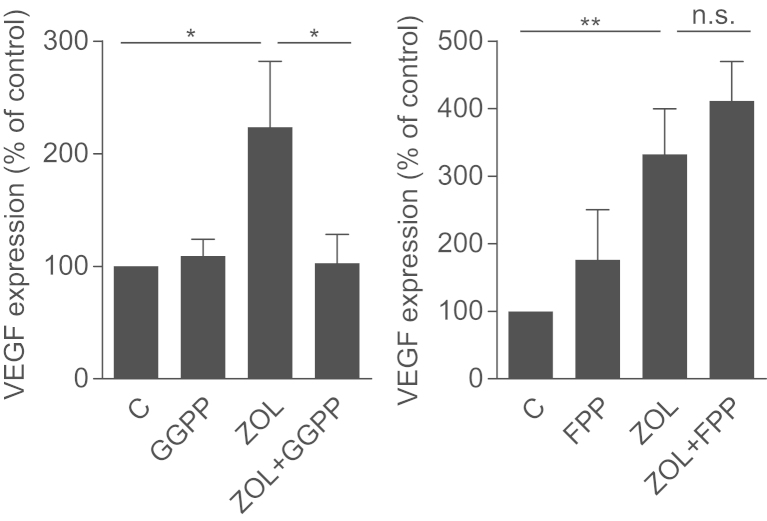
To further clarify the role of the mevalonate pathway in this regulation, MDA-231 cells were treated with increasing concentrations of FTI-277 and GGTI-298, which are specific inhibitors of farnesylation and geranylgeranylation. We observed a dose-dependent increase of VEGF expression after exposure to GGTI-298 (P<0.001), but no significant changes even in the highest concentrations of FTI-277 (Fig. 3A). Of note, the use of different concentrations of FTI-277 and GGTI-298 resulted from their different IC50 values. Relevant inhibition of prenylation of RAP1A (geranylation) and RAS (farnesylation) was verified by Western blot (Fig. 3B). When supplemented with GGPP and FPP, only GGPP successfully reversed the effects of zoledronic acid on VEGF expression. Supplementation of FPP had no significant effect on VEGF production. Together these data show that the VEGF increase is regulated via inhibited geranylgeranylation (Fig. 4).
Fig. 3.
VEGF expression is regulated by inhibited geranylgeranylation. (A) MDA-231 cells were treated with increasing concentrations of 1, 5 or 10 µM of GTI-298 (A) or 0.01, 0.1 or 1 µM of FTI-277 for 24 h. VEGF expression was assessed using qRT-PCR. Data are presented as the percentage expression relative to control treated (PBS, DMSO) cells, and are mean±SD of 3 independent experiments. **P<0.01; ***P<0.001. (B) MDA-231 cells were treated with GTI-298 and FTI-277 as above. Assessment of unfarnesylated RAS (upper band) and ungeranylated RAP1A confirmed mevalonate pathway inhibition. Representative blots are shown. GAPDH is used as a loading control.
Fig. 4.
GGPP prevents VEGF induction by zoledronic acid. MDA-231 cells were treated with either zoledronic acid (100 µM) alone or in combination with GGPP (100 µM) or FPP (100 µM) and VEGF was assessed by qRT-PCR. Data are presented as the percentage expression relative to control treated (PBS, DMSO) cells, and are mean±SD of 3 independent experiments. *P<0.05; **P<0.01.
3.3. VEGF levels decrease in zoledronic acid treated patients in vivo
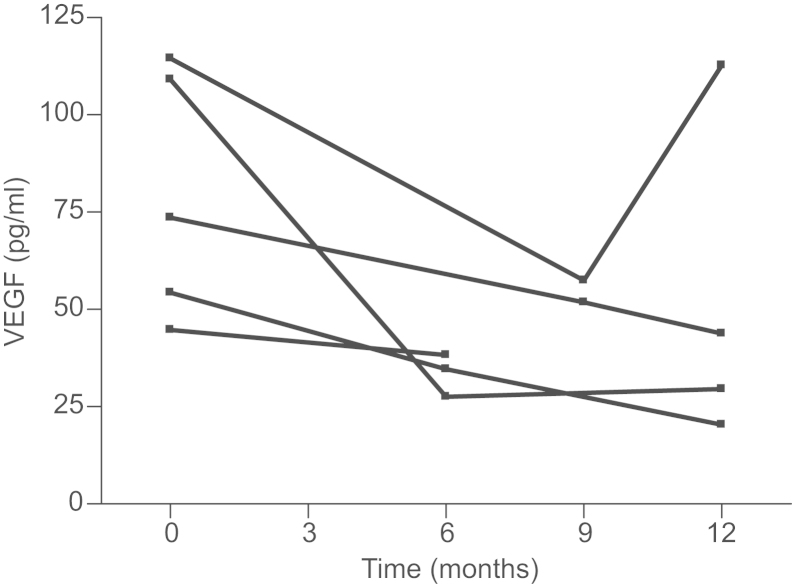
To see if these in vitro results could be confirmed in vivo, we also measured VEGF levels in patients with non-metastatic breast cancer at base line and after 6 (or 9, if 6 months measurement was not available) and 12 months. Base line levels of VEGF varied between 44.7 and 114.5 pg/ml. Following repeated treatment with zoledronic acid VEGF levels decreased in all patients at 6 or 9 month measurement, respectively (Fig. 5). Mean levels of VEGF decreased by 41% compared to baseline values. Of the 4 cases measured 12 months after treatment was initiated, VEGF levels stayed supressed in 3 cases. In one case VEGF levels normalized (98% of baseline value) at 12 months after an initial suppression to 50% was observed after 9 months. No patient had increased VEGF levels after treatment with zoledronic acid.
Fig. 5.
VEGF protein levels decrease in breast cancer patients treated with zoledronic acid. Women with ER-negative non-metastatic disease were repeatedly treated with zoledronic acid (4 mg) every 3 months. VEGF serum levels were measured by ELISA at 6 (or 9 months) and 12 months.
4. Discussion
This study investigated VEGF expression in breast cancer cells following exposure to mevalonate inhibitors. An anti-angiogenic potential of bisphosphonates is generally assumed as several studies have reported decreased serum VEGF levels in patients following treatment with bisphosphonates [14]. This is line with the decrease in serum VEGF levels observed in our pilot study. Although the number of patients included in our study is too low to draw conclusions, it underlines VEGF as a target of the mevalonate pathway. Results from this study require validation in larger trial. Unlike serum assessment of VEGF, direct effects of BP on VEGF production by tumor cells has not been evaluated in detail. Unexpectedly, we did not observe a decrease in breast cancer-derived VEGF mRNA or protein levels following treatment with zoledronic acid or atorvastatin. Instead, both agents resulted in significant increases of VEGF levels in all cell lines tested. It is currently unclear which cell types are mainly responsible for the commonly observed changes in serum VEGF following the treatment of cancer patients with bisphosphonates. It is known that bisphosphonates have direct effects on endothelial cells by inhibiting proliferation sensitizing them to TNF-induced cell death [15]. VEGF levels have also been described to be supressed by zoledronic acid in myeloma-associated macrophages, an effect that was enhanced by co-treatment with the proteasome inhibitor bortezomib [16]. Little is known about the direct effects of zoledronic acid tumor cells. In this study, we show that different breast cancer cell lines increase VEGF levels following exposure to bisphosphonates. However, concentrations needed to achieve significant effects on VEGF were high and comparable to those previously shown to induce apoptosis and growth inhibition [17]. The in vitro concentrations used in our experiments exceeded those normally achievable in clinical routine. While this limits the direct translational relevance of our study, it is noteworthy that zoledronic acid, even when applied in low concentrations, did not suppress VEGF in breast cancer cells. To our knowledge there are no data from animal studies indicating that zoledronic acid increases angiogenesis following zoledronic treatment. However, even when high doses of zoledronic acid are used in animal models, these may still not reach the concentrations required to see effects in vitro. VEGF not only induces vascularization but also acts as a direct survival factor for tumor cells including breast cancer [18]. VEGF has been shown to prevent radiation-induced apoptosis [19] and to up-regulate Bcl-2 [20]. As the concentrations used in this study also increased apoptosis, one explanation may be that the VEGF increase is a result of increased cellular stress. This idea is supported by the finding that VEGF levels were normalized when protein prenylation is restored by supplementation of GGPP. As breast cancer VEGF levels stay unchanged at low levels and increase with high concentrations of zoledronic acid, our study provides some limited evidence that the decreases in VEGF serum levels following BP exposure results from a regulation of other cell types than the tumor cells.
A number of studies have shown that zoledronic acid has the ability to potentiate effects of anti-tumor agents such as doxorubicin [21] or everolimus [22]. In a recent paper the combination of chemotherapy with a single infusion of 4 mg zoledronic acid in patients with breast cancer was shown to result in a greater suppression of VEGF levels at day 5 than with chemotherapy alone [23]. The combination of an anti-angiogenic therapy with zoledronic acid may be an attractive option in some cases. However, this therapy has been associated with an increased rate of osteonecrosis of the jaw (ONJ) [24]. ONJ is a complication of anti-resorptive therapy, while it is rarely seen when BP are given for the treatment of osteoporosis, it is more common when given in oncologic doses [25]. While the pathogenesis of ONJ remains unclear, angiogenic suppression in osteoclasts following BP is one proposed mechanism [26], [27]. This hypothesis is supported by a recent finding, that patients with highest suppression of VEGF levels following BP administration were prone to ONJ [28].
In conclusion, we show that high doses of zoledronic acid may increase VEGF expression in breast cancer cells via inhibited geranylgeranylation. While it is unlikely that tumor cells will be exposed to these concentrations in the clinical setting, this study gives some evidence that the observed decreases in serum VEGF following bisphosphonate exposure is caused by cells other than breast cancer cells.
Role of the funding source
This work was supported by the DAdorW/Amgen Bone Fellowship and the MedDrive Start-Up Grant from the TU Dresden to TDR, and Grants RA 2151/2-1 (to TDR and LCH) and Forschergruppe-1586 SKELMET to LCH from the Deutsche Forschungsgemeinschaft.
Conflict of Interest statement
The authors have received grants or honorarium for advisory boards or lectures to the individual or the institution by Amgen (TDR, LCH, PH), AstraZeneca (PH), Eli Lilly (PH), GlaxoSmithKline (PH), Novartis (TDR, LCH, PH), Pfizer (PH), Roche (PH), Servier (LCH), Merck (LCH, TDR), and Nycomed (LCH). AG, MJ, JH and PBM declare that they have no conflict of interest.
References
- 1.Costa L. Bisphosphonates: reducing the risk of skeletal complications from bone metastasis. Breast. 2007;16(Suppl. 3):S16–S20. doi: 10.1016/j.breast.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E., McCloskey E.V. Bisphosphonates in oncology. Bone. 2011;49:71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. Journal of Bone and Mineral Research. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 4.Gnant M., Clézardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treatment Reviews. 2012;38:407–415. doi: 10.1016/j.ctrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Gnant M., Mlineritsch B., Schippinger W., Luschin-Ebengreuth G., Pöstlberger S., Menzel C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. New England Journal of Medicine. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R.E., Marshall H., Cameron D., Dodwell D., Burkinshaw R., Keane M. Breast-cancer adjuvant therapy with zoledronic acid. New England Journal of Medicine. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 7.Santini D., Vincenzi B., Galluzzo S., Battistoni F., Rocci L., Venditti O. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clinical Cancer Research. 2007;13:4482–4486. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- 8.Ferretti G., Fabi A., Carlini P., Papaldo P., Cordiali Fei P., Di Cosimo S. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69:35–43. doi: 10.1159/000087286. [DOI] [PubMed] [Google Scholar]
- 9.Fournier P., Boissier S., Filleur S., Guglielmi J., Cabon F., Colombel M. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Research. 2002;62:6538–6544. [PubMed] [Google Scholar]
- 10.Yamagishi S., Abe R., Inagaki Y., Nakamura K., Sugawara H., Inokuma D. Minodronate, a newly developed nitrogen-containing bisphosphonate, suppresses melanoma growth and improves survival in nude mice by blocking vascular endothelial growth factor signaling. American Journal of Pathology. 2004;165:1865–1874. doi: 10.1016/s0002-9440(10)63239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croucher P.I., De Hendrik R., Perry M.J., Hijzen A., Shipman C.M., Lippitt J. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. Journal of Bone and Mineral Research. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 12.Tas F., Duranyildiz D., Oguz H., Camlica H., Yasasever V., Topuz E. Effect of zoledronic acid on serum angiogenic factors in patients with bone metastases. Medical Oncology. 2008;25:346–349. doi: 10.1007/s12032-008-9043-5. [DOI] [PubMed] [Google Scholar]
- 13.Benad P., Rauner M., Rachner T.D., Hofbauer L.C. The anti-progestin RU-486 inhibits viability of MCF-7 breast cancer cells by suppressing WNT1. Cancer Letters. 2011;312:101–108. doi: 10.1016/j.canlet.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Metcalf S., Pandha H.S., Morgan R. Antiangiogenic effects of zoledronate on cancer neovasculature. Future Oncology. 2011;7:1325–1333. doi: 10.2217/fon.11.113. [DOI] [PubMed] [Google Scholar]
- 15.Bezzi M., Hasmim M., Bieler G., Dormond O., Rüegg C. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death: evidence for the suppression of sustained activation of focal adhesion kinase and protein kinase B/Akt. Journal of Biological Chemistry. 2003;278:43603–43614. doi: 10.1074/jbc.M308114200. [DOI] [PubMed] [Google Scholar]
- 16.Moschetta M., Di Pietro G., Ria R., Gnoni A., Mangialardi G., Guarini A. Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. European Journal of Cancer. 2010;46:420–429. doi: 10.1016/j.ejca.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Rachner T.D., Singh S.K., Schoppet M., Benad P., Bornhäuser M., Ellenrieder V. Zoledronic acid induces apoptosis and changes the TRAIL/OPG ratio in breast cancer cells. Cancer Letters. 2010;287:109–116. doi: 10.1016/j.canlet.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Byrne A.M., Bouchier-Hayes D.J., Harmey J.H. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) Journal of Cellular and Molecular Medicine. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh O., Tauchi H., Kawaishi K., Kimura A., Satow Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Research. 1995;55:5687–5692. [PubMed] [Google Scholar]
- 20.Pidgeon G.P., Barr M.P., Harmey J.H., Foley D.A., Bouchier-Hayes D.J. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. British Journal of Cancer. 2001;85:273–278. doi: 10.1054/bjoc.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottewell P.D., Woodward J.K., Lefley D.V., Evans C.A., Coleman R.E., Holen I. Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Molecular Cancer Therapeutics. 2009;8:2821–2832. doi: 10.1158/1535-7163.MCT-09-0462. [DOI] [PubMed] [Google Scholar]
- 22.Moriceau G., Ory B., Mitrofan L., Riganti C., Blanchard F., Brion R. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (Everolimus): pivotal role of the prenylation process. Cancer Research. 2010;70:10329–10339. doi: 10.1158/0008-5472.CAN-10-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter M.C., Wilson C., Syddall S.P., Cross S.S., Evans A., Ingram C.E. Neoadjuvant chemotherapy with or without zoledronic acid in early breast cancer—a randomised biomarker pilot study. Clinical Cancer Research. 2013;19:2755–2765. doi: 10.1158/1078-0432.CCR-12-3235. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulou C., Pervena A., Klouvas G., Galani E., Falagas M.E., Tsakalos G. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 25.Khosla S., Burr D., Cauley J., Dempster D.W., Ebeling P.R., Felsenberg D. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 26.Reid I.R., Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nature Reviews Rheumatology. 2011;8:90–96. doi: 10.1038/nrrheum.2011.181. [DOI] [PubMed] [Google Scholar]
- 27.Yin G., Bai Y., Luo E. Angiogenic suppression of osteoclasts may play a role in developing bisphosphonate-related osteonecrosis of the jaw. Medical Hypotheses. 2011;76:347–349. doi: 10.1016/j.mehy.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Vincenzi B., Napolitano A., Zoccoli A., Iuliani M., Pantano F., Papapietro N. Serum VEGF levels as predictive marker of bisphosphonate-related osteonecrosis of the jaw. Journal of Hematology & Oncology. 2012;5:56. doi: 10.1186/1756-8722-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]