Abstract
Aims
To evaluate metastatic lesions within the radiation field using repeated magnetic resonance imaging (MRI) and to compare the imaging findings with pain response following radiotherapy (RT) in patients with spinal metastases (SM) from breast cancer.
Material and methods
32 Patients with SM from breast cancer admitted for fractionated RT were included in this study. MRI examinations of the spine were scored for the extent of bone metastases, epidural disease and the presence and severity of vertebral fractures. Clinical response was defined according to the updated international consensus on palliative RT endpoints.
Results
At 2 and 6 months after RT, 38% and 44% of the patients were classified as responders. None of the patients developed motor deficits. Importantly, a decrease in the intraspinal tumor volume after RT was reported in all patients. Only 6% of the patients showed bone metastases progression within the RT field, whereas 60% of the patients showed disease progression outside the RT portals. 5 Patients developed new fractures after RT, and fracture progression was observed in 21 of the 38 lesions (55%). The pain response to RT did not correlate with the presence of vertebral body fracture before RT, fracture progression or other recorded MRI features of metastatic lesions.
Conclusion
RT provided excellent local tumor control in patients with SM. Most patients benefit from RT even in cases of progressive vertebral fracture. Pain response was not associated with imaging findings and MRI cannot be used to select patients at risk of not responding to RT.
Keywords: Bone metastases, Breast cancer, MRI, Pain response, Radiotherapy
1. Introduction
External beam radiotherapy (RT) is a well-established and efficacious method of palliating painful bone metastases [1], [2]. Patients with bone metastases are at high risk for skeletal-related events such as pathologic fractures or spinal cord compression [3]. This may affect the pain response following RT and lower the quality of life in these patients [4]. However, limited data exist on the local disease control and the incidence of fractures after conventional fractionated RT to the spine [2], [4], [5], [6], [7], [8]. Furthermore, the impact of fractures on pain response is essentially unknown.
Magnetic resonance imaging (MRI) is the modality of choice for the diagnosis and follow-up of cancer patients with spinal metastases (SM). Only a few studies have evaluated pain response and imaging features after RT in patients with SM, and the findings have been inconsistent [9], [10], [11]. Hence, it is important to determine to what extent the RT response rate in SM is correlated with the presence of skeletal complications such as fractures or compression syndromes. Thus, the aim of this study was to evaluate the irradiated metastatic lesions and the rate of local tumor control using repeated MRI and to compare the imaging findings with pain response after RT in patients with SM from breast cancer.
2. Material and methods
2.1. Patients
All consecutive patients with symptomatic SM who were admitted to our institution in 2007 and 2008 were considered for inclusion in a prospective clinical study [12]. The current paper is a retrospective analysis of 32 patients (30 women and 2 men) with SM from breast cancer who were part of the aforementioned clinical study [12]. The inclusion criteria for the present study were as follows: first-time admittance for RT for SM from breast cancer, no motor deficit prior to RT, survival for more than 6 months after RT, available pre- and post-treatment MRI of the vertebral column, age greater than 18 years and signed informed consent. Post-treatment MRIs were conducted to evaluate local disease progression and as a part of routine follow-up for systemic disease status. All patients completed an MRI exam prior to RT and an MRI exam after such treatment. Pretreatment MRIs were performed within 2 months prior to RT. Post-treatment MRIs were performed within 2–6 months of the RT. Patients with paravertebral metastases with direct extension into vertebral bodies and patients with leptomeningeal or intramedullary metastases were not eligible.
All patients were interviewed prior to and at 2 and 6 months after RT using a validated Norwegian version of the Brief Pain Inventory form [12], [13]. The worst, average and least pain experiences during the previous 24 h and the current pain level were recorded using a 10-point scale. The worst pain experience was used as the principal outcome measure. Details on opioid consumption during the previous 24 h, including the drug name, daily dose and administration route, were recorded. All opioids were converted into the oral morphine-equivalent dose (OMED).
Clinical response to treatment was defined according to the updated International Bone Metastases Consensus Working Party palliative RT endpoints [1]. A complete response (CR) was defined as a pain score of 0 with no increase in the OMED. A partial response (PR) was defined either as a pain reduction of 2 or more points measured on a 10-point scale or an OMED reduction of 25% or more. Pain progression (PP) was defined as a pain increase of 2 or more points or an increase in the OMED of 25% or more. Patients not classified as having CR, PR or PP were defined as having an indeterminate response (IR). Patients with either CR or PR were defined as responders, whereas patients with either IR or PP were defined as non-responders [1].
2.2. MRI studies
64 MRI studies were performed in 32 patients. Entire spine MRI examinations were available in all but 2 patients. All images were retrospectively reviewed by one radiologist (MDS) who was blinded to the clinical records. Bone metastases were categorized as either diffuse infiltration of the bone marrow or focal lesions [14], [15]. The metastasis with the largest diameter or that was most suitable to measure was recorded as the target lesion. To avoid partial volume artifacts, only lesions with a diameter equal to or greater than 8 mm were considered measurable (2×slice thickness) [15].
Other recorded features included tumor-conditioned spinal canal stenosis (SCS), compression of the spinal cord, cauda equina and nerve roots. SCS was defined as a narrowing of the cross-sectional area of the spinal canal by a soft tissue tumor extension, bone fragments or both. Compression of the spinal cord was defined as a deviation or indentation of the spinal cord by an epidural tumor or bone fragments. Compression of the cauda equina was defined as an obliteration of the cerebrospinal fluid in the dural sac at the affected level. Nerve root compression was defined as contact between the tumor masses or bone fragments and the spinal nerves in the recess or intervertebral foramen [16].
Vertebral body fractures were recorded both prior to and after RT. A difference of ≥2 mm (2×pixel spacing) between the pre- and post-treatment vertebral height was recorded as fracture progression. Additional studied features included the level of the fractures, the percentage of vertebral height loss, and the percentage of metastatic vertebral body involvement.
The appearance of new lesions, a change in the metastatic pattern from focal to diffuse or at least a 20% increase in the largest diameter of the target lesions after RT was defined as progression. The disappearance of lesions or a reduction at least 30% in the diameters of the target lesions was defined as a response [14], [15]. The radiological response of spinal lesions was evaluated both inside and outside the RT portals. To assess the epidural tumor volume, a cross-sectional area of the spinal canal was measured at the affected level and compared with the cross-sectional area at the same level on post-treatment images.
2.3. Statistical analyses
Data were analyzed using IBM SPSSc Statistics version 21 (IBM, New York, NY, USA). Descriptive statistics including frequency distributions and percentages were used to describe the patient population. Chi-squared tests were used to compare proportions. All reported p values were based on 2-sided tests; p<0.05 was considered statistically significant.
2.4. Ethics
This study was approved by the Regional Ethics Committee, and written informed consent was obtained from all patients.
3. Results
3.1. Patients
The mean patient age at the start of treatment was 58 years (range 82–35). All patients received a radiation dose of 30 Gy delivered in 10 fractions within 2 weeks. RT was given in combination with ongoing chemotherapy (4 patients), hormone treatment (22 patients), bisphosphonates (7 patients) or corticosteroids (7 patients). Importantly, none of the patients developed neurological symptoms at the 2- or 6-month follow-up. All patients were ambulatory prior to and at 2 months after RT; at 6 months after RT, 1 of the 32 patients was non-ambulatory due to poor performance status related to general disease progression.
The mean pain scores were 4.3, 3.9 and 3.7 at baseline, 2 months and 6 months after RT, respectively. The corresponding mean OMED values were 100, 96 and 145 mg, respectively. At 2 and 6 months after RT, 12 patients (38%) and 14 patients (44%) were classified as responders. Age, ongoing chemotherapy, hormone therapy and the use of bisphosphonates were not associated with the pain response; however, patients younger than 65 years of age showed a better response than did the older patients (11 vs. 1 responder at 2 months).
3.2. MRI findings
Pretreatment MRIs were obtained within 1–47 days (mean 16 days) prior to RT. Post-treatment MRIs were obtained within 61–180 days (mean 103 days) after the completion of RT. The recorded MRI findings are presented in Table 1.
Table 1.
Registered MRI features (n=32).
| MRI findings | n | % |
|---|---|---|
| Pre-treatment MRI studies | ||
| 1. Bone metastases | ||
| Focal lesions | 23 | 72 |
| Diffuse infiltration | 9 | 28 |
| 2. Spinal epidural disease | ||
| Spinal canal stenosis | 21 | 66 |
| Compression of the spinal cord or cauda equina | 8 | 25 |
| Nerve root compression | 15 | 47 |
| 3. Fractures | ||
| Single level | 13 | 41 |
| Multiple levels | 9 | 28 |
| Follow-up MRI studies | ||
| 1. Progressive disease (bone marrow) | ||
| Inside the RT field | 2 | 6 |
| Outside the RT field | 19 | 59 |
| Unable to obtaina | 4 | 13 |
| 2. Spinal epidural disease | ||
| Decreased spinal canal stenosis grade | 18 | 56 |
| Increased spinal canal stenosis grade | 2 | 6 |
| Unable to estimateb | 1 | 3 |
| Compression of the spinal cord or cauda equina | 3 | 9 |
| Nerve root compression | 8 | 25 |
| Fracture progression in patients with spinal canal stenosis | 11 | 34 |
| 3. Fractures | ||
| New fractures | 5 | 16 |
| Fracture progression | 17 | 53 |
Incomplete examination (only lumbar spine, n=2) and diffuse infiltration of bone marrow (n=2).
Suboptimal image quality.
On the pretreatment MRIs, 23 patients had focal bony metastases and 9 patients had diffuse metastatic bone marrow infiltration. SCS was noted in 21 patients. For all but 1 patient, SCS was caused by both the epidural tumor and fracture. Compression of the spinal cord/cauda equina was present in 8 patients. Fractures were noted in 22 patients (13 patients at a single spinal level and in 9 patients at multiple levels). In total, 38 fractures were evaluated in 22 patients. All fractures were pathological, tumor-induced fractures.
On the post-treatment MRIs, 2 of the 32 (6%) patients showed a progression of bony disease within the RT field and 19 of the 32 patients (60%) showed a progression of bony disease outside the RT field. None of the patients demonstrated a decrease of more than 30% in the size of target lesions. A decreased epidural tumor volume was observed in all patients (Fig. 1). Only 2 of the 21 patients with pretreatment SCS and fracture showed an increase in the grade of SCS due to fracture progression. The compression persisted after RT in 3 of the 8 patients with pretreatment compression of the spinal cord or cauda equina. Progression of the vertebral fracture was observed in 21 of the 38 lesions (55%) in 17 patients. A total of 5 patients (15%) developed new fractures after RT; 1 patient had a benign (radiation-induced) fracture and 4 patients had malignant (tumor-induced) fractures.
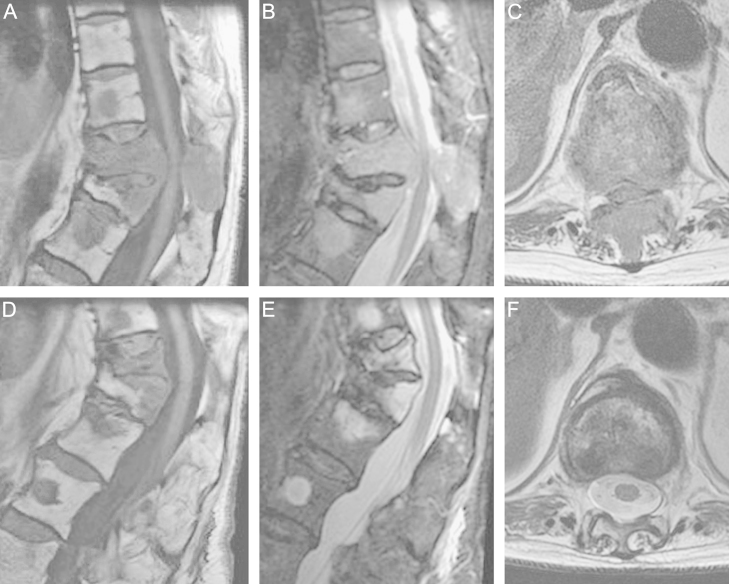
Fig. 1.
Spinal metastases from a breast carcinoma in a 74-year-old woman prior to (A–C) and after radiation therapy (D–F). Sagittal T1-weighted, STIR and transversal T2-weighted MR images of the spine show pathological vertebral fractures, tumor-conditioned spinal canal stenosis and compression of the spinal cord at the Th11 level. After treatment, the cross-sectional area of the spinal canal is improved significantly despite fracture progression at Th11 and increased kyphosis.
3.3. MRI findings and pain response to RT
The distribution of pre- and post-treatment MRI findings among responders and non-responders is presented in Table 2. Patients with advanced disease such as large metastases prior to RT (>75% of vertebral body involvement) or diffuse infiltration of bone marrow, and patients with compression of the medulla spinalis/cauda equina tended to be non-responders more frequently; however, these data did not reach statistical significance. The pain response was not associated with SCS, nerve root compression or progressive metastatic disease outside or within the RT field. The pain response was not associated with the presence of vertebral fractures before RT, fracture progression, the grade of vertebral body height loss or new fractures after RT.
Table 2.
Recorded MRI findings and pain response at 2 and 6 months after RT (n=32).
| MRI findings | Pain response at 2 months |
Pain response at 6 months |
|||
|---|---|---|---|---|---|
| Non-responders | Responders | Non-responders | Responders | ||
| Compression of medulla spinalis/cauda equinaa | Yes (n=8) | 7 | 1 | 6 | 2 |
| No (n=23) | 13 | 10 | 12 | 11 | |
| Nerve root compressionb | Yes (n=15) | 9 | 6 | 7 | 8 |
| No (n=14) | 9 | 5 | 9 | 5 | |
| Progression outside the RT fieldc | Yes (n=19) | 11 | 8 | 11 | 8 |
| No (n=9) | 7 | 2 | 5 | 4 | |
| Progression inside the RT fieldd | Yes (n=2) | 1 | 1 | 1 | 1 |
| No (n=28) | 17 | 11 | 16 | 12 | |
| Fracture progression | Yes (n=17) | 11 | 6 | 9 | 8 |
| No (n=15) | 9 | 6 | 9 | 6 | |
| New fractures | Yes (n=5) | 4 | 1 | 3 | 2 |
| No (n=27) | 16 | 11 | 15 | 12 | |
| Diffuse infiltration of bone marrow | Yes (n=9) | 8 | 1 | 7 | 2 |
| No (n=23) | 12 | 11 | 11 | 12 | |
| Large focal metastases (>75% of vertebral body involvement) | Yes (n=24) | 17 | 7 | 14 | 10 |
| No (n=8) | 3 | 5 | 4 | 4 | |
Unable to estimate in 1 patient due to suboptimal image quality.
Uncertain findings in 3 patients due to image quality.
Unable to obtain in 4 patients due to incomplete examination (only lumbar spine, n=2) and diffuse infiltration of bone marrow (n=2).
Unable to obtain in 2 patients due to diffuse metastatic infiltration of bone marrow.
4. Discussion
This study investigated the relationship between MRI features and the pain response after fractionated RT in a cohort of patients with breast cancer metastases to the spine.
In our series, fracture progression was noted in 53% of the lesions and 5 patients presented with new fractures after RT. Previous studies reported on the rate of pathologic fractures after single or multifraction RT; however, they did not stratify between spine and other locations in the skeleton [2], [4], [5], [6], [7]. Neither the impact of vertebral fractures upon treatment response nor the risk of fracture in different radiation regimens is assessed properly and need to be examined. More pathological fractures seemingly occurred after single fraction RT than after multifraction therapy, but the absolute percentage was low [2], [6], [7]. In general, dose prescription and fractionation should be adjusted to the therapeutic goal and patient prognosis. Patients included in this study had a relatively long expected survival. Consequently, local disease stabilization and prevention of neurological deficits were the main therapeutic goals, and all patients benefited from the fractionated regimen [17].
Vertebral fractures at the initiation of RT were present in 69% of the patients in our series. This percentage is also higher than in previous reports that included patients with various cancer diagnoses [9], [10], and this difference could reflect a higher incidence of vertebral body fractures in patients with breast cancer [18] and tumor biology with a tendency toward osteolytic metastases [19].
The presence of significant bone destruction has been implicated as an indicator of poor prognosis for treatment outcomes [10]; however, the results of previous studies are inconsistent [9], [10], [11]. Zelefsky et al. showed that patients with compression fractures with greater than 50% of vertebral body height loss were less likely to show favorable clinical and radiological responses to RT [10], whereas Maranzano and Latini showed that neither the presence of vertebral body collapse nor the number of spinal compression sites influenced survival or the response to RT [11]. The study by Mitera et al. implied that the pain response after RT did not differ depending on the presence of a pathological fracture or any other imaging features related to the degree of tumor involvement [9]. Our results are to some extent consistent with these reports. Patients with widespread bony metastases (diffuse infiltration of bone marrow or large metastases) and patients with spinal cord compression prior to RT frequently present with more aggressive disease and, according to our data, tend to show poorer responses to RT, not statistically significant. The pain response rate to RT was similar in patients with vertebral fractures, fracture progression or other recorded MRI findings.
Importantly, the epidural tumor volume was reduced in all patients, and all but 3 patients showed an increase in the cross-sectional area of the spinal canal despite fracture progression. Additionally, there was excellent control of bone-only metastases; only 6% of the patients showed disease progression within the RT field, compared with 60% of the patients with progression of SM outside the RT field. The standard treatment protocol in patients included in this study involved a single posterior–anterior (PA) field. Using single PA beam treatment is common in the palliative irradiation of SM. However, dose coverage and heterogeneity may affect the treatment outcome, since distribution decreases in the anterior half of the vertebral body and anterior to the vertebral column [20]. This may partly explain the progression of bone lesions in 1 of 2 patients with progressive disease within the RT field.
The major limitation of this study remains restricted number of patients. Hence, the results should be verified in larger studies. Consequently, precisely predicting treatment response and identifying statistically significant groups is not feasible in small samples. Additional limitations include relatively broad time variations in the post-treatment MRI examinations – as a consequence of repeated MRIs not being part of the prospective study design [12].
In conclusion, RT provided effective local control of spinal metastatic disease in most patients, and our results indicate that most patients with SM benefit from RT even in cases of progressive vertebral fracture. The pain response rate did not correlate with any imaging findings; thus, performing MRI as a part of routine follow-up after RT is likely unnecessary for most patients except for those in whom clinical disease progression is suspected and for those with new symptoms.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Bjørn Naume, and Dr. Erik Løkkevik from the Department of Clinical Oncology, the Norwegian Radium Hospital for their helpful comments. This study was supported by grants from the Norwegian Radium Hospital Foundation, the Norwegian Cancer Society and the Regional Health Authority of South-Eastern Norway.
References
- 1.Chow E., Hoskin P., Mitera G., Zeng L., Lutz S., Roos D. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82:1730–1737. doi: 10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Chow E., Khan L.M., Bruland O.S. Radiotherapy of skeletal metastases. In: Rosen C.J., editor. Primer on the metabolic diseases and disorders of mineral metabolism. ASBMR; Washington (DC): 2013. pp. 754–759. [Google Scholar]
- 3.Lipton A. Clinical features of metastatic bone disease. In: Coleman R.E., Abrahamsson P.A., Hadji P., editors. Handbook of cancer-related bone disease. Bio Scientifica; Bristol: 2012. pp. 59–71. [Google Scholar]
- 4.Hartsell W.F., Scott C.B., Bruner D.W., Scarantino C.W., Ivker R.A., Roach M. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 5.Sande T.A., Ruenes R., Lund J.A., Bruland O.S., Hornslien K., Bremnes R. Long term follow-up of cancer patients receiving radiotherapy for bone metastases: results from a randomized multicentre trial. Radiother Oncol. 2009;91:261–266. doi: 10.1016/j.radonc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Steenland E., Leer J.W., van Houwelingen H., Post W.J., van den Hout W.B., Kievit J. The effect of a single dose fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 7.Sze W.M., Shelley M.D., Held I., Wilt T.J., Mason M.D. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systematic review of randomized trials. Clin Oncol (R Coll Radiol) 2003;15:345–352. doi: 10.1016/s0936-6555(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 8.van der Linden Y.M., Steenland E., van Houwelingen H.C., Post W.J., Oei B., Marijnen C.A. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiotherapy: results on survival in the Dutch Bone Metastasis Study. Radiother Oncol. 2006;78:245–253. doi: 10.1016/j.radonc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Mitera G., Probyn L., Ford M., Donovan A., Rubenstein J., Finkelstein J. Correlation of computed tomography imaging features with pain response in patients with spine metastases after radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:827–830. doi: 10.1016/j.ijrobp.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Zelefsky M.J., Scher H.I., Krol G., Portenoy R.K., Leibel S.A., Fuks Z.Y. Spinal epidural tumor in patients with prostate cancer. Clinical and radiographic predictors of response to radiation therapy. Cancer. 1992;70:2319–2325. doi: 10.1002/1097-0142(19921101)70:9<2319::aid-cncr2820700918>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Maranzano E., Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 12.Zaikova O., Fossa S.D., Kongsgaard U., Kvaloy S., Giercksky K.E., Skjeldal S. Pain after palliative radiotherapy for spine metastases. Clin Oncol (R Coll Radiol) 2010;22:828–836. doi: 10.1016/j.clon.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Klepstad P., Loge J.H., Borchgrevink P.C., Mendoza T.R., Cleeland C.S., Kaasa S. The Norwegian brief pain inventory questionnaire: translation and validation in cancer pain patients. J Pain Symptom Manage. 2002;24:517–525. doi: 10.1016/s0885-3924(02)00526-2. [DOI] [PubMed] [Google Scholar]
- 14.Tombal B., Rezazadeh A., Therasse P., Van Cangh P.J., Vande Berg B., Lecouvet F.E. Magnetic resonance imaging of the axial skeleton enables objective measurement of tumor response on prostate cancer bone metastases. Prostate. 2005;65:178–187. doi: 10.1002/pros.20280. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Switlyk M.D., Hole K.H., Skjeldal S., Hald J.K., Knutstad K., Seierstad T. MRI and neurological findings in patients with spinal metastases. Acta Radiol. 2012;53:1164–1172. doi: 10.1258/ar.2012.120442. [DOI] [PubMed] [Google Scholar]
- 17.Souchon R., Wenz F., Sedlmayer F., Budach W., Dunst J., Feyer P. DEGRO practice guidelines for palliative radiotherapy of metastatic breast cancer: bone metastases and metastatic spinal cord compression (MSCC) Strahlenther Onkol. 2009;185:417–424. doi: 10.1007/s00066-009-2044-2. [DOI] [PubMed] [Google Scholar]
- 18.Kanis J.A., McCloskey E.V., Powles T., Paterson A.H., Ashley S., Spector T. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79:1179–1181. doi: 10.1038/sj.bjc.6690188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozlow W., Guise T.A. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 20.Andic F., Baz Cifci S., Ors Y., Niang U., Dirier A., Adli M. A dosimetric comparison of different treatment plans of palliative spinal bone irradiation: analysis of dose coverage with respect to ICRU 50 report. J Exp Clin Cancer Res. 2009;28:2. doi: 10.1186/1756-9966-28-2. [DOI] [PMC free article] [PubMed] [Google Scholar]