INTRODUCTION
According to the therapeutic effect and strategy of antisense RNA for hepatocellular carcinoma (HCC), we have specifically synthesized partial cDNA of human insulin-like growth factor II (IGF-II) and constructed IGF-II cDNA antisense eukaryotic expression vector. The constructed vector was introduced into hepatoma cell line SMMC-7721 to block the intrinsic IGF-II expression. The biological behavior changes of hepatoma cells were observed. All these would provide scientific basis for IGF-II antisense RNA in the treatment of HCC.
MATERIALS AND METHODS
Plasmid pIGF-II containing partial cDNA of IGF-II was constructed by ourselves[1]. Eukaryotic expression vector pcDNA-3 was a gift from Professor Mao Ji-Fang in the Second Military Medical University. Hepatoma cell line SMMC-7721 was provided by our own lab. Geneticin (G418), 1640 cultivation liquid and Li pofection were provided by Gibco/BRL (USA).
The construction and transduction of IGF-II antisense RNA expression vector: plasmid pIGF-II was doubly digested by Eco-R I/-Xba-I and the 106 bp IGF-IIcDNA fragment was retrieved with DNA purification kit (product of Huashun Company). Plasmid pcDNA was doubly digested by Eco-R I/Xba I and the large fragment was retrieved. The two fragments were co-incubated overnight with T4 DNA ligase under 14 °C, then transferred to Escherichia coli DH5 α. The transformed colony was screened on LB agar containing amoxicillin. The transformed plasmid was extracted and then identified through dot blot (probes were made by ourselves) and PCR. The primer sequences of PCR were 5′-CTAGAGCTTACCGCCCCAGTGAGA-3′, 5′-AATTCTGCGGGCCTGCTGAAGTAG-3′. Because it belonged to the directional coloning, the positively transformed colony was reversely inserted into plasmid fragment, i.e., pIGF-IIAs. According to the method in reference[2], 10 μg pIGF-IIAs was introduced into 5 × 105 SMMC-7721 cells. The screening was performed in 1640 culture solution containing 400 mg/L G418, and pcDNA3 was used as blank vector control.
The influence of pIGF-IIAs on hepatoma cell line SMMC-7721. ① cell growth curve: a total of 1 × 104 logarithmic period growing cells were inoculated in 24-well culture plates. The cells were incubated at 37 °C with 5% CO2 for 24 h, then the cells were collected, 3 wells once, the number of cells was counted after trypan blue staining, and the average value was calculated. ② Cell cycle analysis: 5 × 106 logarithmic period growing cells were washed twice by PBS. One mL 70% ethanol was added to cell sediment. The cells were placed at 4 °C, were stained with PI 12 h later and then measured with Elite Coulter flow cytometry. Cell cycle was analysed by Multcycle software. ③ Soft-agar colony formation experiment was made as reference.
RESULTS
The construction of pIGF-IIAs (Figure 1). IGF-II cDNA was reversely inserted into pcDNA3 vector, PCMV maintained high copies of the original vector in mammalian cells. There were multi-colony sites in BGHpa region, which were favorable for IGF-II cDNA insertion. The inserted sites located in Eco-R I and Xba I region. Neo and Amp were the selective marks of the gene in mammalian cells and bacterial cells. IGF-II cDNA was reversely inserted into pcDNA3 vector, because it belonged to directional colony. The dot blot of the properly transformed plasmid showed that 106 bp hybridization band was amplified by PCR. The positively identified band was pIGF-IIAs (Figure 1).
Figure 1.
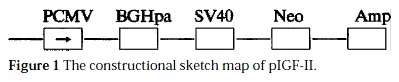
The constructional sketch map of pIGF-II.
pIGF-IIAs and pcDNA3 were introduced into SMMC-7721 cells. After screening with G418 for 4 wk, the number of transformed colonies was 26.00 ± 2.83 and 52.00 ± 4.66 (mean ± SE), respectively.
After screening with G418, the growth curves of SMMC-7721 cells and transgenic cells are shown in Figure 2.
Figure 2.
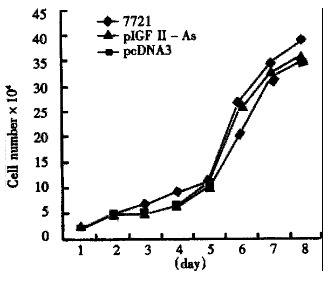
Growth curves of the three cell groups.
Cell cycle analysis was made with flow cytometry (FCM) (Table 1).
Table 1.
Cell cycle analysis with FCM
| Group | G0/1 | S | G2/M |
| 7721 | 72.40 | 21.50 | 6.10 |
| pcDNA3 | 72.40 | 20.30 | 7.20 |
| pIGF-IIAs | 43.00 | 41.20 | 15.80 |
Colony formation in soft-agar cultivation. Cultivated on double layer soft-agar for 14 d, 104 cells were incubated in each culture dish. The cells in group 7721 and pcDNA3 grew well and formed obvious clustered cell colonies. The number of colonies was 205.17 ± 72.02 and 146.50 ± 74.17, respectively, while there was no colony formed in group pIGF-IIAs.
DISCUSSION
Antisense IGF-II gene was introduced into hepatoma cell line SMMC-7721 with antisense technique. The antisense RNA sequence matched with target mRNA, would selectively block the autocrine or paracrine growth stimulation mechanism of hepatoma cells, attaining to the purpose of antisense gene therapy. We found that the number of transformed colonies in pIGF-IIAs transfected SMMC-7721 was significantly less than that in pcDNA3. This may be due to IGF-II antisense RNA suppressing the growth of transfected SMMC-7721. It may imply that IGF-II antisense RNA had the remarkable biological effect of suppressing the growth of hepatoma cells. The growth curves of the three group cells had no significant difference with G418 screening, which was consistent with Cajot′s research on p53 gene transduced lung cancer cell line[3].
The number of S-phase cells increased in pIGF-IIAs transfected group. However, there was no significant difference of the cell cycle in the other two groups. It was infered that IGF-II antisense gene suppressed the overexpression of IGF-II and partially up-regulated IGF-II receptor and IGF-IR, which activated the kinases p34cdc2 related to DNA duplication[4].
Cells of both pcDNA3 and SMMC-7721 groups could form colonies on soft-agar, while cells in the antisense pIGF-IIAs group could not form colonies. It demonstrated that the malignant phenotype of hepatoma cells inclined to disappear. This was the phenomenon of tumor cells reversing gradually to normal cells.
This study demonstrated that the transduction of antisense IGF-II into hepatoma cells had the biological effect of suppressing the carcinogenesis of hepatoma cell in vitro.
Footnotes
Edited by Ma JY
Supported by the National Natural Science Foundation of Guangdong Provi nce, No.940319.
References
- 1.Zhang MQ, Yang DH, Cai ZL, Mao JF, Wang ZQ, Xu C. The synthesis and coloning of IGF-II cDNA. Zhonghua Ganzangbing Zazhi. 1998;6:50–51. [Google Scholar]
- 2.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cajot JF, Anderson MJ, Lehman TA, Shapiro H, Briggs AA, Stanbridge EJ. Growth suppression mediated by transfection of p53 in Hut292DM human lung cancer cells expressing endogenous wild-type p53 protein. Cancer Res. 1992;52:6956–6960. [PubMed] [Google Scholar]
- 4.Kalebic T, Tsokos M, Helman LJ. In vivo treatment with antibody against IGF-1 receptor suppresses growth of human rhabdomyosarcoma and down-regulates p34cdc2. Cancer Res. 1994;54:5531–5534. [PubMed] [Google Scholar]


