Abstract
AIM: To evaluate a culture system for bile acid formation in primary human hepatocytes in comparison with HepG2 cells.
METHODS: Hepatocytes were isolated from normal human liver tissue and were cultured in serum-free William’s E medium. The medium was collected and re newed every 24 h. Bile acids and their precursors in media were finally analysed by gas chromatography-mass spectrometry.
RESULTS: Cholic acid (CA) and chenodeoxycholic acid (CDCA) conjugated with glycine or taurine accounted for 70% and 25% of total steroids. A third of CDC A was also conjugated with sulphuric acid. Dexamethasone and thyroid hormone alone or in combination did not significantly effect bile acid formation. The addit ion of cyclosporin A (10 μmol/L) inhibited the synthesis of CA and CDCA by about 13% and 30%, respectively.
CONCLUSION: Isolated human hepatocytes in primary culture behave as in the intact liver by converting cholesterol to conjugated CA and CDCA. This is in contrast to cultured HepG2 cells, which release large amounts of bile acid precursors and unconjugated bile acids into the medium.
Keywords: bile acid formation, cell culture, cholesterol metabolism, cyclosporin, human hepatocytes
INTRODUCTION
The degradation of cholesterol to bile acids is a major way for elimination of cholesterol from the body. Disturbances of bile acid formation may lead to clinical disorders in humans like atherosclerosis, cholesterol gallstone formation and liver diseases. Primary bile acids-cholic acid (CA) and chenodeoxycholic acid (CDCA) are formed from cholesterol in human liver and excreted via the bile as conjugates to the intestine. In the intestine, the bile acids are partly deco njugated and 7α-dehydroxylated by microbial enzymes to the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA). The bile acids, with the exception of lithocholic acid, are efficiently absorbed from the intestine and return to the liver via the portal vein. In the liver (70%-90%) of the bile acids a re extracted and resecreted to the bile. Thus, the main bile acids in human bile are CA, CDCA, and DCA. Small amounts of LCA and ursodeoxycholic acid (UDCA), a metabolite of CDCA are also found.
Two major pathways of bile acid synthesis from cholesterol have been described[1-5]. The main pathway, also called the neutral pathway, is initiated by a 7α-hydroxylation, catalyzed by the rate-limiting enzyme cholest erol 7α-hydroxylase. This hydroxylation is followed by further tra nsformations of the steroid nucleus and oxidative cleavage of the side chain. An alternative pathway, also called the acid pathway, is initiated by a 27-hydrox ylation of cholesterol. The quantitative importance of the latter pathway is not yet clear. It may be of importance for the formation of particularly CDCA and as a “back-up” pathway in conditions in which the neutral pathway is represse d for some reasons.
Bile acid formation in humans has been studied by different in vivo and in vitro techniques. Also cultures of human hepatoblastoma cells (HepG2) have been employed. However, these cells have been found to be defective in CA formation and conjugation by some authors[6-9]. The cells have also been found to prod uce large amounts of bile acid precursors.
More recently, techniques have been developed for preparation of primary cultures of normal human hepatocytes. These offer now possibilities to study bile acid synthesis in normal hepatocytes under various experimental conditions.
MATERIALS AND METHODS
Preparation and culture of human hepatocytes
Primary hepatocytes were prepared from normal donor liver tissue. The project wa s approved by the Ethics Committee at Huddinge University Hospital and by Human Ethics Committees of the Western Sidney Area Health Service, Australia. Liver se ctions weighing 100-200 g and with only one cut-surface were used. Hepatocyte s were isolated using a 2-step perfusion technique utilizing EGTA and collagena se type IX-S as recently described[10]. They were seeded on 60-mm dis hes at a density of 3.5 × 106 cells per dish. Dishes were precoated with 0.2 mL of matrigel, a laminine-rich extracellular matrix prepared from Engelbreth -Holm-Swarm mouse sarcomas[11]. Hepatocyte viability was > 85% as det ermined by trypan blue exclusion. The cells were then incubated in serum-free W illiam’s E medium (3 mL/dish), supplemented with glutamine (292 mg/L), Na2SeO3 (173 μg/L), insulin (2 mIU/mL), penicillin Gs odium, streptom ycin sulphate and gentamycin. In some experiments, triiodothyronine (T3) (0.1 μmol/L) and/or dexamethasone (0.1 μmol/L) were adde d. In some other experiments, on d 4, cyclosporin A (CsA), an inhibitor of the sterol 27-hydroxylase[12,13], was added to the medium (0-10 μmol/L). The medium was collected and renewed every 24 h and frozen at -20 °C for later analysis of bile acids. At the end of the incubation, the hepatocytes were harvested and stored at 20 °C until extraction.
Analytical procedures
For determination of the primary bile acids CA and CDCA formed, deuterium-labeled bile acids were added to the collected culture medium. After alkaline hydroly sis and extraction, the bile acids were analysed by gas chromatography-mass spectrometry as described recently[10]. For more detailed analysis of neutral and acidic steroids-unconjugated and conjugated with glycine or taurine and/or sulphuric acid-group separation was performed by anion exchange chromat ography after addition of isotopically labeled steroids. Deconjugation was perfo rmed by an enzymatic method. Sulphate groups were cleaved by solvolysis in acidi fied tetrahydrofuran. The identification of steroids was performed by gas chroma tography-mass spectrometry. Further details are given in a recent paper[14].
RESULTS AND DISCUSSION
Formation of bile acids
CA and CDCA were the two dominating steroids formed, accounting for as much as 70% and 25% of total, respectively (Figure 1). CA and CDCA are the two bile acids normally synthesized in human liver but the ratio between the production rates of the two bile acids in the hepatocytes was higher (about 3:1) than that of nor mal subjects (about 2:1). However, the ratio is similar to that obtained in pati ents with complete biliary drainage[15]. Small amounts of two other bile acids, 3β-hydroxy-5-cholenoic acid and 3β, 7β-dihydroxy-5β-cholanoic acid were also detected (about 1% of total of each).
Figure 1.
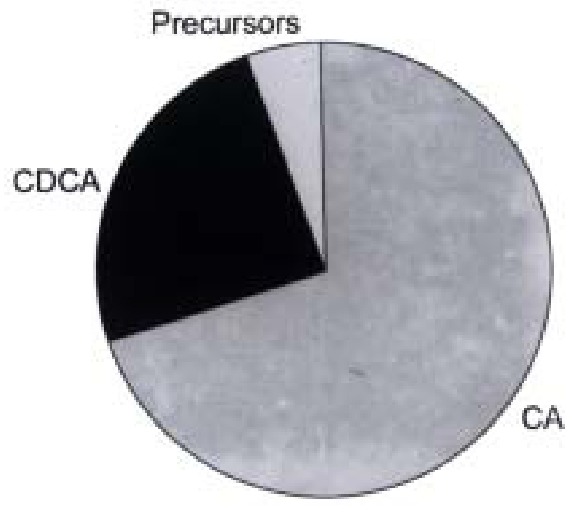
Distribution of cholic acid, chenodeoxycholic acid and precursors formed in primary cultures of human hepatocytes.
Bile acid formation was lowest on d 1 and 2 and then increased several-fold on d 4 to 6, whereafter it declined slightly (Figure 2). The reason for this is not clear. However, it has previously been observed that the activity and mRNA l evels of cytochrome P450 enzymes involved in drug metabolism show a similar prof ile in human hepatocytes e.g., a sharp recovery followed by a steady decline (Liddle C et al unpublised observations).
Figure 2.
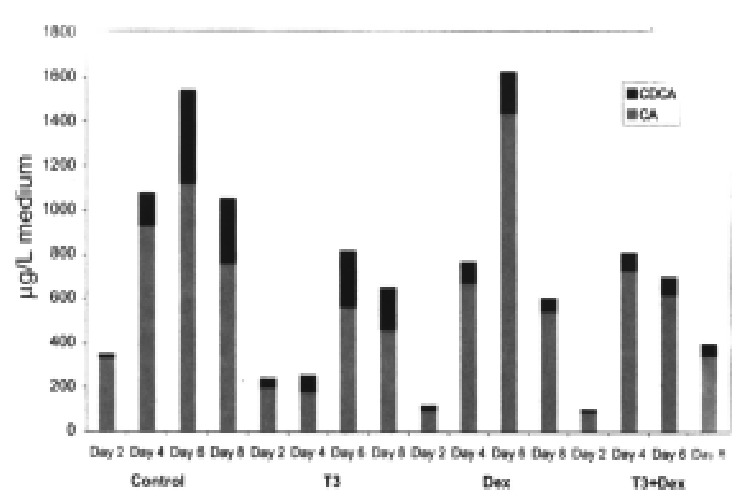
Formation of cholic acid and chenodeoxycholic acid in primary cultures of human hepatocytes during d 2, 4, 6 and 8. Effects of adding T3 and dexa methasone (DEX) alone and in combination.
About 3% of the total amount of the isolated steroids consisted of bile acid pre cursors. Among those, 7α-hydroxy-cholesterol and 7α-hydroxy-4-cholesten-3-one are established intermediates in the neutral pat hway and 27-hydroxycholesterol is the first intermediate in the acidic pathway. Since 7α-hydroxycholesterol can also be formed by autooxidation of cholesterol, 7α-hydroxy-4-cholesten-3-one is a better marker for the neutral pathway. The daily release of 7α-hydroxy-4-cholesten-3-one increases in parallel to those of CA and CDCA, whereas the release of 27-hydroxycholesterol decreases. Since the hepatocytes are incubated in as erum-free medium devoid of lipoprotein cholesterol, the decrease in amounts of 27-hydroxycholesterol is consistent with the hypothesis that cholesterol utili zed for the acidic pathway is mainly derived from plasma lipoproteins[2,7].
As can be seen in Figure 3, HepG2 cells release large amounts of bile acid precursors into the incubation medium. It cannot be excluded that the bile acid synthetic pathways also may be abnormal in cultured HepG2 cells.
Figure 3.
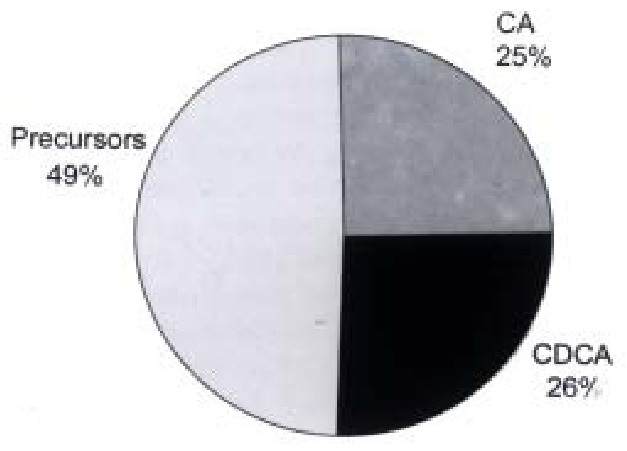
Distribution of cholic acid, chenodeoxycholic acid and precursors formed in cultures of HepG2 cells (data from ref. 7).
Conjugation
CA and CDCA were conjugated with glycine or taurine to more than 99.5% (Figure 4). About one-third of CDCA was also conjugated with sulphuric acid (Figure 5). 3β-Hydroxy-5-cholenoic acid and 3β, 7α-dihydroxy-5β-cholanoic acid were also sulphated. 27-Hydroxych olesterol and other oxysterols were partly sulphated.
Figure 4.
Conjugation of bile acids and precursors formed in primary cultures of human hepatocytes.
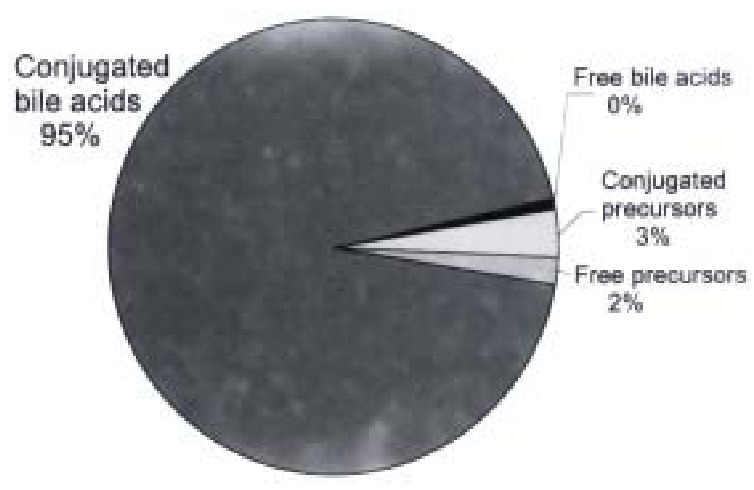
Figure 5.
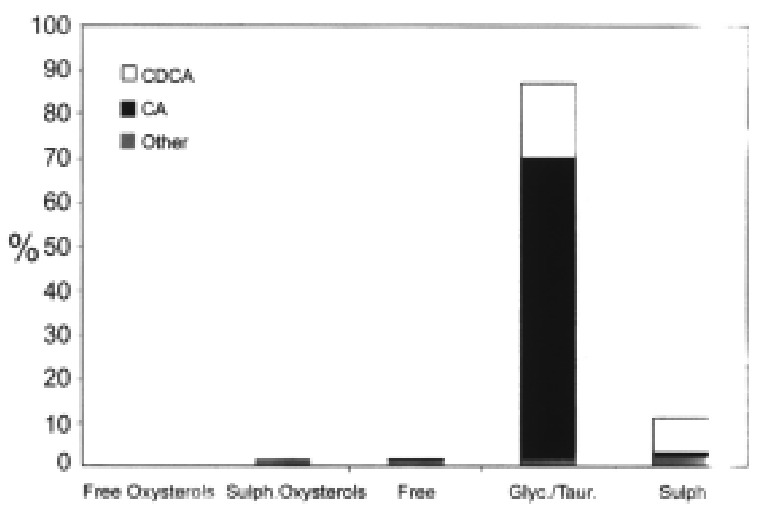
Distribution of free and conjugated bile acids and potential interme diates isolated from medium on the fifth day of incubating primary human hepatoc ytes. CDCA = chenodeoxycholic acid; CA = cholic acid.
In contrast to primary human hepatocytes, Hep G2 cells release large amounts of unconjugated bile acids into the medium (Figure 6).
Figure 6.
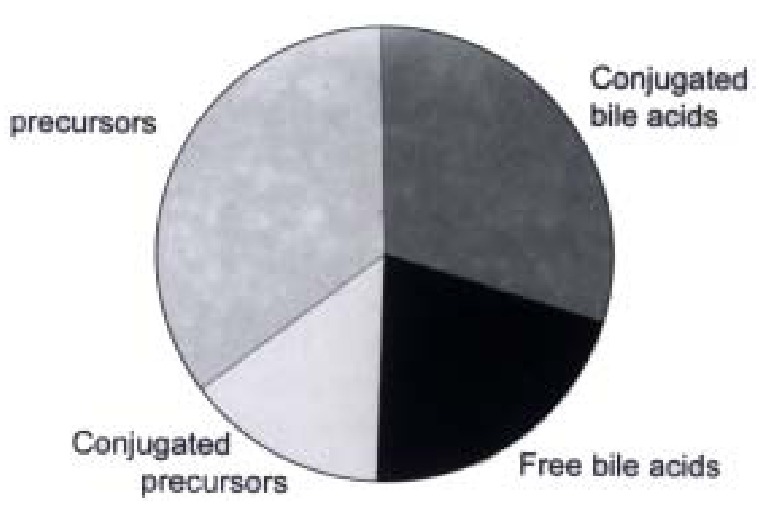
Conjugation of bile acids and precursors formed in cultures of HepG2 cells (data from ref. 7).
Effect of cyclosporin A
Cyclosporin A (CsA) is a potent inhibitor of the sterol 27-hydroxylase activity, especially towards nonpolar substrates like cholesterol[12,13]. When added to the culture medium, 1 μmol/L and 5 μmol/L CsA had l ittle or no effect on the formation of bile acids, but 10 μmol/L Cs A decreased the formation of CA by about 13% and CDCA by about 30% (Figure 7). No accumulation of bile acids occurred in the hepatocytes. However, a significant accumulation of 7α-hydroxy-4-cholestene-3-one and 7α, 12α-dihydroxy-4-cholestene-3-one, an intermediate in CA synthesis, was found indicating that not only the acidic pathway but also the neutral pathway of bile acid formation was affected by CyA. In agreement with thes e results, it has previously been reported that patients with cerebrotendinous xanthomatosis, who lack the sterol 27-hydroxylase[16,17], accumulate 7α, 12α-dihydroxy-4-cholestene-3-one more than other intermediates in the liver[18]. However, the decreased formation of total bile acids (CA and CDCA) during CsA treatment may mainly be due to an inhibitory effect of CsA on the hepatic synthesis of cholesterol[12].
Figure 7.
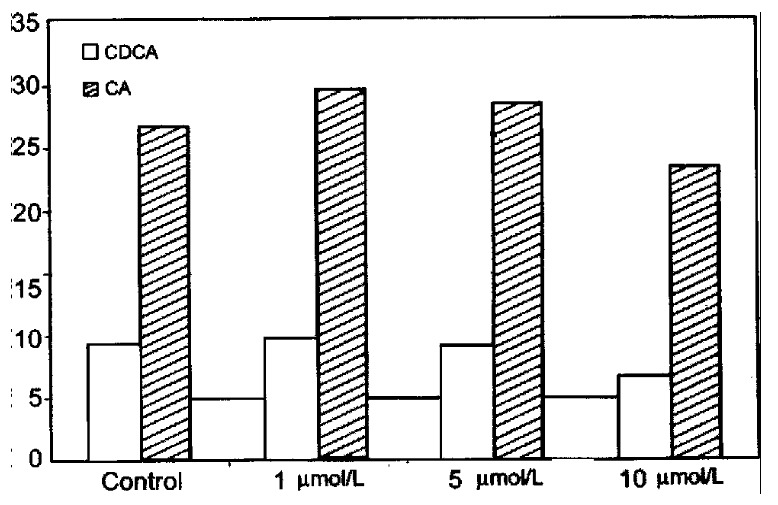
Effect of cyclosporin A on bile acid formation in primary human hepa tocytes.
Influence of hormones
Previous studies have shown that the combined addition of T3 and dexamethasone stimulates bile acid formation, cholesterol 7α-hydroxylase activity and mRNA levels in rat hepatocytes, whereas the addition of T3 or dexamethas one alone has little or no stimulatory effect[10,19]. The addition of T3 and dexamethasone alone or in combination to human hepatocytes did not increase bile acid synthesis (Figure 2). If anything, bile acid formation tended to decrease. The different effects of T3 and dexamethasone in rat and human hepatocytes agree well with recent studies of the hormonal regulation of the cholesterol 7α-hydroxylase gene promoter[20,21]. These studies showed that the rat cholesterol 7α-hydroxylase gene promoter is stimulated by dexamethasone whereas thyroid hormone has no significant effect. The promoter of the human cholesterol 7α-hydroxylase gene, on the other hand , is suppressed by both dexamethasone and thyroid hormone.
In conclusion, isolated human hepatocytes in culture behave as in the intact liver by almost quantitatively converting cholesterol to conjugated CA and CDCA. In contrast, cultured HepG2 cells release large amounts of bile acid precursors an d unconjugated bile acids into the medium. Human hepatocytes in culture represent a useful model for further studies on the synthesis and regulation of bile acids.
Footnotes
Supported by grants from the Swedish Medical Research Council (03X-4793 and 03X-7890)
Edited by Lu J proofread by Mittra S
References
- 1.Bjrkhem I. Mechanism of bile acid biosynthesis in mammalian liver. In: Danielsson H, Sj-vall J, eds.New comprehensive biochemistry,Vol 12. Ams terdam: Elsevier. 1985:231–278. [Google Scholar]
- 2.Axelson M, Sjövall J. Potential bile acid precursors in plasma--possible indicators of biosynthetic pathways to cholic and chenodeoxycholic acids in man. J Steroid Biochem. 1990;36:631–640. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- 3.Björkhem I. Mechanism of degradation of the steroid side chain in the formation of bile acids. J Lipid Res. 1992;33:455–471. [PubMed] [Google Scholar]
- 4.Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992;31:4737–4749. doi: 10.1021/bi00135a001. [DOI] [PubMed] [Google Scholar]
- 5.Princen HMG, Post SM, Twist J. Regulation of bile acid biosynthesis. Current Pharmaceutical Design. 1997;3:59–84. [Google Scholar]
- 6.Everson GT, Polokoff MA. HepG2. A human hepatoblastoma cell line exhibiting defects in bile acid synthesis and conjugation. J Biol Chem. 1986;261:2197–2201. [PubMed] [Google Scholar]
- 7.Axelson M, Mörk B, Everson GT. Bile acid synthesis in cultured human hepatoblastoma cells. J Biol Chem. 1991;266:17770–17777. [PubMed] [Google Scholar]
- 8.Pandak WM, Stravitz RT, Lucas V, Heuman DM, Chiang JY. Hep G2 cells: a model for studies on regulation of human cholesterol 7alpha-hydroxylase at the molecular level. Am J Physiol. 1996;270:G401–G410. doi: 10.1152/ajpgi.1996.270.3.G401. [DOI] [PubMed] [Google Scholar]
- 9.Cooper AD, Craig WY, Taniguchi T, Everson GT. Characteristics and regulation of bile salt synthesis and secretion by human hepatoma HepG2 cells. Hepatology. 1994;20:1522–1531. doi: 10.1002/hep.1840200623. [DOI] [PubMed] [Google Scholar]
- 10.Ellis E, Goodwin B, Abrahamsson A, Liddle C, Mode A, Rudling M, Bjorkhem I, Einarsson C. Bile acid synthesis in primary cultures of rat and human hepatocytes. Hepatology. 1998;27:615–620. doi: 10.1002/hep.510270241. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 12.Princen HM, Meijer P, Wolthers BG, Vonk RJ, Kuipers F. Cyclosporin A blocks bile acid synthesis in cultured hepatocytes by specific inhibition of chenodeoxycholic acid synthesis. Biochem J. 1991;275(Pt 2):501–505. doi: 10.1042/bj2750501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlbäck-Sjöberg H, Björkhem I, Princen HM. Selective inhibition of mitochondrial 27-hydroxylation of bile acid intermediates and 25-hydroxylation of vitamin D3 by cyclosporin A. Biochem J. 1993;293(Pt 1):203–206. doi: 10.1042/bj2930203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelson M, Ellis E, Mörk B, Garmark K, Abrahamsson A, Björkhem I, Ericzon BG, Einarsson C. Bile acid synthesis in cultured human hepatocytes: support for an alternative biosynthetic pathway to cholic acid. Hepatology. 2000;31:1305–1312. doi: 10.1053/jhep.2000.7877. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson I, Einarsson K, Angelin B, Nyberg B, Bergström K, Thulin L. Effects of somatostatin on hepatic bile formation. Gastroenterology. 1989;96:206–212. doi: 10.1016/0016-5085(89)90782-8. [DOI] [PubMed] [Google Scholar]
- 16.Setchell KD, Street JM. Inborn errors of bile acid synthesis. Semin Liver Dis. 1987;7:85–99. doi: 10.1055/s-2008-1040568. [DOI] [PubMed] [Google Scholar]
- 17.Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991;266:7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 18.Björkhem I, Oftebro H, Skrede S, Pedersen JI. Assay of intermediates in bile acid biosynthesis using isotope dilution--mass spectrometry: hepatic levels in the normal state and in cerebrotendinous xanthomatosis. J Lipid Res. 1981;22:191–200. [PubMed] [Google Scholar]
- 19.Hylemon PB, Gurley EC, Stravitz RT, Litz JS, Pandak WM, Chiang JY, Vlahcevic ZR. Hormonal regulation of cholesterol 7 alpha-hydroxylase mRNA levels and transcriptional activity in primary rat hepatocyte cultures. J Biol Chem. 1992;267:16866–16871. [PubMed] [Google Scholar]
- 20.Crestani M, Stroup D, Chiang JY. Hormonal regulation of the cholesterol 7 alpha-hydroxylase gene (CYP7) J Lipid Res. 1995;36:2419–2432. [PubMed] [Google Scholar]
- 21.Wang DP, Stroup D, Marrapodi M, Crestani M, Galli G, Chiang JY. Transcriptional regulation of the human cholesterol 7 alpha-hydroxylase gene (CYP7A) in HepG2 cells. J Lipid Res. 1996;37:1831–1841. [PubMed] [Google Scholar]


