Abstract
The response of nerve cells to synaptic inputs and the propagation of this activation is critically dependent on the cell-surface distribution of ion channels. In the hippocampus, Ca2+ influx through N-methyl-D-aspartate receptors (NMDAR) and/or voltage-dependent calcium channels on dendrites is thought to be critically involved in long-term potentiation, neurite outgrowth, epileptogenesis, synaptogenesis, and cell death. We report that conantokin-G (CntxG), a peptide from Conus geographus venom, competitively blocked with high affinity and specificity NMDAR-mediated currents in hippocampal neurons and is a reliable probe for exploring NMDAR distribution. Fluorescent derivatives of CntxG were prepared and used to directly determine NMDAR distribution on living hippocampal neurons by digital imaging and confocal fluorescence microscopy. In hippocampal slices, the CA1 dendritic subfield was strongly labeled by CntxG, whereas the CA3 mossy fiber region was not. On CA1 hippocampal neurons in culture, dendritic CntkG-sensitive NMDAR were clustered at sites of synaptic contacts, whereas somatic NMDAR were distributed diffusely and in patches. NMDAR distribution differed from the distribution of voltage-dependent calcium channels. A significant fraction of labeled NMDAR on somata and dendrites was found to be highly mobile: rates were consistent with the possible rapid recruitment of NMDAR to specific synaptic locations. The localization of NMDAR and modulation of this distribution demonstrated here may have important implications for the events that underlie neuronal processing and synaptic remodeling during associative synaptic modification.
Full text
PDF
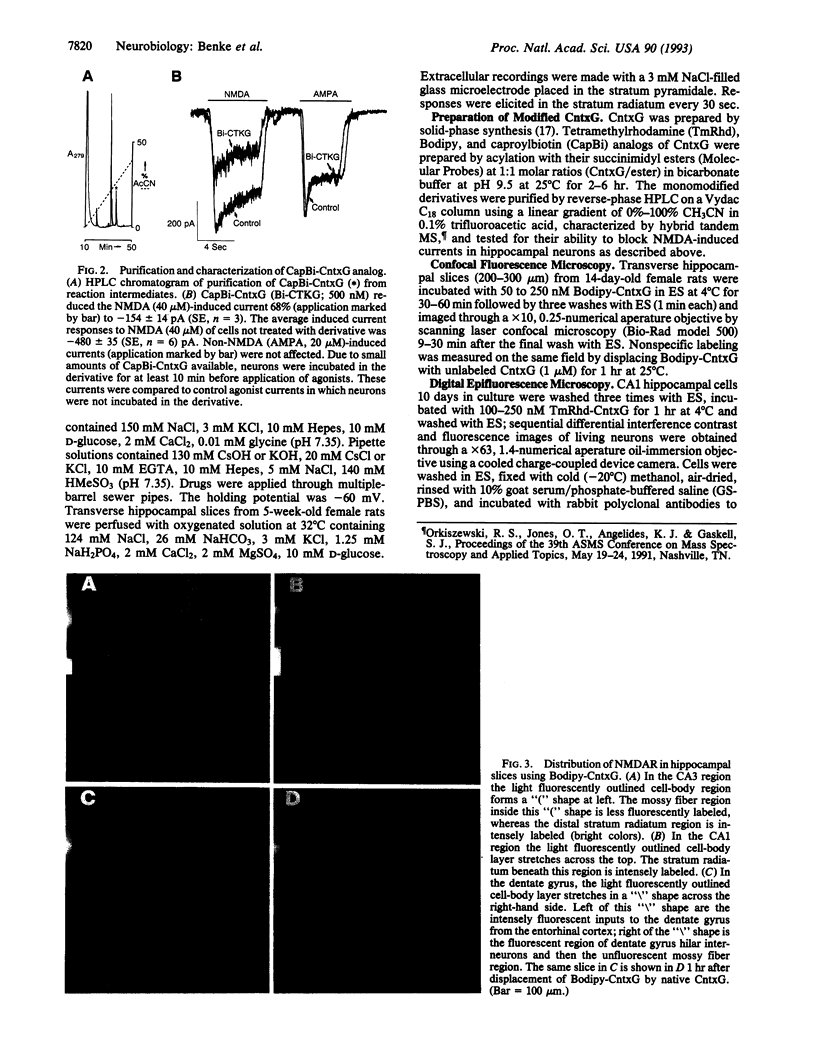
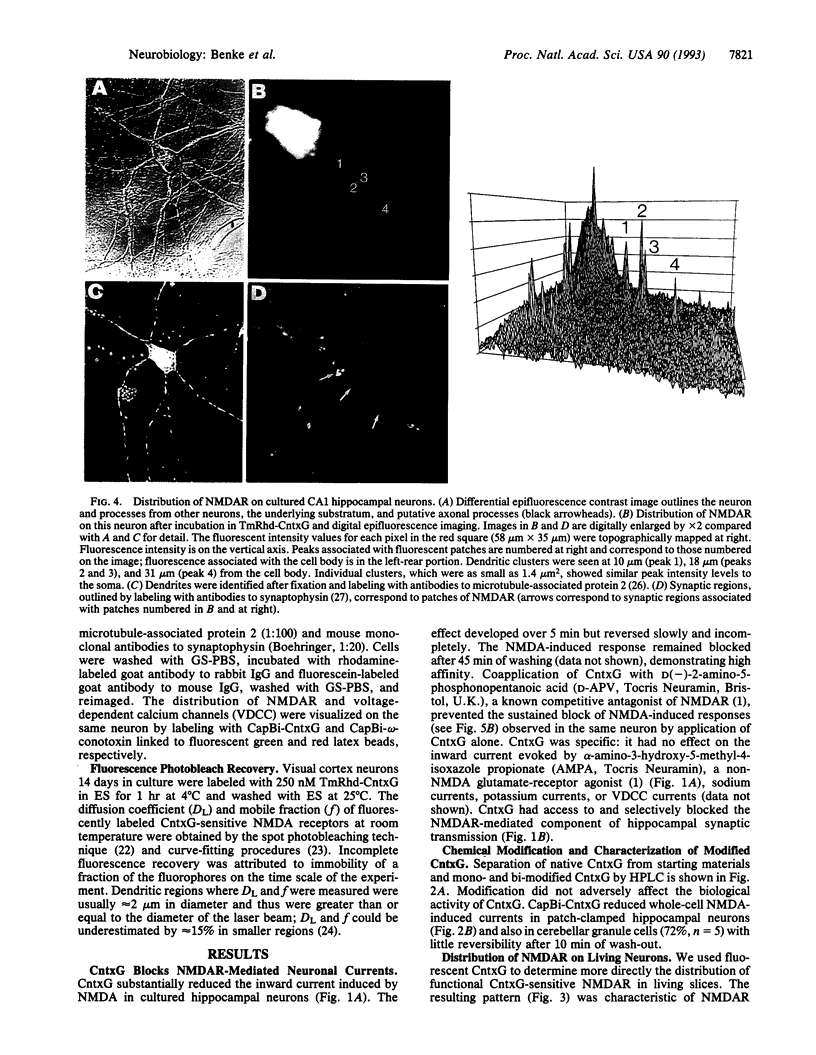
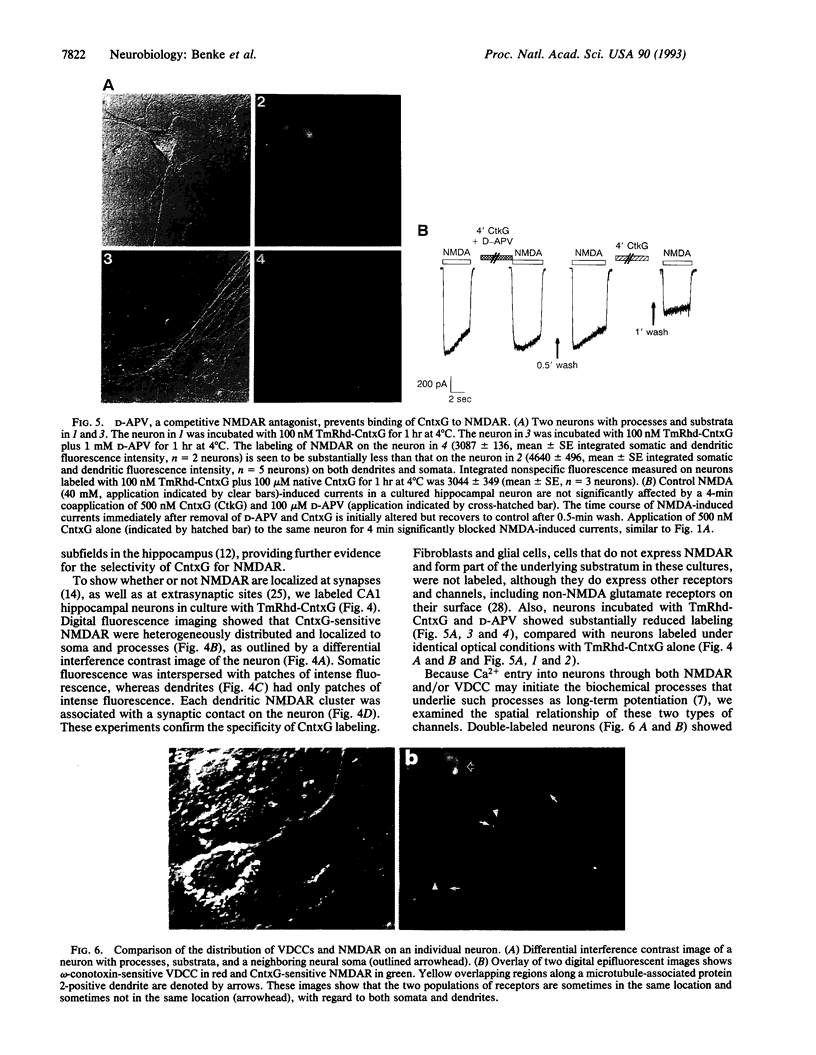

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J., Elmer L. W., Loftus D., Elson E. Distribution and lateral mobility of voltage-dependent sodium channels in neurons. J Cell Biol. 1988 Jun;106(6):1911–1925. doi: 10.1083/jcb.106.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O., MacDermott A. B. Differential distribution of excitatory amino acid receptors on embryonic rat spinal cord neurons in culture. J Neurophysiol. 1991 Apr;65(4):899–913. doi: 10.1152/jn.1991.65.4.899. [DOI] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir Z. I., Alford S., Davies S. N., Randall A. D., Collingridge G. L. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991 Jan 10;349(6305):156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Croucher M. J., Collins J. F., Meldrum B. S. Anticonvulsant action of excitatory amino acid antagonists. Science. 1982 May 21;216(4548):899–901. doi: 10.1126/science.7079744. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Di Stasi A. M., Gallo V., Ceccarini M., Petrucci T. C. Neuronal fodrin proteolysis occurs independently of excitatory amino acid-induced neurotoxicity. Neuron. 1991 Mar;6(3):445–454. doi: 10.1016/0896-6273(91)90252-u. [DOI] [PubMed] [Google Scholar]
- Fromherz P. Self-organization of the fluid mosaic of charged channel proteins in membranes. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6353–6357. doi: 10.1073/pnas.85.17.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie P. B., Segal M., Kater S. B. Independent regulation of calcium revealed by imaging dendritic spines. Nature. 1991 Nov 7;354(6348):76–80. doi: 10.1038/354076a0. [DOI] [PubMed] [Google Scholar]
- Hammerland L. G., Olivera B. M., Yoshikami D. Conantokin-G selectively inhibits N-methyl-D-aspartate-induced currents in Xenopus oocytes injected with mouse brain mRNA. Eur J Pharmacol. 1992 Jul 1;226(3):239–244. doi: 10.1016/0922-4106(92)90067-6. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Protein kinase C activation in mixed micelles. Mechanistic implications of phospholipid, diacylglycerol, and calcium interdependencies. J Biol Chem. 1986 Jun 5;261(16):7184–7190. [PubMed] [Google Scholar]
- Harris K. M., Stevens J. K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989 Aug;9(8):2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. A., Jahn R., Südhof T. C. Transmembrane topography and evolutionary conservation of synaptophysin. J Biol Chem. 1989 Jan 15;264(2):1268–1273. [PubMed] [Google Scholar]
- Jones O. T., Kunze D. L., Angelides K. J. Localization and mobility of omega-conotoxin-sensitive Ca2+ channels in hippocampal CA1 neurons. Science. 1989 Jun 9;244(4909):1189–1193. doi: 10.1126/science.2543080. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Dou P., Kater S. B. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988 Jun;8(6):2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena E. E., Gullak M. F., Pagnozzi M. J., Richter K. E., Rivier J., Cruz L. J., Olivera B. M. Conantokin-G: a novel peptide antagonist to the N-methyl-D-aspartic acid (NMDA) receptor. Neurosci Lett. 1990 Oct 16;118(2):241–244. doi: 10.1016/0304-3940(90)90637-o. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Holets V. R., Toy D. W., Cotman C. W. Anatomical distributions of four pharmacologically distinct 3H-L-glutamate binding sites. Nature. 1983 Nov 10;306(5939):176–179. doi: 10.1038/306176a0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Rabacchi S., Bailly Y., Delhaye-Bouchaud N., Mariani J. Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992 Jun 26;256(5065):1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- Rivier J., Galyean R., Simon L., Cruz L. J., Olivera B. M., Gray W. R. Total synthesis and further characterization of the gamma-carboxyglutamate-containing "sleeper" peptide from Conus geographus venom. Biochemistry. 1987 Dec 29;26(26):8508–8512. doi: 10.1021/bi00400a002. [DOI] [PubMed] [Google Scholar]
- Westenbroek R. E., Ahlijanian M. K., Catterall W. A. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990 Sep 20;347(6290):281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- von Blankenfeld G., Kettenmann H. Glutamate and GABA receptors in vertebrate glial cells. Mol Neurobiol. 1991 Spring;5(1):31–43. doi: 10.1007/BF02935611. [DOI] [PubMed] [Google Scholar]







