TEXT
Since the central nervous system was first demonstrated to be involved in regulation of gastric function by Pavlov[1], and Selye[2] established the stress theory that physicopsychiatric stress altered physiological functions[3], electrical stimulation or lesion of specific brain nuclei identified specific sites in the hypothalamus, limbic system, and medulla that influence gastrointestinal functions. A plethora of peptides have been characterized in the brain by immunohistochemical and molecular biological techniques[4]. The development of retrograde tracing techniques combined with immunohistochemistry reveals that these peptides are localized in the nerve fibers or cell bodies of the hypothalamus and medulla which are important sites for autonomic nervous outflow to the gastrointestinal tract[5,6]. Based on these studies, Taché first reported the effect of central neuropeptides in regulation of gastric functions[7,8]. Since then, more than 40 peptides have been examined and it is well established that many neuropeptides, such as thyrotropin releasing hormone (TRH), corticotropin releasing factor (CRF), neuropeptide Y (NPY), bombesin and somatostatin, mediate a central nervous system induced stimulation or inhibition of gastrointestinal function[9,10]. On the other hand, the liver is also richly innervated[11,12] and retrograde tracing technique has revealed hepatic innervation through the vagus originating in the medulla[13], where abundant neuropeptides exist. This review introduces the current knowledge of central nervous system regulation of hepatic functions by various neuropeptides.
THYROTROPIN RELEASING HORMONE (TRH)
TRH exists in the central nervous system and abundant TRH immunoreactive nerve terminals and TRH receptors are localized in the dorsal vagal complex including the vagal motor nucleus and the nucleus of the solitary truct[14,15]. Neuropharmachological studies demonstrated that TRH displayed a vast array of central nervous system-mediated actions unrelated to its physiological roel in the regulation of the pituitary thyroid axis[16]. TRH injected into the cerebrospinal fluid or into specific brain nuclei exerted a variety of behavioral effects. Accumulated evidence also demonstrated that TRH had potent central nervous system mediated stimulatory effects on gastrointestinal secretion, motility and transit, as well as on the development of gastric ulceration in rats and other animals[7,8,17,18] Mapping studies using microinjection of TRH or TRH analogs into selective nuclei have identified brain sites important for stimulation of gastric secretion and motility. The gastric secretory response has been elicited by microinjection of TRH into the lateral and the ventromedial hypothalamus[19]. More sensitive sites have been identified in the brainstem including the dorsal vagal complex, the nucleus ambiguus and the raphe pallidus[20-22].
Hepatic blood flow is composed of hepatic arterial and hepatic portal blood supplies. In rats portal blood flow constitutes 80% of hepatic circulation. Portal blood flow was altered by electrical stimulation of autonomic nerves and specific brain nuclei which are important sites for autonomic nervous regulation[23]. Stimulation of sympathetic nerves caused constriction of hepatic arterial and portal vessels, resulting in a decrease of liver blood volume and flow in hepatic artery, and an increase in portal pressure[24]. Electrical stimulation of the hypothalamus produced changes in intestinal blood flow and, consequently, in portal vein and in intrahepatic arterial and portal beds[25]. Moreover, stimulation of the medial and posterior hypothalamus has been reported to increase hepatic arterial resistance and decrease portal blood flow through sympathetic nerves[26]. On the other hand, using an in vivo microscopic technique in rats, dilatation of the liver sinusoid following electrical vagal stimulation and acetylcholine application has been observed[27,28].
We studied the effect of central TRH analog on hepatic microcirculation in anesthetized rats[29]. We used the stable TRH analog, RX 77368, which has the same receptor affinity and about the times the potency of natural TRH. In rats under urethan anesthesia, hepatic microcirculation was assessed by the hydrogengas clearance method. Intracisternal injection (the injection into the cisternal magna of the brain) of TRH analog induced stimulation of hepatic blood flow by 17%-74% in a dose dependent manner ranging from 5 ng to 100 ng. This stimulatory response of hepatic blood flow to central TRH analog occurred during the first 15-min observation period, reached a plateau at 30 min and returned to basal value at 60 min. On the other hand, intravenous injection of the TRH analog did not modify hepatic blood flow, confirming the central but not peripheral action of the TRH analog. Although this increased hepatic blood flow by central TRH analog was not modified by spinal cord transection, it was reversed by atropine, vagotomy, and N-G-nitro-L-arginine methyl ester, an inhibitor of nitric oxide and indomethacin, indicating that TRH acted in the brain to stimulate hepatic blood flow through vagal, muscarinic, prostaglandin and nitric oxide pathways. Mapping studies by microinjection of TRH analog into the medullary nuclei revealed that the left but not right dorsal vagal complex was a responsive site for TRH on modulation of hepatic blood flow[30]. This finding agreed well with anatomical evidence that hepatic vagal nerve is originated in the left, but not right, dorsal vagal complex[13].
Although in adult animals the liver is normally in a state of growth arrest, once the liver is damaged or impaired because of hepatic injury or liver resection, hepatic regeneration or proliferation is immediately started. Seventy percent partial hepatectomy[31] performed in rats initiated a very striking response in the liver remnant through hypertrophy and hyperplasia, and returned to its original size in 7-10 d[31]. Hepatocytes in the non stimulated liver were essentially arrested in the G0 state. After partial hepatectomy, growth related events started almost instantly as the cells underwent the transition from G0 to G1 state(prereplicative phase), which lasted about 12 h, at which point DNA synthesis (S state) began and peaked at about 24 h. Mitosis a similar course 6-8 h later[32]. Many factors, such as hormones, peptides and cytokines, were thought to be involved and may interact synergistically to initiate and maintain hepatic proliferation. The autonomic nervous system was also suggested to play a role in the liver regeneration and regulation of hormones and growth factors related to hepatic proliferation. Partial hepatectomy suppressed the sympathetic nerve activity[33] and plasma adrenaline and noradrenaline increased immediately after partial hepatectomy and α-blockade reversed DNA synthesis induced by hepatectomy[34,35], indicating the involvement of an adrenergic effect on hepatic regeneration. The parasympathetic nervous system was also suggested to play an important role in hepatic proliferation. Subdiaphragmatic vagotomy more strongly suppressed DNA synthesis after partial hepatectomyas compared with the splanchnicectomy[36], and selective hepatic branch vagotomy suppressed or delayed liver regeneration in partially hepatectomized rats[37]. Moreover, lesion of the ventromedial hypothalamus has recently induced hepatic DNA synthesis through the vagal nerve[38].
The effect of central administration of TRH analog, RX 77368, on hepatic proliferation has recently been studied in conscious adult rats[39], because central TRH was known to activate vagal efferent fibers[40,41]. Rats was injected with TRH analog intracisternally and hepatic DNA synthesis was assessed by thymidine incorporation into the hepatic DNA fraction and BrdU accumulation 6-72 h later. Hepatic proliferation was stimulated by intracisternal TRH analog (10 ng) with a peak response at 48 h after peptide injection and returned to basal at 72 h. This stimulatory effect by central TRH on hepatic proliferation was dose related, ranging from 5 ng to 100 ng assessed at 24 h. Intravenous TRH analog did not influence hepatic proliferation, confirming a central but not peripheral action of TRH. Stimulation of hepatic proliferation by central TRH was abolished by hepatic branch vagotomy, atropine and indomethacin, suggesting that TRH acts in the brain to stimulate hepatic proliferation through vagal, muscarinic and prostaglandin pathways. However, N-G-nitro-L-arginine methyl ester did not reverse central TRH induced stimulation of hepatic proliferation as it did central TRH induced hepatic circulation, indicating that stimulation of hepatic proliferation is not secondary to the change in hepatic circulation. These data suggest that TRH in the central nervous system may be involved in the vagal regulation of hepatic proliferation.
These findings led us to speculate that central TRH might also protect against experimental liver damage, so effect of central injection of TRH on CCl4 induced liver injury has been investigated[42]. Rats were coadministered CCl4 (2 mL/kg, ip) with TRH analog injected intracisternally and liver damage was assessed by serum alanine aminotransferase (ALT) levels. Intracisternal, bnt not intravenous, injection of TRH analog dose-dependently protected against CCl4 induced liver damage and this protective effect of central TRH was also block by hepatic vagotomy.
CORTICOTROPIN RELEASING FACTOR (CRF)
CRF is one of the brain neuropeptides, and effect of central CRF on physiological, pharmacological, and pathophysiological regulations of the gastrointestinal tract have been reported. Injection of CRF into the cerebrospinal fluid or the brain nuclei, such as paraventricular nucleus or loecus ceruleus inhibited gastric motility and secretion[43-46], and enhanced colonic motility through the autonomic nervous system[47,48]. Since CRF is known to act in the hypothalamus and stimulate the sympathetic nervous outflow, the opposite effect to TRH on hepatic function was expected when injected in the central nervous system.
We studied the effect of central CRF on hepatic microcirculation in anesthetized rats[49]. In rats under urethan anesthesia, hepatic microcirculation was assessed by the hydrogen gas clearance method and laser Doppler. Intracisternal injection of CRF induced inhibition of hepatic blood flow by 18%-36% in a dose dependent manner, ranging from 1 μg to 5 μg. This inhibitory response of hepatic blood flow to central CRF was noted during the first 15 min observation period, reached a plateau at 30-60 min and maintained for more than 120 min. On the other hand, intravenous injection of the CRF did not modify hepatic blood flow, confirming the central but not peripheral action of the TRH analog. Although this deceased hepatic blood flow by central CRF was not modified by atropine and vagotomy, it was reversed by hepatic sympathectomy and 6-hydroxydopamine, which depleted noradrenergic fibers, indicating that CRF acted in the brain to inhibit hepatic blood flow through sympathetic and noradrenergic pathways. The effect of central injection of CRF on CCl4 induced liver injury has also been investigated[50]. Rats were injected with CCl4 (2 mL/kg, sc) and CRF was injected just before and 6 h after CCl4 administration. Liver damage was assessed by serum ALT levels. Intracisternal, injection of CRF dose dependently aggravated CCl4 induced liver damage and this aggravating effect of central CRF was block by 6-hydroxydopamine and chemical sympathectomy.
NEUROPEPTIDE Y (NPY)
NPY, a 36 amino acid peptide of the pancreatic polypeptide family, was first isolated from porcine brain[51,52]. NPY was localized mainly in the peripheral nervous system[53], where it contributed to the innervation of the digestive organs, including the biliary tree[54]. In the brain, NPY nerve fibers and terminals, and NPY receptors were localized in the paraventricular nucleus of the hypothalamus and the dorsal vagal complex[55-57] which are important sites for the autonomic nervous system[58]. Central administration of NPY affected feeding behavior and visceral function[57]. The bile duct was richly innervated by autonomic nerves[59] and electrical stimulation of sympathetic and parasympathetic nerves and stimulation or lesion of certain hypothalamic regions[60] altered bile secretion[61,62]. With respect to the gastrointestinal tract, injection of NPY into the cerebrospinal fluid stimulates gastric acid and pepsin secretion, and pancreatic exocrine and endocrine secretion in rats and dogs[63-65]. Farouk et al[66] and we[67] have found an effect of central NPY on bile secretion in dogs and rats. The effect of intracisternal injection of NPY on bile secretion in urethan anesthetized rats was investigated[67]. Rats were anesthetized with urethan (1.5 g/kg, ip) and the common bile duct was cannulated to collect bile samples. Sodium taurocholic acid (30 μmol·kg·h) was infused intravenously to compensate for bile acid loss due to biliary drainage. Intracisternal NPY (0.02 nmol-0.12 nmol) dose-dependently stimulated bile secretion by 9%-20%. The secretory response occurred within the first 20-40 min after injection and lasted 120 min. On the other hand, intravenous injection of NPY (0.12 nmol) did not modify bile secretion, confirming that NPY did not leak from the cerebrospinal fluid to the peripheral circulation. Thus, central NPY stimulated bile secretion in a bile acid independent and bicarbonate dependent bile flow because central NPY increased only biliary bicarbonate secretion but not biliary bile acid, phospholipid or cholesterol secretion. In other words, central NPY stimulated ductal bile secretion. Although cervical cord transection, bilateral adrenalectomy or pretreatment with N-G-nitro-L-arginine methyl ester did not alter intracisternal NPY induced stimulation of bile secretion, atropine and bilateral cervical vagotomy completely abolished the stimulatory effect of intracisternal NPY on bile secretion, indicating that NPY acted in the brain to stimulate bile acid independent bile secretion through vagal and muscarinic pathways. Mapping studies by microinjection of NPY into the medullary nuclei have shown that the left dorsal vagal complex is a responsive site, like TRH on hepatic circulation, for NPY on stimulation of bile secretion[68].
OTHER NEUROPEPTIDES
Besides TRH, CRF and NPY, a few peptides were suggested to act in the central nervous system to modulate hepatobiliary function. β-endorphin has produced 70% inhibition in liver DNA synthesis in six day old rats[69]. Intracerebroventricular injection of bombesin with a dose of 10 μg induces bicarbonate dependent inhibition of bile secretion in rats[70]. Moreover, intracisternal injection of opioid peptide, DAla-Met enkephalinamide decreased bile secretion in urethan anesthetized rats[71].
SUMMARY AND CONCLUSIONS
Several peptides have been established to act in the brain to influence hepatic function (Figure 1). Bile secretion is modified by central administration of bombesin, opioid peptide and NPY, hepatic blood flow is altered by central CRF and TRH, hepatic proliferation is regulated by central β-endorphin and TRH, and central CRF and TRH interfere experimental liver injury. Among these peptides central TRH is the strongest candidate for playing an important role in hepatic physiological function. Through their use, new knowledge on central and peripheral mechanisms underlying brain regulation of hepatic function will be revealed. Further studies in regard to the physiological relevance of the central action of neuropeptides on specific brain sites should be performed for unraveling the underlying pathways mediating brain liver interaction.
Figure 1.
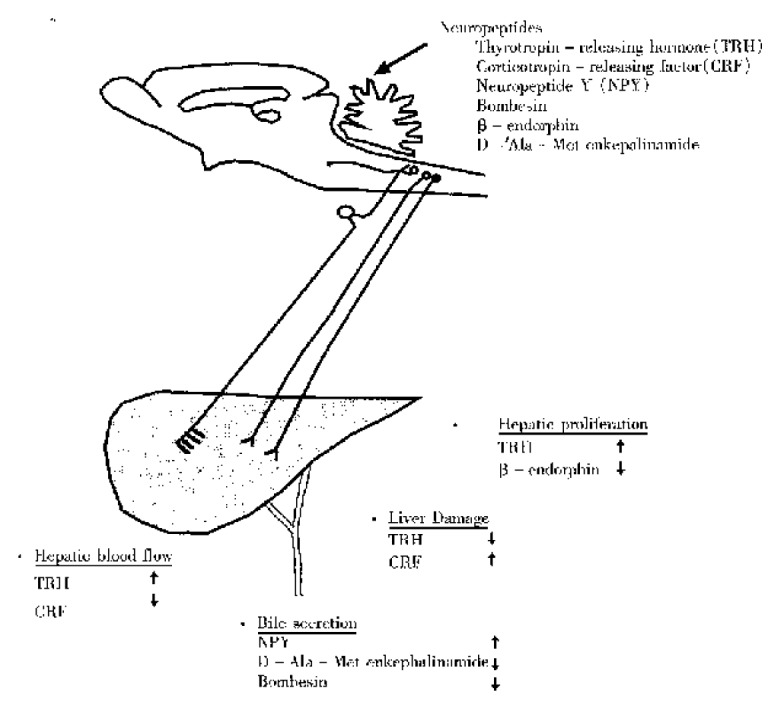
Schematic illustration of interaction bween neuropeptides in the central nervous system and hepatobiliary function.
ACKNOWLEDGEMENT
This work was supported by Japan Research Foundation for Clinical Pharmacology, the Akiyama Foundation, Kanae Memorial Foundation and the Ministry of Education, Culture and Science of Japan (No. 0767554, No. 09670503).
References
- 1.Pavlov I. The work of the digestive glands. (English translation. London: C. Griffin & Co. 1910 [Google Scholar]
- 2.Selye H. Syndrome produced by diverse nocuos agents. Nature. 1932;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 3.Brooks FP. Central neural control of acid secretion. In: C.F. Code, eds. Hand-book of physiology.Washington, D.C.: American Physiological Society. 1967:805. [Google Scholar]
- 4.Krieger DT. Brain peptides: what, where, and why? Science. 1983;222:975–985. doi: 10.1126/science.6139875. [DOI] [PubMed] [Google Scholar]
- 5.Leslie RA. Neuroactive substances in the dorsal vagal complex of the medulla oblongata: nucleus of the tractus solitarius, area postrema, and dorsal motor nucleus of the vagus. Neurochem Int. 1985;7:191–211. doi: 10.1016/0197-0186(85)90106-8. [DOI] [PubMed] [Google Scholar]
- 6.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 7.Taché Y, Vale W, Rivier J, Brown M. Brain regulation of gastric secretion: influence of neuropeptides. Proc Natl Acad Sci USA. 1980;77:5515–5519. doi: 10.1073/pnas.77.9.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taché Y, Vale W, Brown M. Thyrotropin-releasing hormone--CNS action to stimulate gastric acid secretion. Nature. 1980;287:149–151. doi: 10.1038/287149a0. [DOI] [PubMed] [Google Scholar]
- 9.Lenz HJ, Klapdor R, Hester SE, Webb VJ, Galyean RF, Rivier JE, Brown MR. Inhibition of gastric acid secretion by brain peptides in the dog. Role of the autonomic nervous system and gastrin. Gastroenterology. 1986;91:905–912. doi: 10.1016/0016-5085(86)90693-1. [DOI] [PubMed] [Google Scholar]
- 10.Tache Y. Central regulation of gastric acid secretion. In: L.R. Johnson, J. Christensen, E.D. Jacobson and J.H. Walsh, eds. Physiology of the gastrointes-tinal tract.New York: Raven Press. 1987:911–930. [Google Scholar]
- 11.Skaaring P, Bierring F. On the intrinsic innervation of normal rat liver. Histochemical and scanning electron microscopical studies. Cell Tissue Res. 1976;171:141–155. doi: 10.1007/BF00219403. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland SD. An eveluation of cholinesterase techniques in the study of the intrinsic innervation of the liver. J Anat. 1964;98:321–326. [PMC free article] [PubMed] [Google Scholar]
- 13.Kohno T, Mori S, Mito M. Cells of origin innervating the liver and their axonal projections with synaptic terminals into the liver parenchyma in rats. Hokkaido Igaku Zasshi. 1987;62:933–946. [PubMed] [Google Scholar]
- 14.Manaker S, Rizio G. Autoradiographic localization of thyrotropin-releasing hormone and substance P receptors in the rat dorsal vagal complex. J Comp Neurol. 1989;290:516–526. doi: 10.1002/cne.902900406. [DOI] [PubMed] [Google Scholar]
- 15.Rinaman L, Miselis RR. Thyrotropin-releasing hormone-immunoreactive nerve terminals synapse on the dendrites of gastric vagal motoneurons in the rat. J Comp Neurol. 1990;294:235–251. doi: 10.1002/cne.902940208. [DOI] [PubMed] [Google Scholar]
- 16.Taché Y, Stephens RL, Ishikawa T. Central nervous system action of TRH to influence gastrointestinal function and ulceration. Ann N Y Acad Sci. 1989;553:269–285. doi: 10.1111/j.1749-6632.1989.tb46649.x. [DOI] [PubMed] [Google Scholar]
- 17.Garrick T, Buack S, Veiseh A, Tache Y. Thyrotropin-releasing hormone (TRH) acts centrally to stimulate gastric contractility in rats. Life Sci. 1987;40:649–657. doi: 10.1016/0024-3205(87)90266-9. [DOI] [PubMed] [Google Scholar]
- 18.Goto Y, Tache Y. Gastric erosions induced by intracisternal thyrotropin-releasing hormone (TRH) in rats. Peptides. 1985;6:153–156. doi: 10.1016/0196-9781(85)90092-0. [DOI] [PubMed] [Google Scholar]
- 19.Maeda-Hagiwara M, Watanabe H. Inhibitory effects of intrahypothalamic injection of calcitonin on TRH-stimulated gastric acid secretion in rats. Jpn J Pharmacol. 1985;39:173–178. doi: 10.1254/jjp.39.173. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Yang H, Taché Y. Medullary sites of action of the TRH analogue, RX 77368, for stimulation of gastric acid secretion in the rat. Gastroenterology. 1988;95:1470–1476. doi: 10.1016/s0016-5085(88)80065-9. [DOI] [PubMed] [Google Scholar]
- 21.Stephens RL, Ishikawa T, Weiner H, Novin D, Taché Y. TRH analogue, RX 77368, injected into dorsal vagal complex stimulates gastric secretion in rats. Am J Physiol. 1988;254:G639–G643. doi: 10.1152/ajpgi.1988.254.5.G639. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Ishikawa T, Taché Y. Microinjection of TRH analogs into the raphe pallidus stimulates gastric acid secretion in the rat. Brain Res. 1990;531:280–285. doi: 10.1016/0006-8993(90)90785-a. [DOI] [PubMed] [Google Scholar]
- 23.Carneiro JJ, Donald DE. Change in liver blood flow and blood content in dogs during direct and reflex alteration of hepatic sympathetic nerve activity. Circ Res. 1977;40:150–158. doi: 10.1161/01.res.40.2.150. [DOI] [PubMed] [Google Scholar]
- 24.Lautt WW. Hepatic nerves: a review of their functions and effects. Can J Physiol Pharmacol. 1980;58:105–123. doi: 10.1139/y80-019. [DOI] [PubMed] [Google Scholar]
- 25.Folkow B, Rubinstein EH. Behavioural and autonomic patterns evoked by stimulation of the lateral hypothalamic area in the cat. Acta Physiol Scand. 1965;65:292–299. doi: 10.1111/j.1748-1716.1965.tb04276.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsybenko VA, Yanchuk PI. Central nervous control of hepatic circulation. J Auton Nerv Syst. 1991;33:255–266. doi: 10.1016/0165-1838(91)90026-y. [DOI] [PubMed] [Google Scholar]
- 27.Koo A, Liang IY. Microvascular filling pattern in rat liver sinusoids during vagal stimulation. J Physiol. 1979;295:191–199. doi: 10.1113/jphysiol.1979.sp012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo A, Liang IY. Stimulation and blockade of cholinergic receptors in terminal liver microcirculation in rats. Am J Physiol. 1979;236:E728–E732. doi: 10.1152/ajpendo.1979.236.6.E728. [DOI] [PubMed] [Google Scholar]
- 29.Tamori K, Yoneda M, Nakamura K, Makino I. Effect of intracisternal thyrotropin-releasing hormone on hepatic blood flow in rats. Am J Physiol. 1998;274:G277–G282. doi: 10.1152/ajpgi.1998.274.2.G277. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda M, Tamori K, Nakade Y, Takamoto S, Yokohama S, Aso K, Sato Y et al. Thyrotropin-releasing hormone (TRH) in the left dorsal vagal complex (DVC) increasea the hepatic blood flow in rats (abst). Gastroenterology (in press) [Google Scholar]
- 31.Higgins GM, R M.A. Experimental pathology of the liver-I. Restration of the liver of the white rat following partial surgical removal. Arch Path. 1931;12:186–202. [Google Scholar]
- 32.Bucher NL. Liver regeneration: an overview. J Gastroenterol Hepatol. 1991;6:615–624. doi: 10.1111/j.1440-1746.1991.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 33.Iwai M, Shimazu T. Alteration in sympathetic nerve activity during liver regeneration in rats after partial hepatectomy. J Auton Nerv Syst. 1992;41:209–214. doi: 10.1016/0165-1838(92)90060-t. [DOI] [PubMed] [Google Scholar]
- 34.MacManus JP, Braceland BM, Youdale T, Whitfield JF. Adrenergic antagonists, and a possible link between the increase in cyclic adenosine 3',5'-monophosphate and DNA synthesis during liver regeneration. J Cell Physiol. 1973;82:157–164. doi: 10.1002/jcp.1040820204. [DOI] [PubMed] [Google Scholar]
- 35.Cruise JL, Knechtle SJ, Bollinger RR, Kuhn C, Michalopoulos G. Alpha 1-adrenergic effects and liver regeneration. Hepatology. 1987;7:1189–1194. doi: 10.1002/hep.1840070604. [DOI] [PubMed] [Google Scholar]
- 36.Kato H, Shimazu T. Effect of autonomic denervation on DNA synthesis during liver regeneration after partial hepatectomy. Eur J Biochem. 1983;134:473–478. doi: 10.1111/j.1432-1033.1983.tb07591.x. [DOI] [PubMed] [Google Scholar]
- 37.Ohtake M, Sakaguchi T, Yoshida K, Muto T. Hepatic branch vagotomy can suppress liver regeneration in partially hepatectomized rats. HPB Surg. 1993;6:277–286. doi: 10.1155/1993/59691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiba T, Tanaka K, Endo O, Inoue S. Role of vagus nerve in increased DNA synthesis after hypothalamic ventromedial lesions in rat liver. Am J Physiol. 1992;262:G483–G487. doi: 10.1152/ajpgi.1992.262.3.G483. [DOI] [PubMed] [Google Scholar]
- 39.Yoneda M, Tamori K, Sato Y, Yokohama S, Nakamura K, Kono T, Makino I. Central thyrotropin-releasing hormone stimulates hepatic DNA synthesis in rats. Hepatology. 1997;26:1203–1208. doi: 10.1053/jhep.1997.v26.pm0009362363. [DOI] [PubMed] [Google Scholar]
- 40.McCann MJ, Hermann GE, Rogers RC. Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J Auton Nerv Syst. 1989;26:107–112. doi: 10.1016/0165-1838(89)90158-6. [DOI] [PubMed] [Google Scholar]
- 41.Somiya H, Tonoue T. Neuropeptides as central integrators of autonomic nerve activity: effect of TRH, SRIF, VIP and bombesin on gastric and adrenal nerves. Regul Pept. 1984;9:47–52. doi: 10.1016/0167-0115(84)90006-5. [DOI] [PubMed] [Google Scholar]
- 42.Sato Y, Yoneda M, Yokohama S, Tamori K, Nakamura K, Makino I. Protec-tive effect of central thyrotropin releasing hormone (TRH) on CCl4-induced liver damage in rats (abst) Gastroenterology. 1996;110:A1312. [Google Scholar]
- 43.Taché Y, Goto Y, Gunion MW, Vale W, River J, Brown M. Inhibition of gastric acid secretion in rats by intracerebral injection of corticotropin-releasing factor. Science. 1983;222:935–937. doi: 10.1126/science.6415815. [DOI] [PubMed] [Google Scholar]
- 44.Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology. 1988;95:1510–1517. doi: 10.1016/s0016-5085(88)80070-2. [DOI] [PubMed] [Google Scholar]
- 45.Gunion MW, Taché Y. Intrahypothalamic microinfusion of corticotropin-releasing factor inhibits gastric acid secretion but increases secretion volume in rats. Brain Res. 1987;411:156–161. doi: 10.1016/0006-8993(87)90693-7. [DOI] [PubMed] [Google Scholar]
- 46.Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988;94:598–602. doi: 10.1016/0016-5085(88)90229-6. [DOI] [PubMed] [Google Scholar]
- 47.Mönnikes H, Raybould HE, Schmidt B, Taché Y. CRF in the paraventricular nucleus of the hypothalamus stimulates colonic motor activity in fasted rats. Peptides. 1993;14:743–747. doi: 10.1016/0196-9781(93)90107-r. [DOI] [PubMed] [Google Scholar]
- 48.Mönnikes H, Schmidt BG, Tebbe J, Bauer C, Taché Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus nuclei stimulates colonic motor function in rats. Brain Res. 1994;644:101–108. doi: 10.1016/0006-8993(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 49.Nakade Y, Yoneda M, Takamoto S, Yokohama S, Tamori K, Aso K, Sato Y et al. Central corticotropin releasing factor (CRF) decreases the hepatic blood flow in rats (abst). Gastroenterology (in press) [Google Scholar]
- 50.Yokohama S, Yoneda M, Tamori K, Sato Y, Hasegawa T, Nakamura K, Makino I. Effect of central corticotropin releasing factor (CRF) on carbon tetrachloride (CCl4)induced acute liver injury in rat (abst) Gastroenterology. 1997;112:A1201. [Google Scholar]
- 51.Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci USA. 1982;79:5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 53.Lundberg JM, Terenius L, Hökfelt T, Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett. 1983;42:167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- 54.Allen JM, Gu J, Adrian TE, Polak JM, Bloom SR. Neuropeptide Y in the guinea-pig biliary tract. Experientia. 1984;40:765–767. doi: 10.1007/BF01949769. [DOI] [PubMed] [Google Scholar]
- 55.De Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreac-tivity in the rat central nervous system-2. Immunohistochemical analysis. Neuroscience. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 56.Hökfelt T, Lundberg JM, Tatemoto K, Mutt V, Terenius L, Polak J, Bloom S, Sasek C, Elde R, Goldstein M. Neuropeptide Y (NPY)- and FMRFamide neuropeptide-like immunoreactivities in catecholamine neurons of the rat medulla oblongata. Acta Physiol Scand. 1983;117:315–318. doi: 10.1111/j.1748-1716.1983.tb07214.x. [DOI] [PubMed] [Google Scholar]
- 57.Yamazoe M, Shiosaka S, Emson PC, Tohyama M. Distribution of neuropeptide Y in the lower brainstem: an immunohistochemical analysis. Brain Res. 1985;335:109–120. doi: 10.1016/0006-8993(85)90281-1. [DOI] [PubMed] [Google Scholar]
- 58.Gillis RA, Quest JA, Pagani FD, Norman WP. Central centers in the central nervous system for regulating gastrointestinal motility. In: J.D. Wood, eds. Handbook of Physiology. 6, The gastrointestinal system Bethesda: American Physiological Society. 1989:621–683. [Google Scholar]
- 59.Reilly FD, McCuskey PA, McCuskey RS. Intrahepatic distribution of nerves in the rat. Anat Rec. 1978;191:55–67. doi: 10.1002/ar.1091910106. [DOI] [PubMed] [Google Scholar]
- 60.Bogach PG, Lyashchenko PS. Changes in bile secretion during hypothalamic stimulation in dogs. In: eds. Problems of physiology of the hypothalamus. Moscow: Kiev. 1974:56–64. [Google Scholar]
- 61.Cucchiaro G, Yamaguchi Y, Mills E, Kuhn CM, Anthony DC, Branum GD, Epstein R, Meyers WC. Evaluation of selective liver denervation methods. Am J Physiol. 1990;259:G781–G785. doi: 10.1152/ajpgi.1990.259.5.G781. [DOI] [PubMed] [Google Scholar]
- 62.Fritz ME, BROOKS FP. Control of bile flow in the cholecystectomized dog. Am J Physiol. 1963;204:825–828. doi: 10.1152/ajplegacy.1963.204.5.825. [DOI] [PubMed] [Google Scholar]
- 63.Moltz JH, McDonald JK. Neuropeptide Y: direct and indirect action on insulin secretion in the rat. Peptides. 1985;6:1155–1159. doi: 10.1016/0196-9781(85)90443-7. [DOI] [PubMed] [Google Scholar]
- 64.Matsuda M, Aono M, Moriga M, Okuma M. Centrally administered NPY stimulated gastric acid and pepsin secretion by a vagally mediated mechanism. Regul Pept. 1991;35:31–41. doi: 10.1016/0167-0115(91)90251-b. [DOI] [PubMed] [Google Scholar]
- 65.Geoghegan JG, Lawson DC, Cheng CA, Opara E, Taylor IL, Pappas TN. Intracerebroventricular neuropeptide Y increases gastric and pancreatic secretion in the dog. Gastroenterology. 1993;105:1069–1077. doi: 10.1016/0016-5085(93)90951-8. [DOI] [PubMed] [Google Scholar]
- 66.Farouk M, Geoghegan JG, Pruthi RS, Thomson HJ, Pappas TN, Meyers WC. Intracerebroventricular neuropeptide Y stimulates bile secretion via a vagal mechanism. Gut. 1992;33:1562–1565. doi: 10.1136/gut.33.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoneda M, Tamasawa N, Takebe K, Tamori K, Yokohama S, Sato Y, Nakamura K, Makino I, Taché Y. Central neuropeptide Y enhances bile secretion through vagal and muscarinic but not nitric oxide pathways in rats. Peptides. 1995;16:727–732. doi: 10.1016/0196-9781(95)00041-h. [DOI] [PubMed] [Google Scholar]
- 68.Yoneda M, Yokohama S, Tamori K, Sato Y, Nakamura K, Makino I. Neuropeptide Y in the dorsal vagal complex stimulates bicarbonate-dependent bile secretion in rats. Gastroenterology. 1997;112:1673–1680. doi: 10.1016/s0016-5085(97)70050-7. [DOI] [PubMed] [Google Scholar]
- 69.Bartolome JV, Bartolome MB, Lorber BA, Dileo SJ, Schanberg SM. Effects of central administration of beta-endorphin on brain and liver DNA synthesis in preweanling rats. Neuroscience. 1991;40:289–294. doi: 10.1016/0306-4522(91)90191-p. [DOI] [PubMed] [Google Scholar]
- 70.Yao CZ, MacLellan DG, Thompson JC. Intracerebroventricular administration of bombesin inhibits biliary and gastric secretion in the rat. J Neurosci Res. 1989;22:461–463. doi: 10.1002/jnr.490220412. [DOI] [PubMed] [Google Scholar]
- 71.Berbasa NV, Zhou J, Ravi J. Intracisternal (ic) administration of the opioid peptide analogue D-ala-met-enkephalinamide (D-Ala-Met-Enk) modulate bile flow by an opioid receptor-mediated mechanism in the brain (abst) Gastroenterology. 1997;112:A1225. [Google Scholar]


