Abstract
Bone metastases represent common long term complications of patients with breast cancer. Zoledronic acid, an amino-bisphosphonate and mevalonate pathway inhibitor, is an established agent for the treatment of bone metastases. Direct antitumor effects of zoledronic acid have been proposed in breast cancer. Statins are another group of mevalonate pathway inhibitors that have been repeatedly discussed for potential anti-tumor activity. In this study, we tested the hypothesis, whether these agents regulate adhesion of breast cancer cells to extracellular matrix components. Treatment of breast cancer cells with zoledronic acid and atorvastatin, significantly impaired MDA-MB-231 breast cancer cell adhesion on the αvβ3 ligands gelatin and vitronectin, but had no effect on collagen type 1 (α2β1-ligand) and fibronectin (α5β1-ligand). Anti-adhesive effects of zoledronic acid were fully reversed by geranylgeranyl pyrophosphate (GGPP), but not by farnesylpyrophosphate (FPP). Furthermore, effects of zoledronic acid and atorvastatin were mimicked by a specific inhibitor of geranylgeranylation GGTI-298. Functional (using integrin array) and quantitative (using FACS) integrin analyses on MDA-231 cells following zoledronic acid exposure revealed decreased levels of αv and αvβ3 expression. In addition to its effects on integrin mediated adhesion of breast cancer cells, the presence of zoledronic acid caused pronounced morphological changes in MDA-231 cells as seen by F-actin and vinculin rearrangement. Furthermore, phosphorylation of the focal adhesion kinase was inhibited by zoledronic acid. In both cases, changes were fully reversed by GGPP. These results emphasize the role of mevalonate pathway mediated impairment of geranylgeranylation in the anti-adhesive effects of zoledronic acid in breast cancer cells.
Keywords: Zoledronic acid, Atorvastatin, Mevalonate pathway, Adhesion, Breast cancer
1. Introduction
Breast cancer is the most prevalent malignancy in women, and the occurrence of bone metastases is a common long-term complication of this disease [1]. The pathophysiology of bone metastases is complex and a number of steps have to be surpassed by the cancer cell to successfully establish a metastatic lesion, including the adhesion of cancer cells at their metastatic site [2]. Bisphosphonates represent a standard therapy for patients with malignant bone lesions [3]. In addition to their established anti-resorptive effects, direct effects of amino-bisphosphonates on tumor biology have been proposed [4]. Established in vitro and in vivo effects of bisphosphonates on cancer cells include an induction of apoptosis, inhibition of proliferation, migration and invasion as well as anti-angiogenic effects [4], [5], [6]. In contrast to first generation bisphosphonates, which act by forming toxic ATP analogs, amino-bisphosphonates are inhibitors of the mevalonate pathway that block the farnesyl pyrophosphate (FPP) synthase [7]. FPP synthase inhibition leads to a decreased formation of isoprenoid lipids such as FPP and geranylgeranyl pyrophosphate (GGPP) and thereby impairs posttranslational protein prenylation [8]. In fact, many of the observed anti-tumor effects of bisphosphonates have been proposed to be mediated by inhibited protein geranylgeranylation [9]. Statins are the second major class of clinically approved compounds that act by mevalonate pathway inhibition [10]. Statins are widely used for their cholesterol-lowering effects, but have also been associated with potential direct anticancer effects [11].
Cancer cell adhesion is an important step of the metastatic cascade that is essential for the cancer to establish persistence at the site of metastasis. Mevalonate pathway inhibition may impair the adhesive abilities of circulating cancer cells and thereby impact their metastatic potential. Indeed, anti-adhesive effects of bisphosphonates have been described for several cell types, including breast cancer and HUVEC [12], [13], but the underlying molecular mechanisms have not been studied in detail. In this study we investigate the effects of zoledronic acid on integrin mediated adhesion of breast cancer cells in the context of mevalonate pathway inhibition.
2. Materials and methods
2.1. Cells and reagents
Human MDA-MB-231 breast cancer cells were purchased from ATCC (Manassas, VA). MDA-BONE cells (also known as MB-231-TxSA) were obtained from the University of Texas (San Antonio, USA). All cell lines were cultured in DMEM/Ham׳s F-12 (PAA, Pasching, Austria) with 10% fetal calf serum supreme (Lonza, Cologne, Germany) and 1% penicillin/streptomycin (PAA, Pasching, Austria). Cell line authenticity was determined by short tandem repeat profiling and by matching with the known profiles at DSMZ (German Collection of Microorganisms and Cell Cultures). Zoledronic acid, atorvastatin, mevalonate, geranylgeranyl-pyrophosphate (GGPP), farnesyl pyrophosphate (FPP), GGTI-298 and FTI-277 were obtained from Sigma-Aldrich (Munich, Germany). Zoledronic acid was solved in PBS. Mevalonate, FPP and GGPP were solved in methanol:NH4OH solution and atorvastatin, GGTI-298 and FTI-277 were solved in DMSO. Appropriate controls were added to untreated control cells. Breast cancer cell lines were treated with zoledronic acid, atorvastatin, FTI-277 and GGTI-298 for 24 h (unless otherwise indicated). Mevalonate substrates (GGPP, FPP and mevalonate) were supplemented 2 h prior to zoledronic acid treatment to reverse specific pathway inhibition.
2.2. Western blot
Western blot analyses were performed as previously described [14]. Briefly, cells were washed and scraped in a lysis buffer and quantified. Twenty µg of protein were loaded on a SDS–PAGE and transferred onto a 0.2 µm nitrocellulose membrane. Following blocking for 1 h with 5% non-fat dry milk in Tris-buffered saline with 1% tween-20 (TBS-T), membranes were incubated with the primary antibody overnight. After washing, the membrane was incubated for 1 h with the HRP-conjugated secondary antibody. Membranes were washed 3 times with TBS-T again, and proteins were visualized with Super Signal (Pierce, Bonn, Germany) enhanced chemiluminescence. Antibody for RAP1A (sc-1482) was from Santa Cruz (Heidelberg, Germany) and the RAS (610001) antibody was from BD Biosciences (Heidelberg, Germany). Antibodies for FAK and phosphoFAK (Tyr397) were from Cell Signaling Technology (Boston, USA).
2.3. Adhesion assay
The adhesion assay was performed using 96-well microplates coated with vitronectin (R&D Systems, Wiesbaden, Germany), gelatin, collagen or fibronectin (all from BD, Heidelberg, Germany). The microplates were rehydrated with 200 μl PBS/well for 30 min at room temperature prior to use and the PBS removed before adding the cells. MDA-231 breast cancer cells were treated with different inhibitors of the mevalonate pathway for 24 h as indicated. Cells were then stained with the fluorescent dye DilC12(3) (from BD Biosciences, Heidelberg, Germany) for 1 h and washed twice with PBS. DilC12(3) is a fluorescent tracer specifically designed to label viable cells for tumor cell invasion or migration assays. After carefully harvesting the cells with 0.0015 M EDTA/PBS, they were washed again with PBS, counted and reconstituted in DMEM. Cells (125,000/well) were then given on 96-well microplates coated with different surfaces and incubated at 37 °C for 1 h in a CO2 incubator allowing them to adhere. Afterwards the plates were washed gently 3 times with PBS to remove non adherent cells and 100 μl of DMEM added into the wells. The adherent cells were then quantified by their relative fluorescence signal with Fluostar Omega plate reader at 544/590 nm.
2.4. Integrin array
We used the Alpha/Beta (α/β) Integrin-Mediated Cell Adhesion Array Combo Kit (Chemicon/Merck Millipore, Schwalbach, Germany) to examine the adhesion of MDA-231 breast cancer cells in relation to their cell surface integrin subunit expression. This assay contains microplates coated with monoclonal antibodies against the human integrins α1–α5, αv, αvβ3, β1–β4, β6, αvβ5 and α5β1. The attachment to the different integrin subunits is used as an indirect indicator to assess functioning integrin cell surface expression. MDA-231 breast cancer cells were treated as indicated and harvested with 0.0015 M EDTA/PBS, counted and diluted separately to the final concentration of 1×106 cells/ml. Thereafter, 100 µl of the cell suspension was added to each of the anti-integrin coated wells and the control wells and incubated for 1 h at 37 °C. After incubation the wells were washed gently twice with assay buffer. Next, 150 µl of assay buffer and 50 µl of Lysis Buffer/Dye solution provided in the kit were adjoined to each well and incubated for 15 min at room temperature. Finally, 150 µl of the mixture were transferred to a 96-well plate and absorbance was determined with a Fluostar Omega plate reader at 485/530 nm.
2.5. Immunofluorescence
Immunofluorescence (IF) was performed as previously described [15]. Briefly, MDA-231 cells were disseminated on glass slides and treated with zoledronic acid, atorvastatin, GGTI-298, FTI-277 and GGPP as indicated. After washing the cells with PBS, they were fixed for 15 min with 4% paraformaldehyde/PBS and permeabilized after a triple washing step with PBS for 20 min using 0.1% Triton X-100/PBS. Afterwards they were washed again with PBS. Cells were then blocked with 1% BSA, 0.05% Tween/PBS for 1 h. After a triple washing step with PBS, 200 μl of 10 μg/ml Alexa-Fluor-488-Phalloidin (from Cell Signaling Technology, Boston, USA) and 2.5 μg/ml Vinculin (from Sigma-Aldrich, Munich, Germany) in 1% BSA, 0.05% Tween/PBS were added per well and incubated for 1 h at room temperature. Afterwards the cells were washed again three times with PBS and incubated for 1 h at room temperature with Alexa-Fluor-594 antibody (from Life Technologies, Darmstadt, Germany). Following a brief washing step with PBS, the cells were stained with 0.2 μg/ml DAPI for 5 min and then washed several times. The fluorescence-preserving mounting medium Dako was added to the slides to assess the cells thereafter using digital microscopy.
2.6. Flow cytometry
MDA-231 breast cancer cells were treated with zoledronic acid for 24 h, harvested with 0.0015 M EDTA/PBS and resuspended in FACS buffer (1% BSA/PBS). Cells were stained with an αvβ3-FITC antibody or with an αv (CD51) antibody (both from Chemicon/Merck Millipore, Schwalbach, Germany) for 1 h. The αv antibody was consecutively stained with Alexa Fluor 488 antibody (from Life Technologies, Darmstadt, Germany) for 1 h after a washing step with FACS buffer. After washing the cells three times they were resuspended in FACS buffer and analyzed with a FACSCalibur flow cytometer following a standard protocol.
2.7. Statistical analyses
Results are presented as means±standard deviation (SD). All experiments were repeated at least three times. Statistical evaluations were performed using a one-way ANOVA or a Student׳s T-test. P values<0.05 were considered statistically significant.
3. Results
3.1. Zoledronic acid and atorvastatin reduce breast cancer adhesion to the αvβ3 ligands gelatin and vitronectin
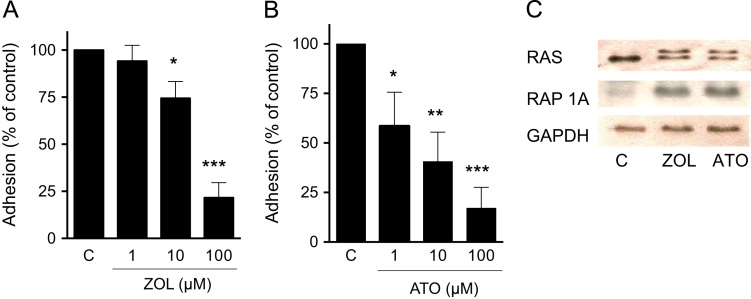
To assess the effects of mevalonate pathway inhibition on breast cancer cell adhesion, MDA-231 cells were pre-treated with increasing concentrations of zoledronic acid or atorvastatin for 24 h prior to the adhesion assay on gelatin. Both agents resulted in a dose dependent inhibition of adhesion, with stronger effects in atorvastatin treated cells (Fig. 1A and B). Effective mevalonate pathway inhibition was proven by assessment of immunoblotting of Rap1A (antibody detects only ungeranylated RAP1A) and RAS (second band indicates unfarnesylated RAS) (Fig. 1C).
Fig. 1.
Zoledronic acid and atorvastatin dose-dependently reduce breast cancer adhesion. ((A) and (B)) MDA-231 breast cancer cells were treated with zoledronic acid (1–100 µM) and atorvastatin (1–100 µM) for 24 h. Data are presented as the percentage adhesion on gelatin relative to control treated (PBS, DMSO) cells, and are mean±SD of 3 independent experiments. (C) Inhibition of mevalonate pathway was verified by assessment of RAS (arrow indicates unfarnesylated band) and ungeranylated RAP1A. GAPDH served as loading control. *p<0.05; **p<0.01; ***p<0.001.
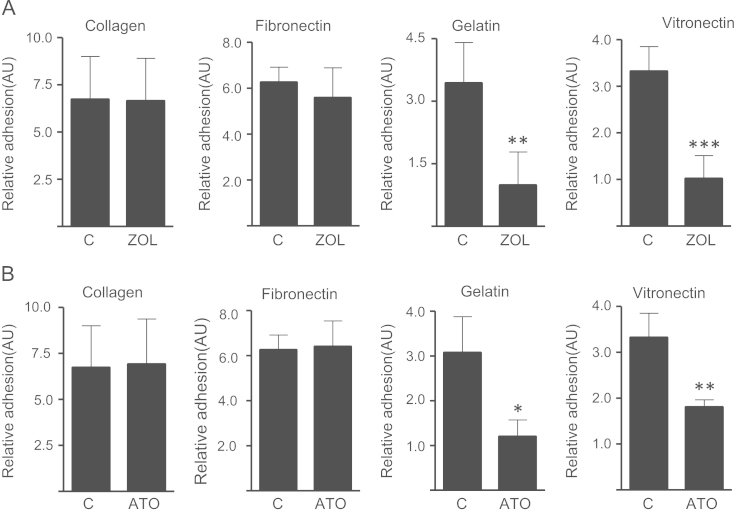
To further clarify the role of integrins in these anti-adhesive effects, adhesion assays of zoledronic acid and atorvastatin treated MDA-231 cells were repeated on different extracellular matrix components. Reductions in adhesion on vitronectin, another αvβ3-ligand, were comparable to those seen on gelatin. Treatment with zoledronic acid reduced adhesion by 69% and atorvastatin reduced adhesion by 45% (p<0.01). However, zoledronic acid and atorvastatin had no effects on collagen type 1 (α2β1-ligand) and fibronectin (α5β1-ligand) (Fig. 2). MDA-Bone cells, a highly osteotropic MDA-231 subclone, showed an even greater reduction in adhesion to gelatin upon exposure to mevalonate pathway inhibitors (Fig. 4A).
Fig. 2.
Zoledronic acid (A) and atorvastatin (B) decrease adhesion of breast cancer cells on αvβ3 ligands. Treatment with zoledronic acid (100 µM) or atorvastatin (10 µM) for 24 h reduces adhesion of MDA-231 breast cancer cells on gelatin and vitronectin (αvβ3-ligands) but not on collagen-I and fibronectin. Data are presented as the relative adhesion relative to control treated (PBS, DMSO) cells, and are mean±SD of 3–4 independent experiments. *p<0.05; **p<0.01; ***p<0.001.
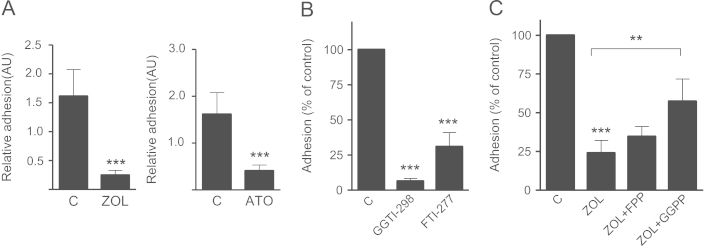
Fig. 4.
Inhibition of mevalonate pathway inhibits adhesion of MDA-Bone breast cancer cells. (A) MDA-Bone cells were treated with zoledronic acid (100 µM) and atorvastatin (10 µM) for 24 h. Both reduced the adhesion on gelatin significantly. Data are presented as the relative adhesion relative to control treated (PBS, DMSO) cells, and are mean±SD of 3–4 independent experiments. (B) MDA-Bone cell adhesion on gelatin was analyzed after treatment with 5 µM GGTI-298 or 0.1 µM FTI-277 for 24 h. (C) MDA-Bone cells were treated either with FPP (100 µM) or GGPP (100 µM) 2 h prior to zoledronic acid (100 µM) or zoledronic acid alone. Data are presented as the percentage adhesion relative to control treated cells, and are mean±SD of 3 independent experiments. **p<0.01; ***p<0.001.
3.2. Anti-adhesive effects of zoledronic acid and atorvastatin are mediated by inhibition of geranylgeranylation
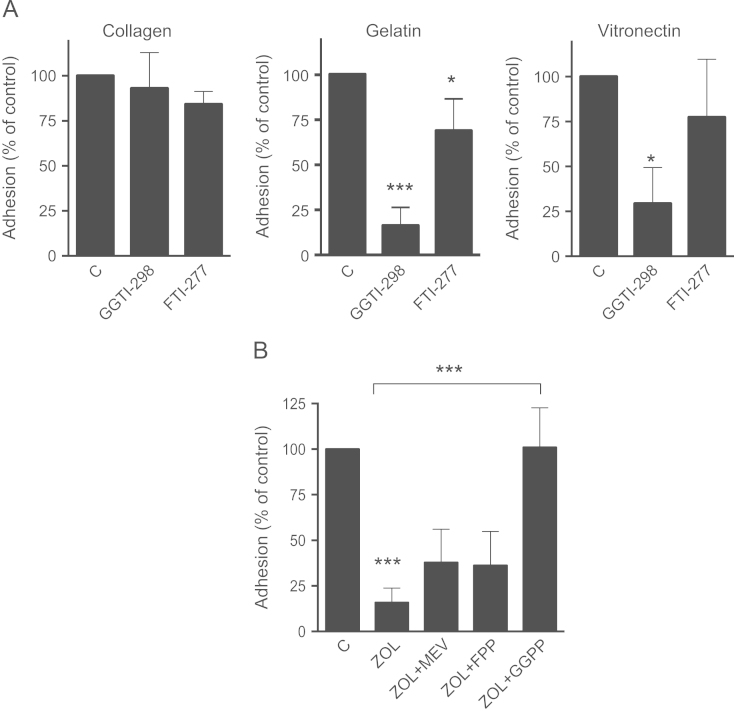
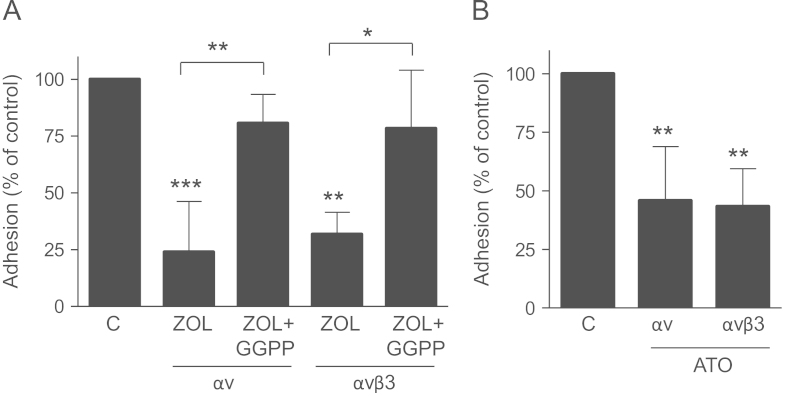
The role of the mevalonate pathway in this observed effect was specified by treating MDA-231 cells with specific inhibitors of farnesylation (FTI-277) and geranylgeranylation (GGTI-298). GGTI-298 caused a strong reduction of adhesion on gelatin (p<0.001) and vitronectin (p<0.05), but had no effects on collagen type 1. Inhibition of farnesylation only had modest effects on MDA-231 adhesion on both gelatin and vitronectin (Fig. 3A). Furthermore, the anti-adhesive effect of zoledronic acid on gelatin could be completely reversed by supplementing geranylgeranyl pyrophosphate. Other mevalonate pathway metabolites such as mevalonate and farnesyl pyrophosphate did not restore the effects of zoledronic acid (Fig. 3B). These results emphasize the dominant role of impaired geranylgeranylation for the observed anti-adhesive effects. Of note, both FTI-277 and GGTI-298 strongly reduced the adhesion of MDA-Bone cells (Fig. 4B). The effect of zoledronic acid was significantly, although not fully reversed by GGPP (Fig. 4C).
Fig. 3.
Anti-adhesive effects are mediated by inhibited geranylgeranylation. (A) MDA-231 cells were treated with 5 µM GGTI-298 or 0.1 µM FTI-277 for 24 h. Adhesion on type I collagen, gelatin and vitronectin coated plates was assessed afterwards. (B) MDA-231 cells were treated either with mevalonate (10 µM), FPP (100 µM) or GGPP (100 µM) 2 h prior to zoledronic acid (100 µM) or zoledronic acid alone. Data are presented as the percentage adhesion relative to control treated cells, and are mean±SD of 3–4 independent experiments. *p<0.05; ***p<0.001.
3.3. Integrin array
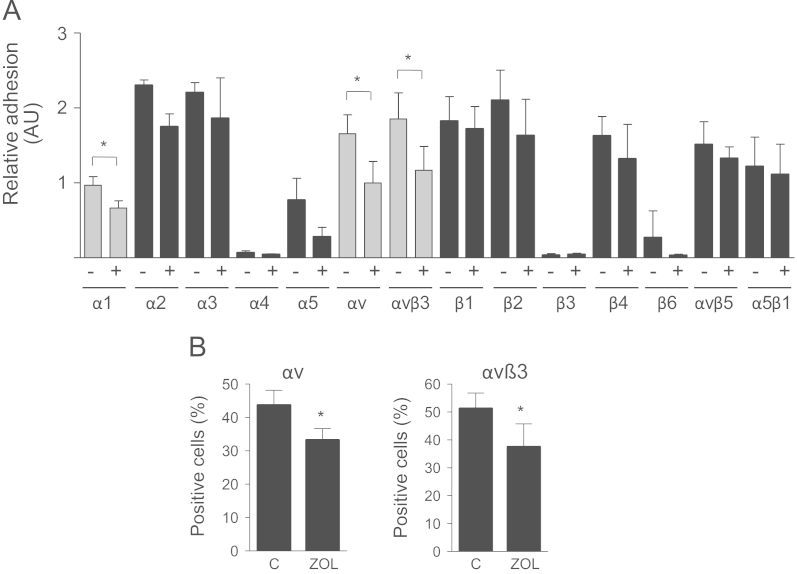
To test whether bisphosphonates have an effect on integrin function, cell surface integrins were analyzed using an α/β integrin-mediated cell adhesion kit. Baseline adhesion to different antibodies varied greatly, indicating differences in integrin expression (Fig. 5A). Adhesion to α4, β3 and β6 was marginal. On the other hand there was a strong attachment to α2, α3, αv, αvβ3, β1, β2, β4, αvβ5 and α5β1 indicating a strong surface expression on MDA-231 cancer cells. After exposure to zoledronic acid the expression of αv and αvβ3 decreased significantly by 40% and 34%, respectively (p<0.05). Of note, adhesion to integrin α5, which showed a lower baseline expression than αv and αvβ3, was also significantly reduced. Other integrins were not significantly affected by treatment with zoledronic acid (Fig. 5A). Expression of αv and αvβ3 on MDA-231 cells was further assessed by flow cytometry. In both cases the number of positively labeled cells significantly decreased by 23% and 25%, respectively (p<0.05) (Fig. 5B). The effect of zoledronic acid on the expression of αv and αvβ3 was largely reversed by the addition of GGPP (Fig. 6A). Atorvastatin also reduced the adhesion of MDA-231 cells to αv and αvβ3 integrins by 54% and 56% (p<0.01) (Fig. 6B).
Fig. 5.
Zoledronic acid reduces the expression of αv and αvβ3. (A) MDA-231 cells were treated with zoledronic acid (100 µM), and adhesion to different integrins was analyzed. Adhesion to α1, αv and αvβ3 decreased after treatment. Data are presented as relative adhesion compared with control treated (PBS, DMSO) cells, and are mean±SD of 3 independent experiments. (B) After treatment with zoledronic acid (100 µM) expression of αv and αvβ3 on MDA-231 cells was assessed using flow cytometry. Data are presented as percentage of positive cells compared with untreated control cells, and are mean±SD of 3–4 independent experiments. *p<0.05.
Fig. 6.
Atorvastatin decreases the expression of αv and αvβ3 and zoledronic acid impairs integrin expression via inhibited geranylgeranylation. (A) MDA-231 cells were treated with GGPP (100 µM) 2 h prior to zoledronic acid (100 µM) or zoledronic acid alone and adhesion to the integrins αv and αvβ3 was assessed afterwards. (B) The adhesion of MDA-231 cells to the integrins αv and αvβ3 was analyzed after treatment with Atorvastatin (10 µM) for 24 h. Atorvastatin reduced the expression of both integrins significantly. Data are presented as the percentage adhesion relative to control treated cells, and are mean±SD of 3–4 independent experiments. *p<0.05; **p<0.01; ***p<0.001.
3.4. Modification of focal adhesion kinase (FAK) and cell morphology
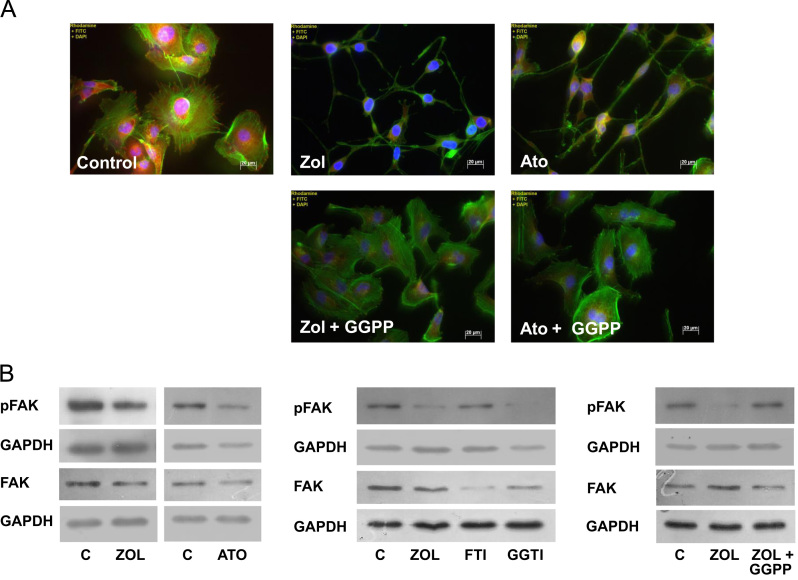
Zoledronic acid and atorvastatin resulted in profound morphological changes in the cytoskeleton of MDA-231 as assessed by immunofluorescence staining of vinculin and f-actin. After 24 h of exposure to mevalonate pathway inhibitors, MDA-231 cells developed a pronounced spindle-like morphological appearance. These changes were fully compensated when supplementing GGPP (Fig. 7A).
Fig. 7.
Inhibition of mevalonate pathway modifies cell morphology and impairs FAK activity in breast cancer cells. (A) MDA-231 cells were treated with zoledronic acid (100 µM) or atorvastatin (10 µM) and GGPP (100 µM). Cell morphology and modification of F-actin and vinculin were assessed by immunofluorescence. (B) After treatment with zoledronic acid alone or with GGPP, atorvastatin, FTI-277 or GGTI-298 as above, FAK activity was assessed by Western Blot analyzing. GAPDH is used as a loading control.
We next assessed the impact of zoledronic acid on focal adhesion kinase (FAK) and cell morphology. Exposure to zoledronic acid reduced the phosphorylation of FAK in MDA-231 cells. Again, this effect was directly mediated by inhibited geranylgeranylation, as it was mimicked by atorvastatin and GGTI-298 and completely restored when co-incubated with GGPP (Fig. 7B). Treatment with FTI-277 did not influence pFAK.
4. Discussion
The adhesion of disseminated cancer cells to their target structure is an essential step of the metastatic process. Both bisphosphonates and statins have been discussed for their direct anti-tumor effects [16], [17]. Here, we assessed the influence of zoledronic acid and atorvastatin on the adhesion of two ER-negative breast cancer cell lines to extracellular matrix proteins. While these agents are primarily used for divergent indications, namely anti-resorptive therapy and cholesterol reduction, they have a common target by inhibiting the mevalonate pathway and a considerable overlap regarding preclinical anti-tumor activity has been noted [5], [18]. Anti-adhesive effects of bisphosphonates in breast cancer have been previously described [19], but the role of the mevalonate pathway in this context has not been described in detail. Prenylation processes are important for the biological activity of numerous cellular signal transduction proteins. By blocking the mevalonate pathway both statins and nitrogen-containing bisphosphonates impair farnesylation and geranylation. In this study, we show that the observed anti-adhesive effects of bisphosphonates are directly mediated by an inhibition of post-translational geranylgeranylation as the effects are fully reversed in the presence of GGPP, which restores geranylgeranylation. Furthermore, the specific inhibition of geranylation using GGTI-298 has similar effects as zoledronic acid, whereas inhibition of farnesylation has no effects. This is in line with a number of previous studies, which have described most anti-tumor effects of bisphosphonates to be mediated by an inhibited geranylgeranylation rather than an inhibition of farnesylation [20]. In combination with the previously described anti-angiogenic effects of zoledronic acid [14], these anti-adhesive effects may negatively influence the metastatic potential of breast cancer cells. Of note, effects were greater in the highly osteotropic MDA-Bone cells, indicating a potential higher sensitivity in breast cancer cells with a higher metastatic risk.
Cell adhesion to extracellular matrix requires the interaction of different molecules like selectins, cadherins and integrins [21]. Especially αvβ3 integrin has been proposed to mediate the adhesion of malignant cells in different entities such as multiple myeloma and breast cancer [22], [23]. Upon exposure to mevalonate pathway inhibitors we observed an impaired adhesion of breast cancer cells that was exclusive to αvβ3 ligands. Consequently, decreased αvβ3 expression was confirmed with two different methods. In HUVEC decreased cell surface levels of αvβ3 and αvβ5 following treatment with zoledronic acid have been previously reported [24] and these effects have been linked to an inhibition of prenylation dependent pathways [12].
This study did not investigate the impact of mevalonate pathway inhibition on the adhesion of estrogen receptor negative breast cancer cells, which is a study limitation. Furthermore, the limiting factor of translating preclinical anti-tumor effects of zoledronic acid are the high concentrations required to achieve these results. Concentrations between 10 to 100 µM are commonly required in vitro to achieve consistent effects and although these concentrations may theoretically accumulate in the bone, they are certainly not reached in serum where bisphosphonate concentrations decline rapidly (within hours) following infusion. In this study zoledronic acid concentrations of 10 µM were required to yield a robust cellular response. Interestingly, considerably lower concentrations of atorvastatin were needed to achieve comparable results. Although statins are not established agents in the treatment of cancer, there is increasing evidence that statins may have tumor modulating activity in vitro [25] and in vivo [26], [27]. Furthermore, some observational studies have shown a reduction in cancer-related mortality by statins [28]. Atorvastatin potently suppressed the adhesion of breast cancer cells in this study supporting the potential anti-cancer effects of statins. The higher potency of statins compared to bisphosphonates may have clinical implications as the high concentrations of zoledronic acid required to achieve similar results may limits its translational potential.
In summary, our results demonstrate that mevalonate pathway inhibition by bisphosphonates and statins exerts anti-adhesive activity on breast cancer cells by impairing αv and αvβ3 expressions. This effect is mediated by inhibited geranylgeranylation and warrants further research on the anti-tumor effects of mevalonate pathway inhibitors including statins which displayed a higher potency in this study than zoledronic acid.
Role of the funding source
This work was supported by the MedDrive start-up grant from the TU Dresden to TDR, and grants RA 2151/2-1 (to TDR and LCH) and Forschergruppe-1586 SKELMET to LCH from the Deutsche Forschungsgemeinschaft.
Conflict of interest statement
The authors have received grants or honorarium for advisory boards or lectures to the individual or the institution by Amgen (TDR, LCH, PH), AstraZeneca (PH), Eli Lilly (PH), GlaxoSmithKline (PH), Novartis (TDR, LCH, PH), Pfizer (PH), Roche (PH), Servier (LCH), Merck (LCH, TDR), and Nycomed (LCH). MW, AG, MR, SF, NS and PBM declare that they have no conflict of interest.
References
- 1.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Mundy G.R., Yoneda T., Hiraga T. Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment. Semin Oncol. 2001;28:35–44. doi: 10.1016/s0093-7754(01)90263-5. [DOI] [PubMed] [Google Scholar]
- 3.Body J.J., Bartl R., Burckhardt P., Delmas P.D., Diel I.J., Fleisch H. Current use of bisphosphonates in oncology. Int Bone Cancer Study Group J Clin Oncol. 1998;16:3890–3899. doi: 10.1200/JCO.1998.16.12.3890. [DOI] [PubMed] [Google Scholar]
- 4.Gnant M., Clézardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev. 2012;38:407–415. doi: 10.1016/j.ctrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Morgan G., Lipton A. Antitumor effects and anticancer applications of bisphosphonates. Semin Oncol. 2010;37:30–40. doi: 10.1053/j.seminoncol.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Clezardin P. Potential anticancer properties of bisphosphonates: insights from preclinical studies. Anti-Cancer Agents Med Chem. 2012;12:102–113. doi: 10.2174/187152012799014977. [DOI] [PubMed] [Google Scholar]
- 7.Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 8.Roelofs A.J., Thompson K., Gordon S., Rogers M.J. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 9.van de Donk N.W., Lokhorst H.M., Nijhuis E.H., Kamphuis M.M., Bloem A.C. Geranylgeranylated proteins are involved in the regulation of myeloma cell growth. Clin Cancer Res. 2005;11:429–439. [PubMed] [Google Scholar]
- 10.Istvan E.S., Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 11.Osmak M. Statins and cancer: current and future prospects. Cancer Lett. 2012;324:1–12. doi: 10.1016/j.canlet.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Hasmim M., Bieler G., Rüegg C. Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation-dependent signaling pathways. J Thromb Haemost. 2007;5:166–173. doi: 10.1111/j.1538-7836.2006.02259.x. [DOI] [PubMed] [Google Scholar]
- 13.Dedes P.G., Ch Gialeli, Tsonis A.I., Kanakis I., Theocharis A.D., Kletsas D. Expression of matrix macromolecules and functional properties of breast cancer cells are modulated by the bisphosphonate zoledronic acid. Biochim Biophys Acta. 2012;1820:1926–1939. doi: 10.1016/j.bbagen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Rachner T.D., Göbel A., Junker M., Hötzel J., Benad-Mehner P., Hadji P. Regulation of VEGF by mevalonate pathway inhibition in breast cancer. J Bone Oncol. 2013;2:110–115. doi: 10.1016/j.jbo.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salbach J., Kliemt S., Rauner M., Rachner T.D., Goettsch C., Kalkhof S. The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways. Biomaterials. 2012;33:8418–8429. doi: 10.1016/j.biomaterials.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Green J.R. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 17.Graaf M.R., Richel D.J., van Noorden C.J., Guchelaar H.J. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Demierre M.F., Higgins P.D., Gruber S.B., Hawk E., Lippman S.M. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 19.Boissier S., Magnetto S., Frappart L., Cuzin B., Ebetino F.H., Delmas P.D. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res. 1997;57:3890–3894. [PubMed] [Google Scholar]
- 20.Goffinet M., Thoulouzan M., Pradines A., Lajoie-Mazenc I., Weinbaum C., Faye J.C. Zoledronic acid treatment impairs protein geranyl-geranylation for biological effects in prostatic cells. BMC Cancer. 2006;6:60. doi: 10.1186/1471-2407-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D.M., Feng Y.M. Signaling mechanism of cell adhesion molecules in breast cancer metastasis: potential therapeutic targets. Breast Cancer Res Treat. 2011;128:7–21. doi: 10.1007/s10549-011-1499-x. [DOI] [PubMed] [Google Scholar]
- 22.Ria R., Vacca A., Ribatti D., Di Raimondo F., Merchionne F., Dammacco F. Alpha(v)beta(3) integrin engagement enhances cell invasiveness in human multiple myeloma. Haematologica. 2002;87:836–845. [PubMed] [Google Scholar]
- 23.Sloan E.K., Pouliot N., Stanley K.L., Chia J., Moseley J.M., Hards D.K. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellahcène A., Chaplet M., Bonjean K., Castronovo V. Zoledronate inhibits alphavbeta3 and alphavbeta5 integrin cell surface expression in endothelial cells. Endothelium. 2007;14:123–130. doi: 10.1080/10623320701347187. [DOI] [PubMed] [Google Scholar]
- 25.Wong W.W., Dimitroulakos J., Minden M.D., Penn L.Z. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 26.Knox J.J., Siu L.L., Chen E., Dimitroulakos J., Kamel-Reid S., Moore M.J. Phase I trial of prolonged administration of lovastatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or of the cervix. Eur J Cancer. 2005;41:523–530. doi: 10.1016/j.ejca.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Kawata S., Yamasaki E., Nagase T., Inui Y., Ito N., Matsuda Y. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886–891. doi: 10.1054/bjoc.2000.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen S.F., Nordestgaard B.G., Bojesen S.E. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]