Abstract
AIM: To study the effect of Helicobacter pylori (H. pylori) infection on gastric epithelial proliferation in the progression from normal mucosa to gastric carcinoma.
METHODS: Gastric biopsy specimens from normal controls (n = 11), superficial gastritis (n = 32), atrophic gastritis with intestinal metaplasia (n = 83), dysplasia (n = 25) and gastric carcinoma (n = 10) were studied by immunohistochemical stianing of proliferating cell nuclear antigen (PCNA).
RESULTS: The gastric epithelial proliferation, expressed as PCNA labeling index (LI)%, was progressively increased in successive stages from normal mucosa to gastric carcinoma regardless of H. pylori status. There was significant difference in PCNA LI% among all groups (P < 0.01). The analysis pursuing the effect of H. pylori infection on gastric epithelial proliferation in the progression from normal mucosa to gastriccarcinoma showed that in superficial gastritis and mild atrophic gastritis groups, PCNA LI% in H. pylori positive patients were 13.14 ± 1.6 and 19.68 ± 2.22 respectively, significantly higher than 6.95 ± 0.78 and 11.34 ± 1. 89 in H. pylori negative patients (P < 0.01); but there was no such difference in other groups (P > 0.05).
CONCLUSION: H. pylori infection causes increased gastric epithelial proliferation in the stages of superficial and mild atrophic gastritis and may play a part in triggering gastric carcinogenesis.
Keywords: helicobacter infections, gastric mucosa/microbiology, stomach neoplasms/microbiology, gastric mucosa/pathology
INTRODUCTION
Gastric carcinoma is one of the leading causes of malignancy-related death in China, and its etiology has not been fully elucidated. The epidemiological and histopathological studies have shown that Helicobacter pylori (H. pylori) infection is closely associated with gastric carcinogenesis[1-5]. However, the mechanism and the stages in which H. pylori participates in the process of gastric carcinogenesis are largely unknown. An increase in epithelial cell proliferation is one of the earliest mucosa changes in the development of gastric cancer, and may serve as a risk indicator for it[6,7]. The proliferation nuclear cell antigen (PCNA) expression is a reliable marker for evaluation of cell proliferation[8,9]. In this study, we used PCNA as a marker to investigate the effect of H. pylori infection on gastric epithelial proliferation in the progression from normal mucosa to gastric carcinoma.
MATERIALS AND METHODS
Subjects
Archival gastric biopsy specimens used in this study were randomly selected from those kept in the pathological department of Shanghai Institute of Digestive Diseases between January and December 1996. The specimens were taken from 161 subjects, and the diagnosis was made based on the endoscopic and histological findings. Subjects were assigned to one of the following five study groups according to the histological diagnosis: Group 1, normal gastric mucosa, H. pylori negative; group 2, chronic superficial gastritis; group 3, chronic atrophic gastrits with intestinal metaplasia; group 4, dysplasia; and group 5, gastric carcinoma (intestinal type). The atrophy and intestinal metaplasia in gastric mucosa were graded as mild, moderate and severe respectively according to the criteria proposed in Sydney system, and scored 1, 2 and 3. Atrophic gastritis was usually accompanied by intestinal metaplasia, so the scores of atrophy and intestinal metaplasia in each patient were added up, and then further classified as mild for total score equal or less than 2, moderate for scores 3-4, and severe for scores more than 4. The patients with chronic gastritis accompanied by dysplasia all belonged to group 4.
PCNA staining
PCNA staining of antral biopsy specimens was performed using immunohistochemial ABC method. Briefly, PCNA staining was proceeded, after deparaffinizing in xylene, clearing in ethanol, rehydrating through graded ethanol and washing in PBS. Endogenous peroxidase was blocked by 0.3% H2O2, and then washed slightly in PBS. The sections were preincubated with diluted normal goat serum, and then incubated with anti-PCNA antibody (PC10, mouse anti-human, DAKO Company) diluted 1:80 at 4 °C overnight. After draining, the sections were incubated with a biotinylated anti-mouse IgG diluted 1:50 for 60 min, then incubated with ABC working solution for 30 min according to manufaturer’s instruction (Sino-American Biotechnology Company). After another washing, the sections were incubated with 3-3’diaminobenzidine tetrahydrochloride (DAB) solution under microscopic monitoring, and then counterstained with hematoxylin. The stained PCNA positive tissue (gastric polyp) sections served as positive controls. A negative control, where primary antibody was replaced by PBS, was also stained parallelly.
Analysis of gastric epithelial proliferative activity
PCNA positive cells were counted only in well oriented sections with visible entrire gastric pits. A mean number of 10 pits were examined for each specimen, and greater than 500 cells were analysed. Labeling index per cent (LI%) was measured by counting the percentage of the number of PCNA positive cells of the total number of cells.
Identification of H. pylori infection
H. pylori was detected under microscopy on the histological sections stained with a modified Giemsa staining method.
Statistical analysis
LI% was compare among groups, and the significance was analysed using Student’ t test for unpaired data, P values less than 0.05 were considered statistically significant.
RESULTS
Table 1 shows the demographic profileand H. pylori status in 161 subjects. Figure 1 shows PCNA LI% in the study groups regardless of H. pylori status. PCNA LI% in normal gastric mucosa, superficial gatritis, atrophic gastritis, dysplasia and gastric carcinoma group was 6.31 ± 1.67 (x- ± s), 10.04 ± 1.32, 17.11 ± 2.55, 32.46 ± 4.16 and 46.05 ± 4.63, respectively. PCNA LI% was progressively increased from normal mucosa to gastric carcinoma, and there was significant difference between the groups (P < 0.01).
Table 1.
The demographic profile and H. pylori status in 161 subjects
| Study groups | No. of patients | Femal/male | Average ages (yrs) | No. of Hp positive | No. of Hp negative |
| Normal gastric mucosa | 11 | 2/9 | 44.5 | 0 | 11 |
| Superficial gastritis | 32 | 10/22 | 43.6 | 16 | 16 |
| Atrophic gastritis | |||||
| Mild | 32 | 11/21 | 42.1 | 16 | 16 |
| Moderate | 26 | 11/15 | 51.0 | 15 | 11 |
| Severe | 25 | 8/17 | 56.1 | 10 | 15 |
| Dysplasia | 25 | 7/18 | 53.2 | 13 | 12 |
| Gastric Carcinoma | 10 | 3/7 | 66.2 | 5 | 5 |
Figure 1.
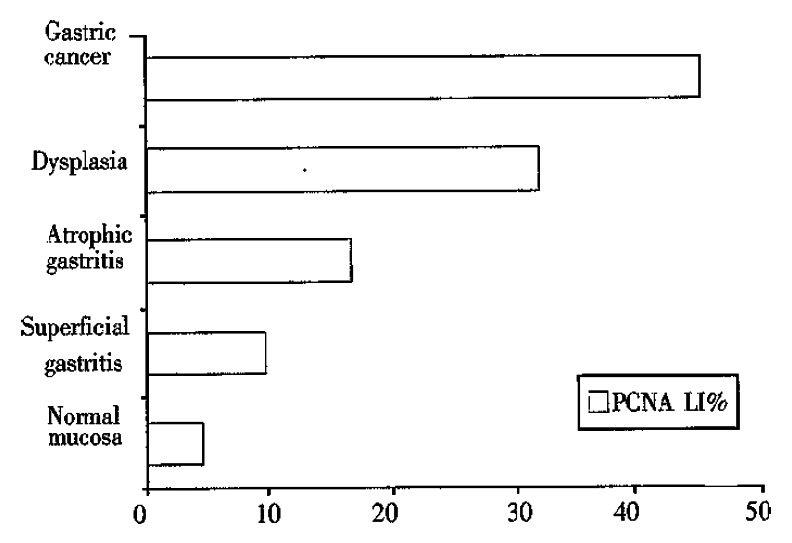
PCNA LI% in the study groups from normal gastric mucosa to gastric carcinoma regardless of H. pylori status.
Figure 2 shows the PCNA LI% in the groups associated with H.pylori status. Atrophic gastritis group was further classified into mild, moderate and severe subgroups based on the scores of atrophy and intestinal metaplasia. In superficial gastritis and mild atrophic gastritis groups, PCNA LI% in H. pylori positive patients was 13.14 ± 1.6 and 19.68 ± 2.22 respectively, significantly higher than 6.95 ± 0.78 and 11.34 ± 1.89 in H. pylori negative patients (P < 0.01); but there was no such difference in other groups (P > 0.05).
Figure 2.
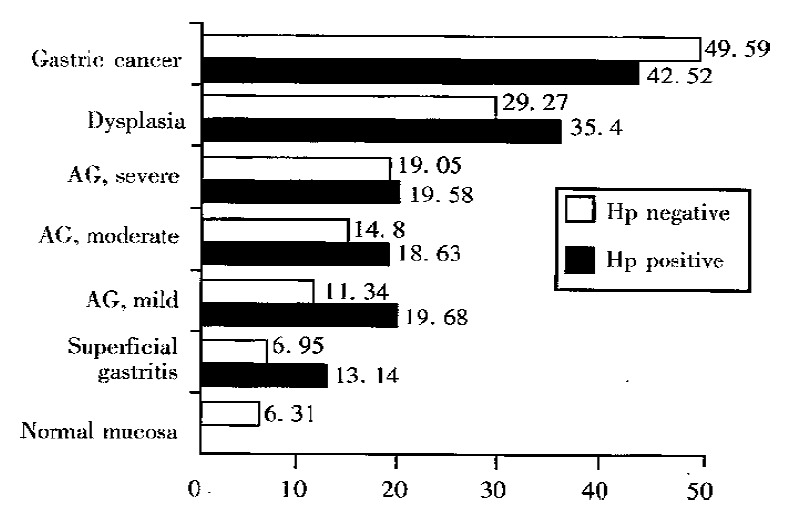
PCNA LI% in study groups associated with H. pylori status from normal mucosa to gastric carcinoma. AG = atrophic gastritis.
DISCUSSION
The etiology of gastric carcinoma has not been fully elucidated. In 1988, Correa[10] proposed a human model of gastric carcinogenesis based on the epidemiological, pathological and clinical findings. He postulated that gastric cancer develops through a complex sequence of events from normal to superficial gastritis, atrophic gastritis, intestinal metaplasia, dysplasia and finally to intestinal type gastric carcinoma, and that the chronic gastritis in the first step in the progression to malignancy. This postulation was proved correct later by several studies[11,12].
H. pylori is the primary etiological cause of chronic gastritis[13,14], and is associated with a sixfold increased risk of gastric carcinoma[2]. Long-term studies of H. pylori infection have provided evidence of a progression from H. pylori gastritis to atrophic gastritis, intestinal metaplasia, and dysplasia. H. pylori has been listed by the WHO as class 1 carcinogen for gastric cancer. But the exact mechanisms that H. pylori participates in gastric carcinogenesis are not clear.
Excessive cell proliferation increases the chance of spontaneous error of DNA replication, and potentiates the action of any carcinogen targetting DNA, and therby enhences the risk of neoplastic transformation of cells[15]. The assessment of epithelial cell proliferation as an indicator of risk has been validated in gastric carcinoma, even before H. pylori was found to be associated with chronic gastritis[6]. Several recent studies have shown that H. pylori infection can promote gastric epithelial proliferation[16,17] so that H. pylori infection links to increased risks of gastric carcinogenesis. But few studies have pursued the effect of H. pylori infection on gastric epithelial proliferation in the progression from normal mucosa to gastric carcinoma[18].
Our results showed that gastric epithelial proliferative activity expressed as PCNA LI% increased progressively from normal mucosa to superficial gastritis, atrophic gastritis, dysplasia and gastric carcinoma, suggesting that increased gastric epithelial proliferation is associated with gastric precancerous changes and gastric cancer, and can be used as an indicator for evaluating the risk of gastric carcinogenesis.
The analysis of the effect of H. pylori infection on gastric epithelial proliferation in progression from normal mucosa to gastric carcinoma indicated that the increase in gastric epithelial proliferation associated with H. pylori infection was only seen in superficial gastritis and mild atrophic gastritis, and in other groups, there was no significant difference in PCNA LI% between H. pylori positive and negative patients. The results suggested that H. pylori infection causes increased gastric epithelial proliferation primarily in the stages of superficial and mild atrophic gastritis, and it may not have so strong influence in the late stages of gastric carcinogenesis. There, H. pylori infection may be the precipitating factor in triggering gastric carcinogenesis.
Once H. pylori gastritis develops to the stage of gastric atrophy, the gastric acid secretion will decrease markedly, leading to changes in gastric bacterial flora, and subsequently promoted the endogenous formation of carcinogenic N-nitrosocompounds[19,20], and gastric epithelial proliferation. This may explain why H. pylori infection has less effect on gastric epithelial proliferation in late stages of gastric carcinogenesis.
Footnotes
Project supported by the Shanghai Municipal Commission of Science and Technolog, No. 954119023.
References
- 1.Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 3.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 4.Craanen ME, Blok P, Dekker W, Tytgat GN. Helicobacter pylori and early gastric cancer. Gut. 1994;35:1372–1374. doi: 10.1136/gut.35.10.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaka M, Kimura T, Kato M, Kudo M, Miki K, Ogoshi K, Kato T, Tatsuta M, Graham DY. Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer. 1994;73:2691–2694. doi: 10.1002/1097-0142(19940601)73:11<2691::aid-cncr2820731107>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Xu YL, Zheng ZT, Liu DX. Cell proliferation kinetics in chronic gastritis and gastric cancer. Chin Med J (Engl) 1984;97:526–531. [PubMed] [Google Scholar]
- 7.Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res. 1988;48:235–245. [PubMed] [Google Scholar]
- 8.Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Yoshitake K, Sato M, Ahnen DJ. Proliferating cell nuclear antigen expression in normal, preneoplastic, and neoplastic colonic epithelium of the rat. Gastroenterology. 1992;103:160–167. doi: 10.1016/0016-5085(92)91109-h. [DOI] [PubMed] [Google Scholar]
- 10.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 11.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 12.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. [PubMed] [Google Scholar]
- 13.Jiang SJ, Liu WZ, Zhang DZ, Shi Y, Xiao SD, Zhang ZH, Lu DY. Campylobacter-like organisms in chronic gastritis, peptic ulcer, and gastric carcinoma. Scand J Gastroenterol. 1987;22:553–558. doi: 10.3109/00365528708991897. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt JI. Histopathology of gastroduodenal inflammation: the impact of Helicobacter pylori. Histopathology. 1995;26:1–15. doi: 10.1111/j.1365-2559.1995.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 15.Ames BN, Gold LS. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science. 1990;249:970–971. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- 16.Fan XG, Kelleher D, Fan XJ, Xia HX, Keeling PW. Helicobacter pylori increases proliferation of gastric epithelial cells. Gut. 1996;38:19–22. doi: 10.1136/gut.38.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch DA, Mapstone NP, Clarke AM, Sobala GM, Jackson P, Morrison L, Dixon MF, Quirke P, Axon AT. Cell proliferation in Helicobacter pylori associated gastritis and the effect of eradication therapy. Gut. 1995;36:346–350. doi: 10.1136/gut.36.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahill RJ, Kilgallen C, Beattie S, Hamilton H, O'Morain C. Gastric epithelial cell kinetics in the progression from normal mucosa to gastric carcinoma. Gut. 1996;38:177–181. doi: 10.1136/gut.38.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37–S43. [PubMed] [Google Scholar]
- 20.Sobala GM, Pignatelli B, Schorah CJ, Bartsch H, Sanderson M, Dixon MF, Shires S, King RF, Axon AT. Levels of nitrite, nitrate, N-nitroso compounds, ascorbic acid and total bile acids in gastric juice of patients with and without precancerous conditions of the stomach. Carcinogenesis. 1991;12:193–198. doi: 10.1093/carcin/12.2.193. [DOI] [PubMed] [Google Scholar]


