INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies in China. To date, surgery is still the best solution to it. However, metastatic recurren ces after curative hepatic resections are very common. Tang et al[1] have repor ted that recurrence rate within 5 years of curative hepatic resection is 61.5%. As curative hepatic resection has a high tendency for metastatic recu rrence, therapeutic interventions such as transarterial embolization and antiang i ogenesis have been tried to further improve prognosis of HCC patients. Therefore, establishing a dependable, sensitive, easy, and economical method to predict me tastatic recurrence following curative hepatic resection is of clinical urgency.
Neovascularization has been shown to be essential for the growth and metastasis of solid tumors. Vascular endothelial growth factor (VEGF), a dimeric hepari n-binding glycoprotein with a molecular weight of about Mr 45000, is one of the most important angiogenic factors. In addition to increasing permeability of blood vessels, VEGF has potent mitogenic effect on vascular endothelial cells[2-9]. Serum VEGF levels have previously been shown to be raised in patients with various tumors, including brain, renal, melanoma, breast, gastrointest inal, and liver malignancies particularly in metastatic diseases[10-15].Because VEGF plays an essential role in tumor angiogenesis and hence the meta stasis and recurrence of HCC, its elevation in serum may be a candid at ebiomarker of metastatic recurrence. Con-sequently, we set out to study whether preoperative serum VEGF could be used as a biomarker of metastatic recurrence following curative hepatic resection in HCC. Since 84.6% of HCC patients have acc ompanied cirrhosis to some extent we also examined serum concentrations of VEGF in cirrhotic patients and normal healthy controls[16]. In addition, we studied the relationship between serum VEGF concentrations and immuno histological expressions of two known metastatic recurrence parameters-p53 and PCNA in tumor tissues.
MATERIALS AND METHODS
Subjects
The current study registered 12 normal healthy controls, 12 patients with cirr hosis, 8 patients with benign liver tumors including hemangioma and focal nodular hyperplasia, and 85 HCC patients who received curative resection. The healthy controls were selected randomly from people coming to our hospital for a medical checkup and found to be healthy. Cirrhotic patients were diagnosed clinically. HCC and benign liver tumor were diagnosed histologically. All HCC patients had u nderlying cirrhosis to some degree as confirmed by operations. Thrombi, intra- and extra-hepatic dissemination, were confirmed by operation, and/or ultrasonog raphy, and computed tomography. According to generally recognized standards, we set HCC patients with thrombi, intra- and extra-hepatic dissemina tion, and tumor size larger than 5 cm as high-tendency metastatic recurrence (HTMR) group, and less than 5 cm as low-tendency metastatic recurrence (LTMR) group. Hepatic resection with no signs of tumor lesion within the liver, and no metastatic lesion outside the liver after operation as well as no tumor thrombi in major bran ches of portal, hepatic vein, and intrahepatic biliary ducts before operation wa s considered as curative hepatic resection.
Blood samples were taken from all subjects. The serum was separated after 20-30 min of coagulation at room temperature and was stored at -80 °C until the assay. Repeated thawing and freezing of samples was avoided.
VEGF assay
The VEGF determinations were performed in duplicate following the manufacturer’s instructions using the R&D Systems Quantikine enzyme-linked immunosorbent assay (ELISA) kit. The VEGF concentration in a sample was determined by computer software-generated interpolation (Microsoft Origin software) from the standard curve. The internal VEGF standards ranged from 0 to 2000 ng/L, and the intens ity of chromogen was measured at a wavelength of 450 nm with a reference wavelength of 595 nm using the dual wavelength mode on the BIO-RAD 450 Microplate Reader. Standard curve was generated and plotted using a log-log linear regression.
Immunohistochemistry
For the immunohistochemical demonstration of p53 and PCNA protein, formalin fixed, paraffin embedded sections were deparaffinized in xylene and alcohol and placed for 15 min in alcohol-H2O2 for blocking endogenous peroxidase. The samples were processed in a microwave oven, placed in a thermoresistant plas tic box with 10 mmol/L pH 6.0 citrate buffer. Tissue sections were treated in the oven twice for 5 min while the buffer was boiling. Tissue sections were left a t room temperature in the buffer solution for 20 min without drying. Section s were treated with bovine serum albumin to prevent background staining and incu bated for 1 h with a primary nondiluted ready-to-use murine anti-p53 a ntibody (Dako, Carpentaria, CA) or murine anti-PCNA antibody (Dako, Carpentaria, CA) diluted at 1:500 at room temperature in a humidified chamber. Slides were rinsed with phosphate buffered saline for 3 min and incubated first with the biotinylated linked goat anti-mouse antibody for 30 min and then with the l abeling reagent, peroxidase conjugated streptavidine, for 30 min. After the slides were rinsed, the peroxidase label was demonstrated using 3-amino-9-eth ylcarbazol (AEC) for 15 min, and counterstained with Mayer hematoxylin. AEC produced a red product which was soluble in alcohol and was used with an aqueous mounting media. Positive and negative controls were included in each experiment. Specifically, for the latter the primary antibody was substituted with nonspec ific mouse IgG. p53 or PCNA immunopositivity was recorded when more than 15 carcinoma cell nuclei were stained in one or more fields[10].
Statistics
Analyses were performed using SAS (Version 6.12; SAS Institute, Inc., Cary, NC ). Student’s t test and Oneway ANOVA were used to determine the differences between the means of different groups. Results were expressed as mean ± SD. Th e level of significance was P < 0.05.
RESULTS
The VEGF concentrations in the normal controls and groups of cirrhotic, benign liver tumor, and HCC patients were 158.46 ± 41.84 ng/L, 90.00 ± 22.42 ng/L, 156 .34 ± 41.32 ng/L, 164.42 ± 76.07 ng/L, respectively (Table 1). Cirrhotic patients had the lowest levels of VEGF in the four groups. Compared with the cirrhotic group, HCC group had a significantly higher level of VEGF in the serum (P < 0 .01). Yet, no significant differences could be found between serum levels of VEG F in HCC and benign liver tumor or normal healthy control group (P > 0.05) (Figure 1). Since large HCC has a high tendency to recur after hepatic resection, we next divided HCC patients into small HCC and large HCC group. The VEGF concent rations of large HCC group were a little higher than those of small HCC patients (173.52 ± 52.34 ng/L vs 154.46 ± 37.23 ng/L, P > 0.05). However, this diffe rence was not significant. In patients with thrombi, VEGF levels were significan tly higher than those in patients without (182.46 ± 35. 61 ng/L vs 157.62 ± 53. 42 ng/L, P < 0.05). On dividing the HCC patients into HTMR and LTMR groups, HT MR patients were observed to have significantly higher VEGF concentrations in th e serum than LTMR patients (185.33 ± 92.88 ng/L vs 144.75 ± 51.37 ng/L, P < 0.05) (Figure 2). Anotable case observed was that of a female patient having a tumor growth of just 1.8 cm diameter with no thrombi but with the highest levels of VEGF (819.37 ng/L), she was the first to metastasize (within three months). As p53 is reported to play a role in regulating the production of VEGF, we f urther divided HCC patients into p53 positive and p53 negative groups. We found that serum VEGF levels in p53 positive patients were significantly h igher than those in p53 negative patients (176.56 ± 53.29 ng/ L vs 149.26 ± 41.29 ng/L, P < 0.05). Despite PCNA being a commonly used clinical indicator of metastatic recurrence after curative hepatic resection in HCC, we did not fi nd any significant difference in VEGF levels between PCNA positive and PCNA nega tive groups (176.56 ± 53.29 ng/L vs 165.26 ± 54.29 ng/L, P > 0.05).
Table 1.
Serum VEGF levels in different HCC groups which received curative hepatic resect ion, benign liver tumor group, and normal control group (-x ± s)
| Group | Case (n) | VEGF concentrations (ng/L) |
| Normal control | 12 | 158.46 ± 41.84 |
| Liver cirrhosis | 12 | 90.00 ± 22.42 |
| Benign | 8 | 156.34 ± 41.34 |
| HCC | 85 | 164.42 ± 76.07ab |
| Small HCC | 34 | 154.46 ± 37.23 |
| Large HCC | 51 | 173.52 ± 52.34c |
| Without thrombi | 71 | 157.62 ± 53.42 |
| Thrombi | 14 | 182.46 ± 35.61d |
| p53 negative | 34 | 149.26 ± 41.29 |
| p53 positive | 38 | 176.56 ± 53.29e |
| PCNA negative | 36 | 165.26 ± 54.29 |
| PCNA positive | 31 | 176.56 ± 53.29f |
P < 0.01, vs liver cirrhosis;
P > 0.05, vs benign;
P < 0.05, vs small HCC;
P < 0.05, without thrombi;
P < 0.05, vs p53 negative;
P > 0.05, vs PCNA negative.
Figure 1.
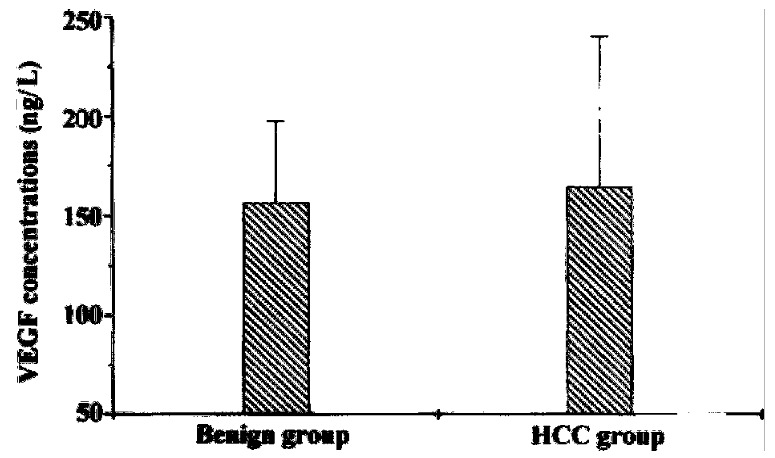
Serum VEGF levels in benign liver tumor and HCC (hepa tocellular carcinoma) groups.
Figure 2.
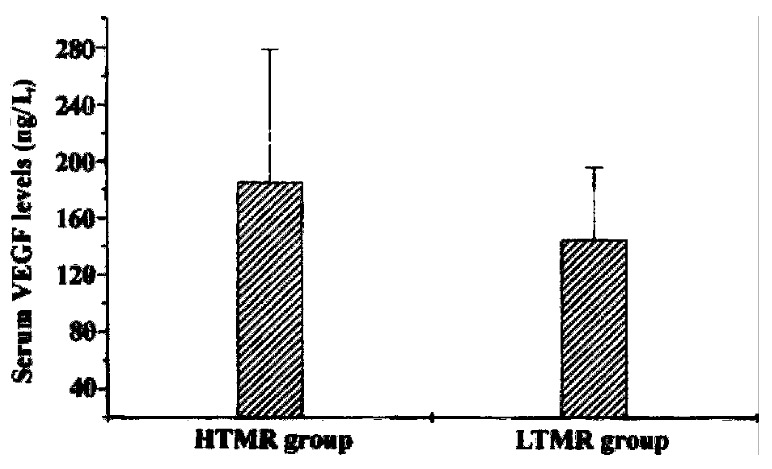
Serum VEGF levels in HTMR and LTMR groups. HTMR: high-tendency metastatic recur rence group; LTMR: low tendency metastatic recurrence group.
DISCUSSION
VEGF is produced by a wide variety of tumor cells, helping the growth and diss emination of the solid tumor by making it more vascular. In HCC, it acts in a paracrine fashion and plays an essential role in tumor angiogenesis[2-5,17].
The prognostic value of VEGF has been shown in breast and gastric cancer based on VEGF expression in tumor tissue detected by immunohistochemistry, with VEGF concentrations being high in highly vascular rich breast tumors. The VEGF positi vity in gastric cancer correlates with vessel involvement, lymph node metastasis as well as liver metastasis and is associated with an overall poor prognosis[18-23]. Because local tumor invasion and metastatic spread are angiogenes is-dependent, it is hypothesized that metastatic recurrence after curative hep atic resection in HCC may be associated with up-regulation of angiogenic factors. Our present study showed that HTMR patients had significantly higher levels o f VEGF than LTMR patients. This indicates that VEGF is a potential biomarker of metastatic recurrence in HCC patients after curative hepatic resection. The sens itive elevation of VEGF in one female patient further strengthens the hypothesis that raised VEGF levels may predict metastatic recurrence in HCC. The range of serum VEGF levels among healthy controls was from undetectable to 481.02 ng/L. The relevance of normal levels of VEGF is not clear at present and further studies are required to clarify it. As about 84.6% of Chinese HCC patients have some degrees of cirrhosis, it would be proper to compare VEGF levels between cirrhos is group and HCC group, rather than normal healthy controls and HCC patients[16]. Compared with cirrhotic patients, HCC patients had significantly hig her levels of VEGF in their serum. It indicates that VEGF could play an importan t role in transforming liver cirrhosis into HCC. Unexpectedly, the mean serum le vels of VEGF in HCC and benign liver tumor patients were observed to be very clo se. This suggests that VEGF can not be used as a marker to distinguish benign li ver tumor from malignant one (HCC). The main reason for this phenomenon may be that all of the benign liver patients in our study have noncirrhotic liver. Since tumor thrombi is a putative indicator of early metastatic recurrence following curative hepatic resection and poor prognosis[24,25], we detected VEGF levels in HCC patients with thrombi and found that consistent high levels of VEG F reflected a high tendency towards metastatic recurrence in the thrombi group. Meanwhile, we found that there was no significant difference between small HCC group and large HCC group regarding VEGF concentrations although mean serum VEGF levels in big HCC patients were higher than those in small HCC patients. Despite the fact that tumor size is a commonly used prognostic indicator of HCC, our re sult does not sufficiently reflect its role as an important parameter of metastatic recurrence in HCC[26,27]. Here the point to contemplate is that the ability of metastatic recurrence of HCC cannot be predicted by a single parameter alone, such as presence of tumor thrombi, intrahepatic dissemination or tumor size, rather all of them combined together can give a more precise indication.
p53 and PCNA have been reported to be indicators of HCC metastatic recurr ence[28-31]. Meanwhile, p53 also plays an important role in regula ting the production of VEGF. Wild-type p53 down-regulates whereas mutated p53 up-regulates VEGF expression according to some studies[32-35] . In the present study, p53 positive patients had significantly higher levels of VEGF than p53 negative counterparts. confirming previous reports[25]. However, the difference in VEGF levels between PCNA positive and PCNA neg ative groups was not significant. The role of PCNA in the regulation of VEGF is currently being investigated in our lab.
In conclusion, this study demonstrates that serum VEGF is a potential biomarke r of metastatic recurrence in HCC patients following curative hepatic resection. However, it can not distinguish HCC from benign liver tumor. p53 positive patients have a significantly higher VEGF level in the serum than their counterp arts. Further follow-up studies are needed to delineate the ability of VEGF in predicting metastatic recurrence after curative hepatic resection in HCC.
ACKNOWLEDGEMENTS
We thank Professor Yin-Kun Liu and Dr. Li-Neng Zhang for their help and sugge stions.
Footnotes
Supported by the Shanghai Leading Medical Subjects Grant (No 983001) and State Key Basic Research Grant (No G1998051211) for financial supports.
Edited by Zhu QR proofread by Mittra S
References
- 1.Tang ZY, Yu YQ, Zhou XD. An important approach to prolong-ing surviv alfurther after radical resection of AFP positive hepa-tocellular carcinoma. J Exp Clin Cancer Res. 1984;3:359–366. [Google Scholar]
- 2.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 3.Nicosia RF, Lin YJ, Hazelton D, Qian X. Endogenous regulation of angiogenesis in the rat aorta model. Role of vascular endothelial growth factor. Am J Pathol. 1997;151:1379–1386. [PMC free article] [PubMed] [Google Scholar]
- 4.Miller JW. Vascular endothelial growth factor and ocular neovascularization. Am J Pathol. 1997;151:13–23. [PMC free article] [PubMed] [Google Scholar]
- 5.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 7.Yue WB, Wang LD, Ding I. Detection of angiogenic growth fac-tors in patients with precancerous and cancerous lesions of esopha-gus from high risk- area in Henan, China. World J Gastroenterol. 1998;4 Suppl 2:109–111. [Google Scholar]
- 8.He P, Tang ZY, Ye SL, Liu BB. Relationship between expression of alpha-fetoprotein messenger RNA and some clinical parameters of human hepatocellular carcinoma. World J Gastroenterol. 1999;5:111–115. doi: 10.3748/wjg.v5.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun HC, Li XM, Xue Q, Chen J, Gao DM, Tang ZY. Study of angiogenesis induced by metastatic and non-metastatic liver cancer by corneal micropocket model in nude mice. World J Gastroenterol. 1999;5:116–118. doi: 10.3748/wjg.v5.i2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, Ramakrishnan S. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997;80:98–106. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997;79:206–213. doi: 10.1002/(sici)1097-0142(19970115)79:2<206::aid-cncr2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Kumar H, Heer K, Lee PW, Duthie GS, MacDonald AW, Greenman J, Kerin MJ, Monson JR. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res. 1998;4:1279–1285. [PubMed] [Google Scholar]
- 13.Toi M, Kondo S, Suzuki H, Yamamoto Y, Inada K, Imazawa T, Taniguchi T, Tominaga T. Quantitative analysis of vascular endothelial growth factor in primary breast cancer. Cancer. 1996;77:1101–1106. doi: 10.1002/(sici)1097-0142(19960315)77:6<1101::aid-cncr15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 15.Baccala AA, Zhong H, Clift SM, Nelson WG, Marshall FF, Passe TJ, Gambill NB, Simons JW. Serum vascular endothelial growth factor is a candidate biomarker of metastatic tumor response to ex vivo gene therapy of renal cell cancer. Urology. 1998;51:327–332. doi: 10.1016/s0090-4295(97)00498-6. [DOI] [PubMed] [Google Scholar]
- 16.Ying YY. Advances in primary liver cancer pathological research. In: Tan g ZY.Research and advance of primary liver cancer. Shanghai: Shanghai Medical University Publishing House 1990.p 68-71 [Google Scholar]
- 17.Stapleton AM, Zbell P, Kattan MW, Yang G, Wheeler TM, Scardino PT, Thompson TC. Assessment of the biologic markers p53, Ki-67, and apoptotic index as predictive indicators of prostate carcinoma recurrence after surgery. Cancer. 1998;82:168–175. doi: 10.1002/(sici)1097-0142(19980101)82:1<168::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, Senger DR. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255–1262. [PMC free article] [PubMed] [Google Scholar]
- 19.Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, Sakahara H, Mori T. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76:1221–1227. doi: 10.1038/bjc.1997.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton DP, Cai A, Wendt K, Young M, Gamero A, De Cesare S. Angiogenic protein expression in advanced epithelial ovarian cancer. Clin Cancer Res. 1997;3:1579–1586. [PubMed] [Google Scholar]
- 22.Masood R, Cai J, Zheng T, Smith DL, Naidu Y, Gill PS. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci USA. 1997;94:979–984. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(sici)1097-0142(19960301)77:5<858::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Bu W, Tang ZY, Sun FX, Ye SL, Liu KD, Xue Q, Chen J, Gao DM. Effects of matrix metalloproteinase inhibitor BB-94 on liver cancer growth and metastasis in a patient-like orthotopic model LCI-D20. Hepatogastroenterology. 1998;45:1056–1061. [PubMed] [Google Scholar]
- 25.Niu Q, Tang ZY, Ma ZC, Chen L, Qin LX, Zhang LH. Serum vascular endothelial growth factor is a potential predictor of metastatic recurrence after curative hepatic resection in hepatocellular carcinoma. Zhonghua Shiyan Waike Zazhi. 1999;16:493–494. [Google Scholar]
- 26.Chen MF, Hwang TL, Jeng LB, Wang CS, Jan YY, Chen SC. Postoperative recurrence of hepatocellular carcinoma. Two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg. 1994;129:738–742. doi: 10.1001/archsurg.1994.01420310070012. [DOI] [PubMed] [Google Scholar]
- 27.The liver cancer study group of Japan. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer. 1994;74:2772–2780. doi: 10.1002/1097-0142(19941115)74:10<2772::aid-cncr2820741006>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Qin LX, Tang ZY, Liu KD. The relationship between p53 point mutation and invasion of hepatocellular carcinoma. Zhonghua Zhongliu Zazhi. 1995;17:405–408. [Google Scholar]
- 29.Tang ZY. Advances of the treatment and research of metastasis as well as recurrence in liver cancer. In: Liver Cancer Institute. Advances in clinical research of liver cancer. Shanghai: Shanghai Medical University Publishing House. 1998:1–11. [Google Scholar]
- 30.Jia L, Chen TX, Sun JW, Na ZM, Zhang HH. Relationship between microvessel density as well as PCNA expression and clinical prognosis in colon cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:74–76. [Google Scholar]
- 31.Xu QW, Li YS, Zhu HG. Relationship between expression p53 protein, PCNA and CEA in colorectal cancer and lymph node metastasis. World J Gastroenterol. 1998;4:218–220. [Google Scholar]
- 32.Mukhopadhyay D, Tsiokas L, Sukhatme VP. Wild-type p53 and v-Src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res. 1995;55:6161–6165. [PubMed] [Google Scholar]
- 33.Agani F, Kirsch DG, Friedman SL, Kastan MB, Semenza GL. p53 does not repress hypoxia-induced transcription of the vascular endothelial growth factor gene. Cancer Res. 1997;57:4474–4477. [PubMed] [Google Scholar]
- 34.Bochner BH, Esrig D, Groshen S, Dickinson M, Weidner N, Nichols PW, Skinner DG, Cote RJ. Relationship of tumor angiogenesis and nuclear p53 accumulation in invasive bladder cancer. Clin Cancer Res. 1997;3:1615–1622. [PubMed] [Google Scholar]
- 35.Takahashi Y, Bucana CD, Cleary KR, Ellis LM. p53, vessel count, and vascular endothelial growth factor expression in human colon cancer. Int J Cancer. 1998;79:34–38. doi: 10.1002/(sici)1097-0215(19980220)79:1<34::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]


