Abstract
AIM: Several triggering receptors have been described to be involved in natural killer (NK) cell-mediated target cytotoxicity. In these studies, NK cells derive d from blood or spleen were used. Pit cells are liver-specific NK cells that possess a higher level of natural cytotoxicity and a different morphology when com pared to blood NK cells. The aim of this study was to characterize the role of t he NK-triggeringmoleculesNKR-P1A,ANK61antigen,and CD45 in pit cell-media ted killing of target cells.
METHODS: 51Cr-release and DNA fragmentation were used to quantify target cell lysis and apoptosis, respectively.
RESULTS: Flow cytometric analysis showed that pit cells expressed CD45, NK R-P1A, and ANK61 antigen. Treatment of pit cells with monoclonal antibody (mAb) to CD45 (ANK74) not only inhibited CC531s or YAC-1 target lysis but also apopto sis induced by pit cells. The mAbs to NKR-P1A (3.2.3) and ANK61 antigen (ANK61) had no effect on pit cell-mediated CC531s or YAC-1 target cytolysis or apoptosis, while they did increase the Fcγ receptor positive (Fcγ R+) P815 cytolysis and apoptosis. This enhanced cytotoxicity could be inhibited by 3,4-dichloroi socoumarin, an inhibitor of granzymes.
CONCLUSION: These results indicate that CD45 participates in pit cell-med iated CC531s and YAC-1 target cytolysis and apoptosis. NKR-P1A and ANK61 antig en on pit cells function as activation structures against Fcγ R+ P 815 cells, which was mediated by the perforin/granzyme pathway.
Keywords: hepatic NK cells, pit cells, cytolysis, apoptosis, perforin/granzyme pathway
INTRODUCTION
Natural killer (NK) cells can kill certain tumor cells or virus-infected cells without prior sensitization and thus play an important role in host anti-tumor defense[1]. Cytotoxic activity of NK cells is achieved by two distinct mechanisms, i.e. necrosis (cytolysis) and apoptosis, and is believed to be regulated by triggering and inhibiting receptors on NK cells[1-3]. Several candidate receptors involved in triggering a cytotoxic reaction, such as NKR-P1A (CD161A) and an NK-activation structure recognized by monoclonal antibody (mAb ) ANK61 (ANK61 antigen), have been described on rat NK cells[2,4,5].
NKR-P1A is a lectin-like surface molecule expressed on all rat NK cells[4]. The ligands for NKR-P1A are oligosaccharides. Interactions of these oligo saccharides on target cells with NKR-P1A on NK cells are crucial for target cell recognition and for NK cytotoxicity[6]. Monoclonal antibody to NKR -P1A (3.2.3) has be en shown to stimulate NK cell phosphoinositide turnover, calcium mobilization, degranulation and NK cytotoxicity against certain Fcγ receptor posit ive (Fcγ R+) tumor target cells[4,7]. These findings demonstrate that NKR-P1A is an activating molecule on rat NK cells that recognizes target cells and induces cytotoxicity.
Recently, a rat NK-activation structure (ANK61 antigen) recognized by mAb ANK61 has been described[5]. Cross-linking of the ANK61 antigen and Fcγ R+ tumor targets with mAb ANK61 resulted in enhanced killing of the targets by NK cells[5].
CD45, which is expressed by most leukocytes, including NK cells, has been shown to be involved in NK cell-mediated target cell cytotoxicity[8-10]. It is reported that mAbs against CD45 inhibit NK cellmediated target cell killing[8,9] and CD45-negative NK cells fail to lyse tumor targets[10]. The cytoplasmic domain of CD45 has tyrosine phosphatase activity, which is believed to play an important role in the regulation of NK activity[8,11] .
Pit cells are liver-specific NK cells[12]. Compared to blood NK cells, rat pit cells possess a higher level of cytolytic activity against a variety of tumor target cells, including NK resistant P815 cells, which is comparable to the cytotoxicity level of lymphokine-activated killer (LAK) cells[13]. These data indicate that pit cells have different characteristics from blood NK cells. Although much is known about the NK receptors that participate in NK cell a ctivation and target cell killing, the surface receptors involved in pit cell-mediated target cell killing have not yet been clarified. Moreover, involvement o f the above mentioned antigens in NK cell-mediated target cell apoptosis has re mained elusive, since 51Cr-release assay employed in the previous stu dies[4,5,10] indicates only cytolysis, rather than apoptosis. In the pre sent work, we used mAbs against CD45, NKR-P1A, and ANK61 antigen to evaluate their roles in pit cell-mediated target cell lysis and apoptosis.
MATERIALS AND METHODS
Isolation and purification of pit cells
Male Wistar rats (Proefdierencentrum, Leuven, Belgium) weighing (250-280) g were used at an age between 8 to 12 wk. Hepatic NK cells (pit cells) were iso lated according to protocols described before[14]. The purity of the rec overed pit cells was at least 90%, as evaluated by morphology on May-Giemsa-st ained cytospin preparations and by flow cytometric analysis using mAb 3.2.3. The viability of the recovered cells was more than 95%, as determined by Trypan blue exclusion. The procedures used in this study were approved by the local ethical committee (license No LA1230212).
Tumor cell line
CC531s, a dimethylhydrazine-induced colon carcinoma of Wag/Rij (inbred strain of Wistar) rats[15], P815, a murine mastocytoma, and YAC-1, a mouse T cell lymphoma, were maintained in culture medium RPMI-1640 (Gibco, Life Technolog ies, Gent, Belgium), supplemented with 10% fetal calf serum (Eurobiochem, Bierges, Belgium), benzylpenicillin (100 kU/L), streptomycin (100 mg/L) , and glutamine (0.2 mmol/L) (Gibco, Life Technologies, Gent, Belgium).
Reagents and antibodies
The following mouse anti-rat mAbs were used: 3.2.3 (anti-NKR-P1A, IgG2b or IgG1)[4], ANK74 (anti-CD45, IgG1)[8],and ANK61 (anti-rat NK activation structure, IgG1)[5] were developed in Department of S urgery and Pathology, Leiden University Medical Center, the Netherlands. 3,4-d ichloroisocoumarin (DCI), a granzyme inhibitor[16]was purchased from Sigma (Bornem, Belgium).
Flow cytometry
The expression of surface antigens on the cells was measured by one or two-colo r flow cytometric analyses as described previously[17]. Briefly, a quantity of 0.5 × 106 cells per sample was incubated (30 min, 4 °C) with the prim ary antibodies, 3.2.3, ANK74, and ANK61. Cells were then washed thrice with cold phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.02% sodium azide. Subsequently, cells were incubated with fluorescein-conjuga ted anti-mouse IgG1 and biotin-conjugated anti-mouse IgG2b (Gilbertsv ille, PA). After incubation and washing, cells were incubated with streptavidin-phycoerythrin (Gilbertsville, PA). Then, cells were washed and fixed with 2% p araformaldehyde in PBS and analyzed (FACstar, Becton Dickinson, Mountain View, CA). Isotype-matched irrelevant antibodies were used as a control.
51Cr-release assay
Cytolysis was measured after 4 h incubation of P815 and YAC-1 cells, and 18 h for CC531s cells. 51Cr-release assay was performed using round -bottomed 96-well microplates as described previously[18]. Briefly, 1 × 106 target cells/0.5 mL were labeled with 9.25 MBq Na 51CrO4 (A mersham Belgium, Gent, Belgium) for 80 min at 37 °C. Before the coincubation, freshly isolated pit cells were preincubated with either saturating amounts of the mAbs (final concentration for each mAb was 10 mg/L), 3.2.3, A NK61, ANK74, or irrelevant mouse IgG1 for 15 min at room temperature. The mAbs were al so present during the assay and the treatments with these mAbs did not affect the viability of the cells. The target cells were seeded at a concentration of 1 × 104 cells/ well. Suspensions of effector cells were then added to the wells at effector to target (E:T) ratios of 10:1 in a final volume of 200 μL. All assays were done in triplicate. For the experiments using DCI, pit cells were preincu bated with 50 μmol/L DCI for 30 min at 37 °C. After washing twice with medium, the cells were coincubated with the target cells as mentioned above. After a coincubation at 37 °C for 4 or 18 h, 100 μL of supernatant per well was aspi rated and counted in a gamma counter to determine experimental release. Results were expressed as percentage of specific lysis according to the formula:
Math 1
Math 1.

Math(A1).
Experimental release represents cpm (counts per minute) release from target cells in the presence of effector cell and mAbs. Spontaneous release was obtained fr om wells containing labeled target cells and medium only. Maximal release of 51Cr-labeled target cells was determined after adding 20 μL 10% sodium dodecyl sulfate (SDS) detergent to the labeled target cells.
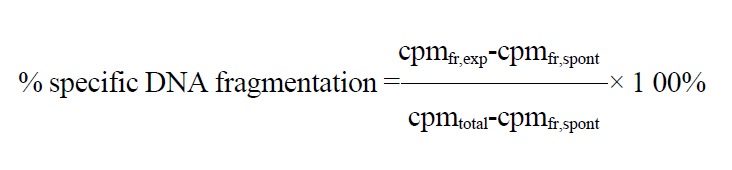
Quantitative DNA fragmentation assay
DNA fragmentation in the target cells was determined as described previously[19]. Briefly, to label the DNA of the target cells, 5 × 105 cells in 250 μL medium were incubated with 0.37 MBq [methyl-3H] thymidi ne ([3H]-TdR) (Amersham, Buckinghamshire, U.K) for 3 h at 37 °C. Before the coincubation, freshly isolated pit cells were preincubated with the mAbs as mentioned above. The pit cells (105 cells in 100 μL) and the target cells (104 cells in 100 μL) (E:T = 10:1) were placed in trip licate in 1.5 mL microcentrifuge tubes. The total volume per microcentrifuge tub e was 200 μL . For the experiments using DCI, pit cells were preincu bated with 50 μmol/L DCI for 30 min at 37 °C. After washing twice with medium, the cells were coincubated with the target cells as mentioned above. After 3 h coincubation at 37 °C and centrifugation at 300 × g for 10 min, the incubation medium was removed from the tubes. Sub sequently, the pelleted cells were lysed with 0.5 mL of cold lysis buffer (4 °C) (5 mmol/L Tris, 2 mmol/L EDTA, 1% Triton X-100, pH 7.4) for 30 min at 4 °C. Then, the lysates were ultracentrifuged (10000 × g for 15 min at 4 °C) to se parate fra gmented DNA from intact DNA. Radioactivity (cpm) in the incubation medium, in th e 10000 × g supernatant and in the 10000 × g pellet was determined in a beta count er (Beckman, Fullerton, CA, USA). The percentage fragmented DNA was calculated using the following formula:
Math 2
Math 2.
Math(A1).
in which: cpmfr = the radioactivity in the incubation medium plus the cpm in the 10000 × g supernatant; cpmtotal = cpmfr+radioac tivity in the 10000 × g pellet; exp = experimental (CC531s cells with pit cells); spont = spont aneous (CC531s cells and medium only).
Hoechst 33342 (HO 342)/propidium Iodide (PI) staining
The method of HO 342 and PI staining has been described previously[19]. In short, target cells, at a concentration of 1 × 104 cells per well in a 96m ultiwell plate, were coincubated with pit cells (E:T = 10:1). After 3 h coinc ubation, the cells were stained with HO 342, which stains DNA blue, and PI, which only penetrates necrotic cells through the damaged cell membrane and stains DN A red. Cells were viewed under a Leica DM IRB/E inverted fluorescence microscope (Leica, Heidelberg, Germany) with ultraviolet excitation at 340 to 380 nm. The apoptotic target cells were determined by their characteristic apoptotic morphol ogical changes of the nucleus, i.e. condensation of chromatin and nuclear fragmentation. Target cells alone acted as spontaneous apoptosis control.
Statistical analysis
Results were given as the mean with the corresponding standard deviation. Statistical analysis was performed by Students’t test or one-way ANOVA with post-hoc multiple comparison analysis made by Duncan test, using SPSS statistical package (SPSS Inc., Chicago, IL, USA). Statistical significance between two groups was considered at the level of P < 0.05.
RESULTS
Expression of surface antigens on pit cells
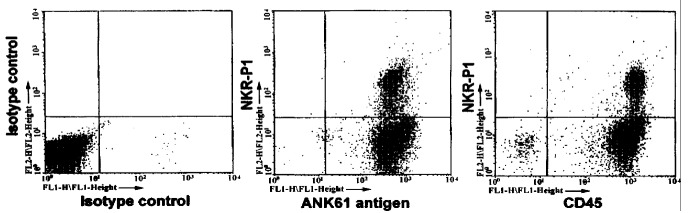
Flow cytometric analysis was used to determine the expression of surface antigens on pit cells and target cells. In two-color flow cytometric analysis, mAb 3.2 .3 was used to stain pit cells[20]. Pit cells were shown to express CD45 , which was recognized by mAb ANK74, and an NK activation structure, which was recognized by mAb ANK61 (Figure 1). CC531s, P815, and YAC-1 cells did not express these NK-associated antigens (data not shown).
Figure 1.
Expression of CD45 and ANK61 antigen on hepatic NK cells (pit cells). The cells were washed out of the liver by sinusoidal lavage. After Ficoll-Paque gradient centrifugation and nylon-wool adherence to remove erythrocytes, granul ocytes, monocytes and B lymphocytes, the cells were stained with anti-NKR-P1A mAb (3.2.3) (phycoerythrin) and anti-ANK61 antigen (ANK61), anti-CD45 mAb (ANK 74) (fluorescein) and analyzed by two-color flow cytometry. CD45 and ANK61 anti gen were expressed on the x-axis (fluorescein), NKR-P1A on the y-axis (phycoe rythrin).
Effect of CD45, NKR-P1A, and ANK61 antigen on pit cell-mediated target cell lysis
To investigate the function of these surface antigens in pit cell-mediated cyto lysis of targets, the mAbs against these antigens were used in 51Cr-r elease assays. In the presence of anti-CD45 mAb ANK74, the lysis of CC531s and YAC-1 cells by pit cells was significantly inhibited (P < 0.01, P < 0.05) ( Figure 2A, 2B). The presence of mAbs 3.2.3 and ANK61 had no effect on pit cell-mediated lysis against-CC531s and YAC-1 targets (Figure 2A, 2B). These data in dicate that CD45 is involved in pit cellmediated cytolysis against CC531s and YAC-1 targets.
Figure 2.
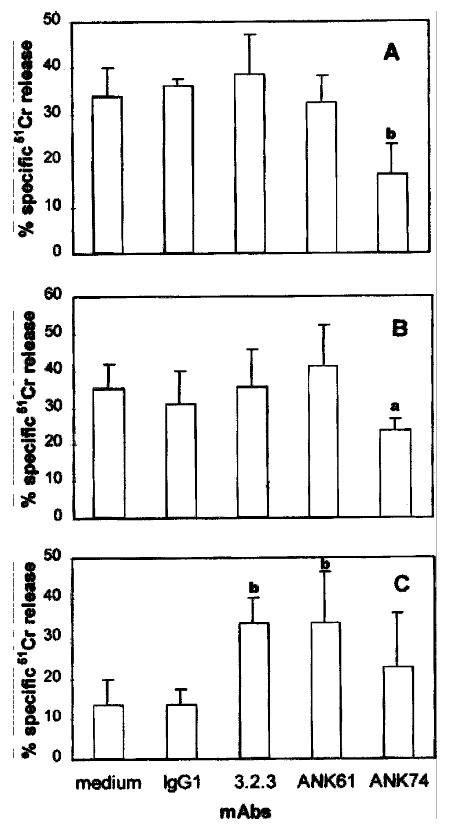
Effect of the mAbs on pit cell-mediated CC531s (A), YAC-1 (B) and P81 5 (C) target cell cytolysis.51Cr-labeled target cells were incubated at an E:T ratio of 10:1 with freshly isolated pit cells in the absence or prese nce of the mAbs. (A), Cytolysis of CC531s cells was measured in an 18h 51Cr-release assay. (B), Cytolysis of YAC-1 cells was measured in a 4-h 51Cr-release assay. (C), Cytolysis of P815 cells was measured in a 4-h 51Cr-release assay. Values are mean ± SD from four different experiments. aP < 0.05, bP < 0.01 vs the control
It is reported that mAbs 3.2.3 or ANK61 can induce cytolysis in Fcγ R+ target cells by NK cells by redirected antibody-dependent cellular cytotoxicity (rADCC )[4,5]. In order to investigate whether these mAbs play a similar role in pit cells, cytolytic activity of pit cells was measured by a 4-h 51Cr-release assay using the Fcγ R+ P815 cell line[21], a murine mast ocytoma, as target cells. As shown in Figure 2C, the mAbs 3.2.3 and ANK61 signif icantly increased the cytolysis of P815 cells by pit cells (P < 0.01). Ant i-CD45 mAb ANK74 did not significantly increase the cytolysis (Figure 2C).
Effect of CD45, NKR-P1A, and ANK61 antigen on pit cell-mediated target apoptosis
When CC531s[19], P815 (Vermijlen D, unpublished data) or YAC-1 targets (Figure 3) were coincubated with pit cells, these target cells showed typical mo rphological characteristics of apoptosis like nuclear fragmentation. To address the effect of the surface antigens in pit cell-mediated apoptosis of these targets, a quantitative DNA fragmentation assay was used. The results showed that the anti-CD45 mAb ANK74 significantly inhibited pit cell-mediated DNA fragmentat ion of CC531s and YAC-1 cells (P < 0.05, P < 0.01) (Figure 4A, 4B). The pre sence of mAbs 3.2.3, ANK61 had no effect on DNA fragmentation of CC531s and YAC-1 cells by pit cells (Figure 4A, 4B), but increased the DNA fragmentation of P 815 cells induced by pit cells (P < 0.01) (Figure 4C). The anti-CD45 mAb ANK7 4 did not significantly affect the DNA fragmentation of P815 cells by pit cells (Figure 4C).
Figure 3.
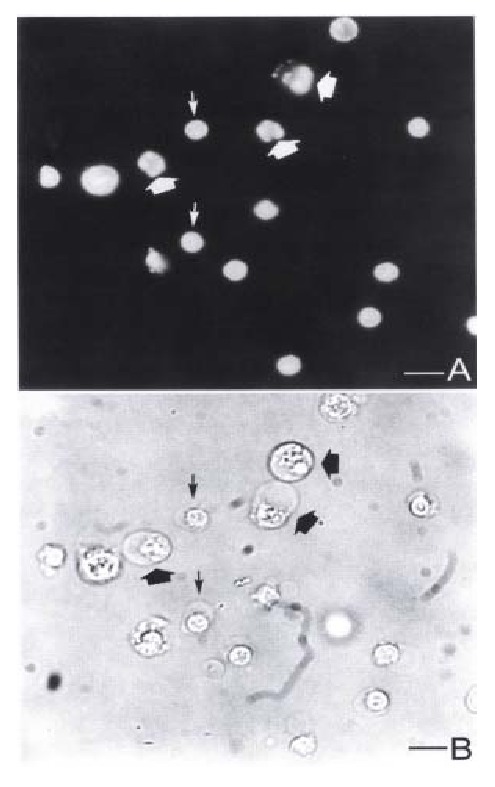
Fluorescence and light micrographs of YAC-1 cells coincubated with pit cells at an E:T ratio of 10:1 for 3 h. (A) Fluorescence micrograph showing the apoptotic YAC-1 cells with fragmented nuclei (thick arrows) and pit cells ( thin arrows). Light micrograph shows the same field as (A). Bar = 5 μm.
Figure 4.
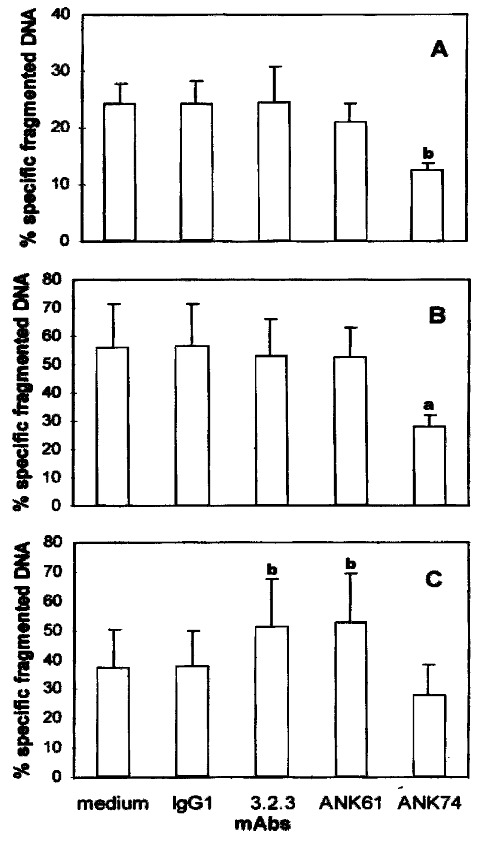
Effect of the mAbs on pit cell-induced CC531s (A), YAC-1 (B) and P8 15 (C) target cell apoptosis. [3H]-TdR labeled target cells were incubated at an E:T ratio of 10:1 with freshly isolated pit cells for 3 h in the absence or presence of the mAbs. Values are mean ± SD from four different ex periments.aP < 0.05, bP < 0.01 vs the control.
Inhibition of anti-NKR-P1A and ANK61 antigen mAb-enhanced P815 cell cytotoxicity by DCI
To address whether mAbs 3.2.3 and ANK61 enhanced Fcγ R+ P815 cell killing by pit cells via the perforin/granzyme pathway, we used DCI, an inhibitor of granzymes in intact cells, to investigate the role of granzymes in the process. When pit cells were preincubated with 50 μmol/L DCI, the mAb-enhanced P81 5 cytolysis and apoptosis by pit cells were completely inhibited (P < 0.05, P < 0.01) (Figure 5A, 5B). These results suggest that mAbs 3.2.3 or ANK61 induce cytotoxicity in Fcγ R+ P815 cells by pit cells via the perforin/granzyme pathway.
Figure 5.
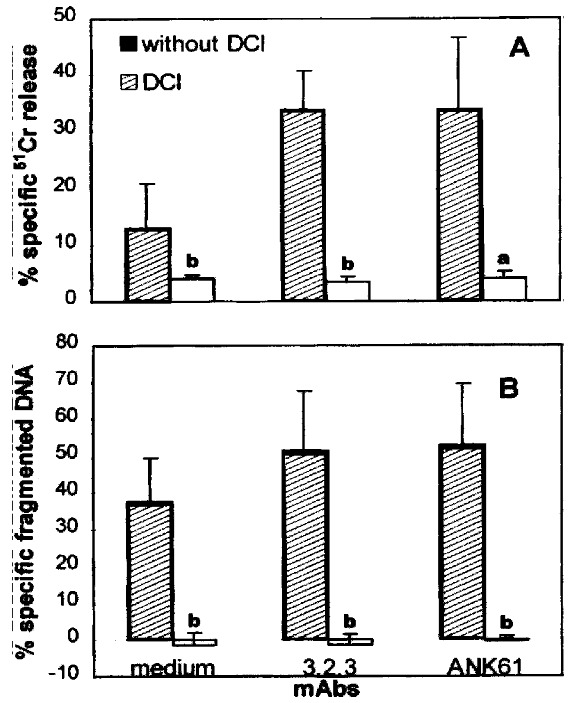
Effect of DCI on mAbs 3.2.3 and ANK61-enhanced Fcγ R+ P815 cell cytolysis (A) and apoptosis (B) by pit cells. Pit cells were preincubated with 50 μmol/L DCI for 30 min at 37 °C. After washing twice, the cells were coincubated with P815 cells as mentioned in materials and methods. Values are mean ± SD from three different experiments. aP < 0.05, bP < 0.01 vs the corresponding control.
DISCUSSION
Pit cells are liver-specific NK cells and, together with Kupffer cells, constit ute the natural cellular defense against invading cancer cells in the liver[12]. It has been shown that pit cells are four to eight times more cytotoxi c against YAC-1 and CC531s cells than blood NK cells and are able to kill NK-r esistant, LAK-sensitive P815 cells[13]. However, the surface receptors involved in pit cell-mediated cytotoxicity against tumor targets have remained elusive. Present data show that the mAbs against the NK-triggering molecules NK R-P1A and ANK61 antigen increased not only cytolysis but also apoptosis of FcγR+ P815 cells, but had no effect on Fcγ R YAC-1 and CC531s cytotoxicity ind uced by pit cells. These results indicate that NKR-P1A and ANK61 antigen on pit cells function as triggering molecules against Fcγ R+ target cells, but not against Fcγ R-targets. It has been demonstrated that mA bs 3.2.3 and ANK61 enhance cytolytic activity of NK and LAK cells only against Fcγ R+ tumo r target cells by rADCC[4,5,7]. Other evidence to support this observati on was that, in our experiments, cross-linking of NKR-P1A on pit cells with mAb 3.2.3 did not induce granule exocytosis from pit cells, as observed in May-Giemsa-stained cytospin preparations (data not shown). This is in contradiction with a report describing that mAb3.2.3caninduce degranulation and release of granzymes fro m NK and IL-2 activated NK cells[22]. Furthermore, after incubation of pit cells with mAb 3.2.3, the medium and the pit cells were separated by centrifugation. The medium did not kill CC531s and YAC-1 cells, whereas the pit cells’cytotoxicity against these targets was comparable to freshly isolated pit cells (data not shown). Moreover, it has also been shown that NKR-P1A negative NK clones are able to lyse the same type of target cells as NKR-P1A positive NK clones [23] and expression of NKR-P1A is not an absolute requirement for NK activity[24]. These data suggest that NKR-P1A is not the sole activation molecule on NK cells. Actually, a diversity of activation molecules on NK cells has been described[2,3,5,25]. NK cells might use these differe nt activation molecules to lyse different target cells.
It is believed that mAbs 3.2.3 or ANK61-enhanced cytotoxicity in Fcγ R+ target cells by NK cells is a rADCC reaction[4,5]. The present data show further that these mAb-enhanced c ytolysis and apoptosis of P815 cells by pit cells are mediated by the perforin/granzyme pathway because this enhanced cytotoxicity can be completely inhibited by the granzyme inhibitor DCI.
Although the function of CD45 is well known in NK cells[8-11], its rol e in pit cells has not yet been clarified. We showed here that CD45 was involved in the pit cell induced cytolysis and apoptosis of CC531s and YAC-1 cells but not of P815 cells. The reason that CD45 on pit cells had different effects on pi t cell-mediated cytotoxicity against different targets is not clear. It has bee n reported that CD45 is not essential for Fcγ R-mediated function in NK cells, since NK cells in CD45-/-mice have normal cytotoxic activities, comparable to normal mice (CD45+/+)[26]. Another possible explanation is that CD4 5 plays a regulatory role in signal transduction through surface receptors on NK cells, such as NKR-P1A, LFA-1, and killer cell inhibitory receptors (KIR) in NK cell-mediated target killing[8,10,11]. A diversity of receptors is found to be involved in NK cell activation[2,3]. It could be that CD45 plays a different regulating role in different NK activation receptors. On the other ha nd, the regulatory role of CD45 in the inhibitory signal pathway via KIR is dependent on the expression level of MHC class I on target cells[8]. The ant i-CD45 mAb inhibits only the lysis of target cells expressing low level of MHC class I, such as CC531s[8] and YAC-1[27], whereas the lysis of target cells expressing high level of MHC class I, such as P815 [27], is not inhibited[8].
In summary, CD45 on pit cells participated in pit cell-mediated CC531s and YAC-1 but not P815 cytolysis and apoptosis. NKR-P1A and ANK61 antigen on pit cells were involved in pit cell-mediated Fcγ R+ P815 cell cytolysis a nd apoptos is, but not in Fcγ R-CC531s and YAC-1 cell cytotoxicities. The mAbs 3.2.3 an d ANK61-induced cytotoxicities of Fcγ R+ P815 cells by pit cells were mediated by the perforin/granzyme pathway. These findings provide evidence that the same m olecules on pit cells and blood or spleen-derived NK cells regulate target cell lysis and apoptosis. The difference in cytotoxic capacity between these cell ty pes therefore may be on a quantitative rather than a qualitative level.
ACKNOWLEDGEMENTS
We thank Carine Seynaeve and Marijke Baekeland for their technical help, Karen C rits for her technical assistance in flow cytometry, Ronald De Zanger for his help with the statistical analysis, and Chris Derom for her photographic support.
Footnotes
Supported by the grants 3.0053.92, 3.0050.95, 9.0038.96, 1.5.411. 98 from the Nat ional Foundation for Scientific Research (FWO) and the grants 194.322.1740, 195.332.1310, 196.322.0140, and OZR.230 from the Research Council of the Free University of Brussels
Edited by Zhu QR proofread by Mittra S
References
- 1.Brittenden J, Heys SD, Ross J, Eremin O. Natural killer cells and cancer. Cancer. 1996;77:1226–1243. doi: 10.1002/(sici)1097-0142(19960401)77:7<1226::aid-cncr2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 3.Timonen T, Helander TS. Natural killer cell-target cell interactions. Curr Opin Cell Biol. 1997;9:667–673. doi: 10.1016/s0955-0674(97)80120-2. [DOI] [PubMed] [Google Scholar]
- 4.Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giezeman-Smits KM, Gorter A, Nagelkerke JF, van Vlierberghe RL, van Eendenburg J, Eggermont AM, Fleuren GJ, Kuppen PJ. Characterization of three new membrane structures on rat NK cells which are involved in activation of the lytic machinery. Immunobiology. 1997;197:429–443. doi: 10.1016/S0171-2985(97)80077-0. [DOI] [PubMed] [Google Scholar]
- 6.Bezouska K, Yuen CT, O'Brien J, Childs RA, Chai W, Lawson AM, Drbal K, Fiserová A, Pospísil M, Feizi T. Oligosaccharide ligands for NKR-P1 protein activate NK cells and cytotoxicity. Nature. 1994;372:150–157. doi: 10.1038/372150a0. [DOI] [PubMed] [Google Scholar]
- 7.Ryan JC, Niemi EC, Goldfien RD, Hiserodt JC, Seaman WE. NKR-P1, an activating molecule on rat natural killer cells, stimulates phosphoinositide turnover and a rise in intracellular calcium. J Immunol. 1991;147:3244–3250. [PubMed] [Google Scholar]
- 8.Giezeman-Smits KM, Gorter A, van Vlierberghe RL, v Eendenburg JD, Eggermont AM, Fleuren GJ, Kuppen PJ. The regulatory role of CD45 on rat NK cells in target cell lysis. J Immunol. 1999;163:71–76. [PubMed] [Google Scholar]
- 9.Starling GC, Hart DN. CD45 molecule cross-linking inhibits natural killer cell-mediated lysis independently of lytic triggering. Immunology. 1990;71:190–195. [PMC free article] [PubMed] [Google Scholar]
- 10.Bell GM, Dethloff GM, Imboden JB. CD45-negative mutants of a rat natural killer cell line fail to lyse tumor target cells. J Immunol. 1993;151:3646–3653. [PubMed] [Google Scholar]
- 11.Poggi A, Pardi R, Pella N, Morelli L, Sivori S, Vitale M, Revello V, Moretta A, Moretta L. CD45-mediated regulation of LFA1 function in human natural killer cells. Anti-CD45 monoclonal antibodies inhibit the calcium mobilization induced via LFA1 molecules. Eur J Immunol. 1993;23:2454–2463. doi: 10.1002/eji.1830231012. [DOI] [PubMed] [Google Scholar]
- 12.Wisse E, Luo D, Vermijlen D, Kanellopoulou C, De Zanger R, Braet F. On the function of pit cells, the liver-specific natural killer cells. Semin Liver Dis. 1997;17:265–286. doi: 10.1055/s-2007-1007204. [DOI] [PubMed] [Google Scholar]
- 13.Vanderkerken K, Bouwens L, Wisse E. Characterization of a phenotypically and functionally distinct subset of large granular lymphocytes (pit cells) in rat liver sinusoids. Hepatology. 1990;12:70–75. doi: 10.1002/hep.1840120112. [DOI] [PubMed] [Google Scholar]
- 14.Kanellopoulou C, Seynaeve C, Crabbé E, Baekeland M, Vermijlen D, Vermoe sen A, Braet F, Zanger RD, Wisse E. Isolation of pure pit cells with a magnetic cell sorter and effect of contaminating cells on their cytolytic capability agai nst CC531. In: Wisse E, Knook DL and Balabaud C, editors. Cells of hepatic sinu soid. The Kupffer cell foundation. Leiden, The Netherlands. 1997;6:471–4 7 3. [Google Scholar]
- 15.Marquet RL, Westbroek DL, Jeekel J. Interferon treatment of a transplantable rat colon adenocarcinoma: importance of tumor site. Int J Cancer. 1984;33:689–692. doi: 10.1002/ijc.2910330521. [DOI] [PubMed] [Google Scholar]
- 16.Odake S, Kam CM, Narasimhan L, Poe M, Blake JT, Krahenbuhl O, Tschopp J, Powers JC. Human and murine cytotoxic T lymphocyte serine proteases: subsite mapping with peptide thioester substrates and inhibition of enzyme activity and cytolysis by isocoumarins. Biochemistry. 1991;30:2217–2227. doi: 10.1021/bi00222a027. [DOI] [PubMed] [Google Scholar]
- 17.Luo D, Vanderkerken K, Bouwens L, Kuppen PJ, Baekeland M, Seynaeve C, Wisse E. The role of adhesion molecules in the recruitment of hepatic natural killer cells (pit cells) in rat liver. Hepatology. 1996;24:1475–1480. doi: 10.1002/hep.510240629. [DOI] [PubMed] [Google Scholar]
- 18.Bouwens L, Wisse E. Hepatic pit cells have natural cytotoxic (NC) activity against solid tumor derived target cells. In: Wisse E, Knook DL, Decker K, editors. Cells of the Hepatic Sinusoid. The Kupffer Cell Foundation, Rijswijk. The Netherlands. 1989;2:215–2 2 0. [Google Scholar]
- 19.Vermijlen D, Luo D, Robaye B, Seynaeve C, Baekeland M, Wisse E. Pit cells (Hepatic natural killer cells) of the rat induce apoptosis in colon carcinoma cells by the perforin/granzyme pathway. Hepatology. 1999;29:51–56. doi: 10.1002/hep.510290143. [DOI] [PubMed] [Google Scholar]
- 20.Luo D, Vanderkerken K, Bouwens L, Kuppen PJ, Crabbé E, Wisse E. The number and distribution of hepatic natural killer cells (pit cells) in normal rat liver: an immunohistochemical study. Hepatology. 1995;21:1690–1694. [PubMed] [Google Scholar]
- 21.van de Griend RJ, Bolhuis RL, Stoter G, Roozemond RC. Regulation of cytolytic activity in CD3- and CD3+ killer cell clones by monoclonal antibodies (anti-CD16, anti-CD2, anti-CD3) depends on subclass specificity of target cell IgG-FcR. J Immunol. 1987;138:3137–3144. [PubMed] [Google Scholar]
- 22.Velotti F, Palmieri G, D'Ambrosio D, Piccoli M, Frati L, Santoni A. Differential expression of granzyme A and granzyme B proteases and their secretion by fresh rat natural killer cells (NK) and lymphokine-activated killer cells with NK phenotype (LAK-NK) Eur J Immunol. 1992;22:1049–1053. doi: 10.1002/eji.1830220426. [DOI] [PubMed] [Google Scholar]
- 23.Brissette-Storkus C, Appasamy PM, Hayes LA, Kaufman CL, Ildstad ST, Chambers WH. Characterization and comparison of the lytic function of NKR-P1+ and NKR-P1-rat natural killer cell clones established from NKR-P1bright/TCR alpha beta-cell lines. Nat Immun. 1995;14:98–113. [PubMed] [Google Scholar]
- 24.Pinard D, Olsson NO, Chambers WH, Martin F. High expression of NKR-P1 is not an absolute requirement for natural killer activity in BDIX rats. Cancer Immunol Immunother. 1996;42:15–23. doi: 10.1007/s002620050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 26.Yamada H, Kishihara K, Kong YY, Nomoto K. Enhanced generation of NK cells with intact cytotoxic function in CD45 exon 6-deficient mice. J Immunol. 1996;157:1523–1528. [PubMed] [Google Scholar]
- 27.Haridas V, Saxena RK. Role of major histocompatibility complex class I antigens in modulating the performance of murine tumour cells in cold target competition assays. Immunology. 1995;84:86. [PMC free article] [PubMed] [Google Scholar]