Abstract
AIM: To study the relationship between pre-formation of galls tone and the kinetics and ultra-structure of sphincter of Oddi.
METHODS: Adult female rabbits were used and divided into 3 groups, and fed with either normal or high cholesterol diet for four or eight weeks. Each group contained eight rabbits. The manometry of sphincter of Oddi, biliary cineradiography, gallbladder volume measurement and ultrastructure observatio n under electron microscope were performed.
RESULTS: In groups I and II, the basal pressure in low-pressu re ampulla or high pressure zone of sphincter of Oddi was elevated, the amplitude of phasic contraction was decreased and the volume of gallbladder were increased, with a significant difference (P < 0.01, from those of control. Gallstones were found in group II rabbits(7/8). Under cineradiography, low-press ure ampulla showed a spasmodic status without apparent peristaltic contraction. Under electron microscope, inside the muscular cells of sphincter of Oddi, loosening of microfilament and swelling of plasmosomes which congregated at the top were observed. The amount showed no obvious change under nitric oxide synthase (NOS) stain.
CONCLUSION: Twisting of the microfilament and disarrangement of kink macula densa inside the muscular cells suggested that the sphincter of Oddi was under spasmodic status. The impaired diastolic function caused and aggravated the stasis of cystic bile. The swelling plasmosome could be one of the important factors in elevating the tonic pressure of sphincter of Oddi.
Keywords: hypercholesterolemia, cholithiasis, sphin cter of Oddi/ultrastructure
INTRODUCTION
Gallstone formation results from many complex factors working together. Among them the bile stasis caused by impaired gallbladder emptying is thought to be the fundamental kinetic factor. Because the sphincter of Oddi (SO) is the only gate through which bile discharged into the duodenum, the bile filling and excretion of the gallbladder are closely related with the motility state of SO. Hyperchole sterolemia is a crucial factor of lithogenesis. But up to date, there has been no report on the effect of hypercholesterolemia on SO and the causal relationship between abnormality of SO and gallstone formation. The aim of this study is to investigate the relationship between SO motility and its ultra-morphology, and gallstone formation under the influence of hypercholesterolemia.
MATERIAL AND METHODS
Animal experiment
Twenty-four adult female rabbits weighing between 2.0 kg and 2.5 kg were provided by the Laboratory Animal Center of the Fourth Military Medical University. They were divided randomly into three groups (n = 8 each). Control group, fed with standard diet; Group I, standard diet plus cholesterol 1 g/d, 6 times/week, sacrificed after 4 weeks; and Group II standard diet plus cholesterol with 1 g/d, 6 times/ week, sacrificed after 8 weeks.
The cholesterol (98% purification, 0.1% phosphatide) was purchased from Changsha Biochemical Pharmaceutical Factory, China. Before the study, the serum cholesterol was tested and only animals with a concentration lower than 3 mmol/L were included in the study. After feeding, a concentration higher than 10 mmol/L was evaluated as hypercholesterolemia. During laparotomy, if crude cholesterol crystal was observed in naked eye in the bile and the surface of gallbladder mucosa, it was interpreted as gallstone formed[1].
Manometry and equipment
Manometry catheter was modified from Cook’s 5F (outer-diameter 1.6 mm ) a ngiographic catheter. The tip hole was blocked, and a side-hole (0.4 mm diameter) was made from 10 mm of the tip. During manometry, the catheter lumen was infused with 9 g/L saline at 0.25 mL/min using a miniature complaint hydraulic infusion pump[2]. The baseline resistance for duodenum pressure was set to zero during infusion. Recordings were obtained using a 4-channel RM-6200C polygraphic recorder (Chengdu Instrument Inc., Chengdu, China) with a PT-14M transducer (Gaolian Transducer Science Inc., Shanghai, China). Following an overnight fast (except water), the animals were anesthetized with ketamine (30 mg/kg) (Shaanxi Medical Research Institute) and maintained with 15 mg/kg per 30 min. After anesthesia the animals were tied supine on a board and underwent laparotomy. The extra-hepatic bile duct system was readily visible. The duodenum was cut open, and the catheter was cannulated into common bile duct through the duodenal papilla of billiary duct. The catheter was withdrawn at 1 mm-2 mm increments, and measurement was started after 5 min pause until the tip of the catheter dropped out into the duodenum.
After manometry, 300 g/L meglucamine diatrizoate was injected into biliary tract of controls and group I animals. The cineradiographic recording of biliary tract was obtained at a frame rate of 25/s when the tract was full of contrast.
Nitric oxide synthetase histochemistry and imaging analysis
SO specimens from three rabbits of each group were subjected to histochemistry and image examinations after manometry. Each specimen was fixed in 40 g/L paraformal dehyde PBS solution at 4 °C for 16-18 h, then put into 100 g/L sourose solution and preserved at low temperature according to the method of Thune A[2]. After NPDPH stain, the total number of NADPH, mean string length and spacing were measured with MPIAS 500 Multimedia Color Pathologic Imag e Pattern Analysis System (Qing Ping Imaging Corp.).
Statistical analyses
All results were expressed as -x ± s. Differences were tested for sign ificance by Student’s t test, and P < 0.05 was considered as significan t difference.
RESULTS
Serum cholesterol
Before the cholesterol-feeding, the total serum cholesterol had no difference between control and groups I and II ( < 3 mmol/L ), while the concentration was elevated significantly ( > 10 mmol/L) after four or eight weeks of cholesterol feeding, reaching the standard of hypercholesterolemia. There was significant difference between control and experimental groups (P < 0.01), but no difference was observed between groups I and II (P > 0.05), (Table 1).
Table 1.
Serum cholesterol concentration (mmol/L)
| Group | Pre-feeding | Post-feeding |
| Control | 1.43 ± 0.61 | 1.36 ± 0.42 |
| Group I | 1.57 ± 0.56a | 30.61 ± 6.32a |
| Group II | 1.40 ± 0.68b | 29.03 ± 2.59b |
Post
P < 0.01, t = 13.06,
P < 0.01, t = 32.17 vs control.
Gallbladder volume and bile characteristics
Gallbladder volume of control, group I and II was 2.0 mL ± 1.0 mL, 3.4 mL ± 1.0 mL, 3.9 mL ± 0.9 mL respectively, and volume of gallbladder of group I and II increased more significantly than control group (IP < 0.01, t = 2.8 vs control. IIP < 0.01, t = 3.96 vs control), and there was no difference between group I and II (bP > 0.05, t = 1.04 vs group I).
Cineradiography
The calibra of low-pressure ampulla segment in control animals was uneven, and enlarged significantly at the site where contrast media concentrated, while in group I it was smaller than that of the controls and the changes of caliber were not prominent during excretion of contrast (Figures 1 and 2).
Figure 1.
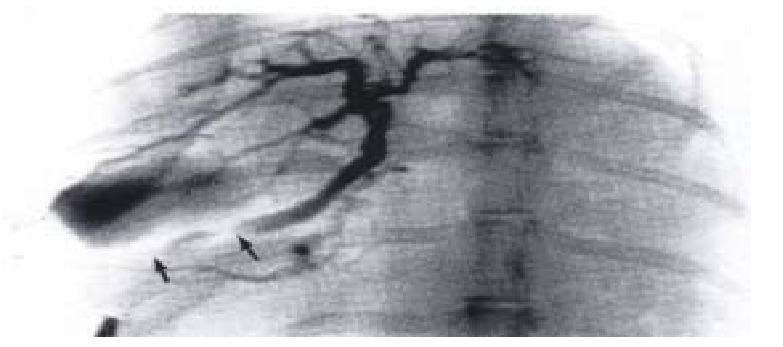
The cineradiographic recording of same bili ary tract of one control. The contrast clustering the proximal segment (low-pre ssure ampulla) which enlarged significantly during excretion of contrast indicat ed that part was diastoled. (↑ → ↑: External segment of SO)
Figure 2.
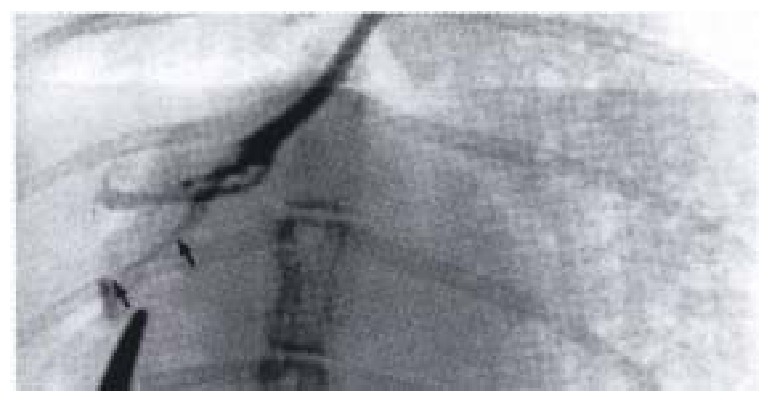
Biliary tract cineradiographic image of gr oup I rabbit. The caliber of proximal segment decreased, retained a fixed spasm state. (↑ → ↑: External segment of SO)
Manometry
In the control, manometry showed a constant high-pressure zone ( HPZ ) at the terminal of SO segment. It featured a high basal pressure and inconspicuous amplitude fluctuation. A low-pressure zone existed beyond the HPZ (proximal to the SO), with a lower basal pressure but relatively more prominent rhythmic peristalsis. These results coordinated with Toouli’s report[3].
The results obtained in groups I and II are compared and listed in Table 2. The basal pressure in proximal segment of groups I and II was higher than that of controls (P < 0.01), but there was no difference between the two groups II (P > 0.05). The amplitude of peristalsis in these segments were lower than the controls (P < 0.01), with no difference between groups I and II.
Table 2.
Manometry of rabbits SO (mmHg)
|
Proximal segment |
HPZ |
|||
| Basal pressure | Amplitude | Basal pressure | Amplitude | |
| Controls | 11.7 ± 2.8 | 18.1 ± 5.9 | 69.3 ± 9.8 | 8.7 ± 3.6 |
| Group I | 20.9 ± 6.1 | 6.4 ± 4.5a | 138.4 ± 45.5 | 4.2 ± 2.2a |
| Group II | 25.6 ± 9.1 | 6.7 ± 4.8b | 144.5 ± 40.5 | 3.4 ± 2.2b |
Proximal: Basal pressure
P < 0.01, t = 4.20,
P < 0.01, t = 5.11 vs controls; Amplitude aP < 0.01, t = 4.47, bP < 0.01, t = 4. 24 vs control. HPZ: Basal pressure aP < 0.01, t = 4.20, bP < 0.01, t = 5.11 vs control; Amplitude aP < 0.01, t = 3.15, bP < 0.01, t = 3.17 vs control.
The basal pressure in HPZ of groups I and II was higher than the controls, but the amplitudes were lower than the control. There was no difference in basal pressures between groups I and II (P > 0.05), (Figures 3, 4 and 5).
Figure 3.
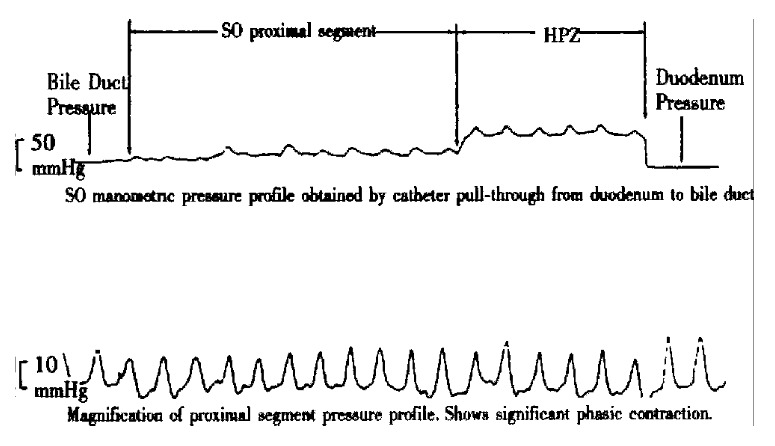
Pressure curve of SO segment of control rabbit.
Figure 4.
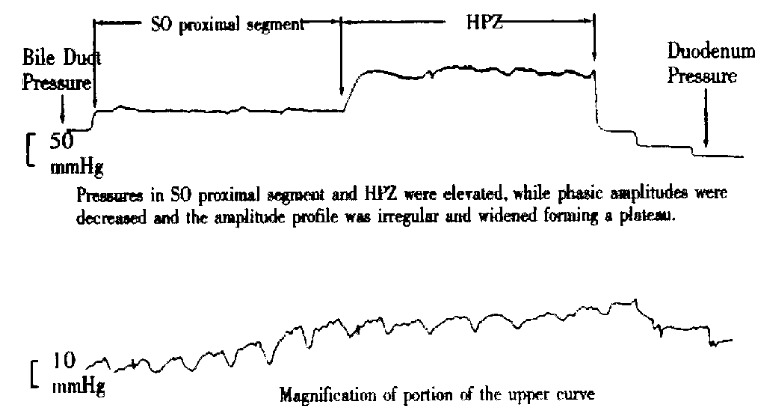
Pressure curve of SO segment of group I rabbit.
Figure 5.
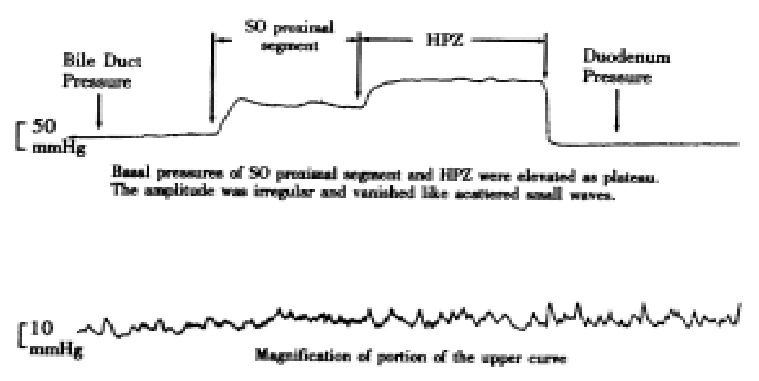
Pressure curve of SO segment of group II rabbit.
NADPH stain imaging analysis
As shown in Table 3, there was no difference in the total number, total area, area density, area percentage, mean string length and mean spacing among the groups (P > 0.05).
Table 3.
Rabbit’s smooth muscle of SO NADPH stain imaging analysis (μm)
| Total (number) | Total (area) | Area (density) | Area (percentage) | Mean (string) | Mean (spatial range) | |
| Controls | 107.6 ± 64.4 | 330.5 ± 133.4 | 0.0079 ± 0.0048 | 2.4 ± 1.0 | 1.6 ± 0.4 | 77.2 ± 67.7 |
| Group I | 117.6 ± 43.4 | 319.5 ± 126.5 | 0.0087 ± 0.0032 | 2.4 ± 0.9 | 1.5 ± 0.3 | 69.7 ± 26.2 |
| Group II | 109.6 ± 45.4 | 360.4 ± 190.4 | 0.0081 ± 0.0033 | 2.7 ± 1.4 | 1.6 ± 0.4 | 79.9 ± 61.7 |
Reference area is 13550.6 μm.
Pathologic examination
Two SO specimens of each group were examined histologically. Each specimen was fixed in formalin and then HE stained. Under light microscope, the smooth muscle arranged regularly. There was no increase of inflammatory cell and collagenous or elastic fibers.
Three SO smooth muscle specimens of each group were collected randomly. They were cut into 1mm small specimens, soaked in 30 g/L glataraldehyde promptly and pre-fixed for 2-4 h under 4 °C, then post-fixed, dehydrated, embedded, ultra-thin sliced, lead/uranium double stained and observed under JEM-200EX transmission electronic microscope. It was found that SO smooth muscle cells’microfilament was loose and irregularly arranged, kink macula densa reduction, swelling and intercristal space disappeared or vacuolized and congreg ated at the top of the nuclear of plasmosome in groups I and II, but no such ch anges in the controls (Figures 6, 7 and 8).
Figure 6.
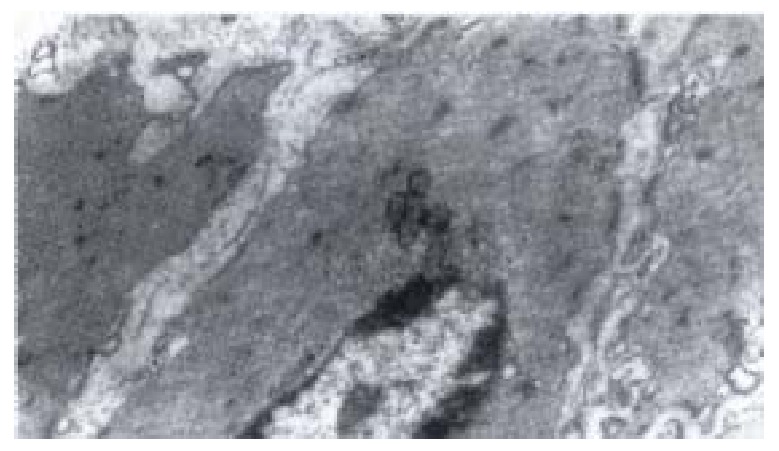
Electron-microscopic scanning of a control ‘s SO segment smooth muscle. The myofilaments were regularly arranged, kink mac ula densa clear and dense, plasmosome was normal.
Figure 7.
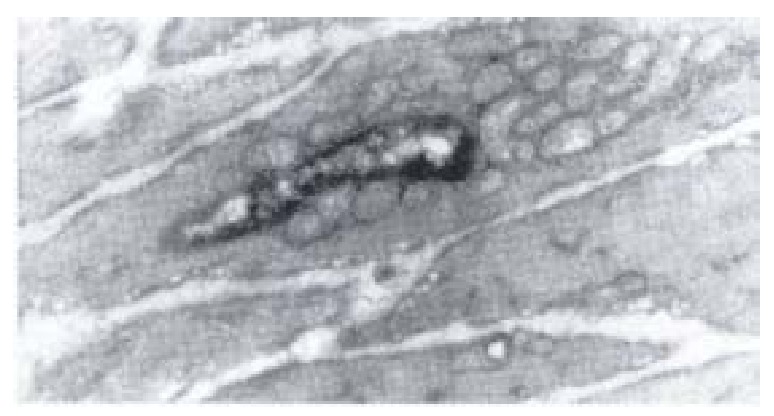
Electron-microscopic scanning of a group I rabbit’s SO smooth muscle. Image showed the swelling of plasmosome and disap pear of intercristal space, decrease of kink macula densa and twisting of myofil aments.
Figure 8.
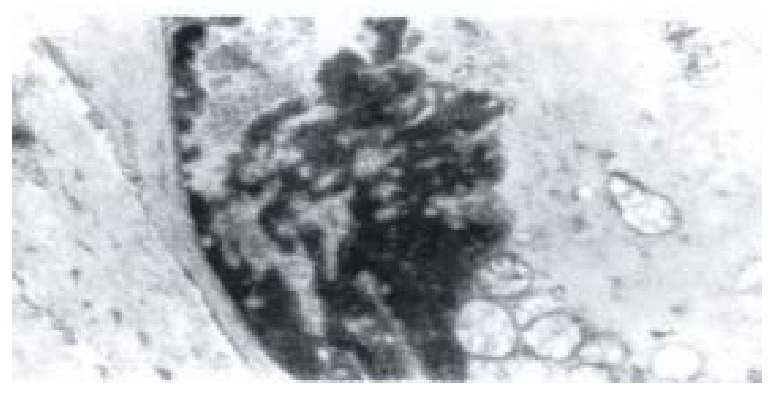
Electron-microscopic scanning of a group II rabbit’s SO segment smooth muscle. It shows the swelling of plasmosome, irre gular arranged or fragmented intercristal space, some vacuolized, congregated at one end of nuclear, and disarragement of kink macula densa.
DISCUSSION
The biliary tract system can be treated as a half-opened and low-flow system according to its anatomic features, and SO is the only excreting gate to the duo denum. According to Dodd WJ et al[4], at fasting state only 50%-75% of hepatic bile enters the gallbladder while the remainder flows directly into the duodenum. Obviously, SO plays an important role in the participating in hepatic bile flow and remaining the pressure gradient of biliary system, consequently the regulating gallbladder filling and emptying.
The pathologic agents that related to the gallstone formation were still the hot spot of research. Most researchers hold that hypotonus of gallbladder and bile stasis are the main factors of chololithiasis while the dyskinesis may be the result of gallbladder pathologic lesion itself[5]. However, Lange K et al[6] found that the tension of gallbladder was not decreased, but increased during the early stage of chololithiasis formation. Pitt A et al[7] and Li YF et al[8] had shown that sphincterectomy may prevent the formation of gallbladder stone and improve the contractility of gallbladder partially, but has no effect on the cholesterol crystal genesis. All these suggested that the dysfunction of the SO may result in the abnormal motility of gallbladder.
Toouli J et al[3] and Wei JG et al[9] reported their findings on SO dynamics anatomy, which indicated that choledochus sphincter’s phys iological anatomy consisted of a high pressure distal segment and a low-pressu re proximal ampulla segment. The low-pressure ampulla segment’s rhythmic contractile and diastole activity are the primary kinetic sources during fasting. Our results showed that there was no difference in basal pressure of low-pressure segment and total pressure (basal+phasic amplitude) of HPZ between experimental groups and the control. It suggested that hypercholesterolemia did not impair the construct ion foundation of the contractile activity of SO. But the contractile amplitude of functional ampulla in the experimental rabbits was decreased to those of the controls. The amplitude of low-pressure ampulla/ total ( basal + amplitude ) was 60.7% in the control rabbits, but was only 23.4% and 25.5% in groups I and II. Obviously that elevated basal pressure of low-pressure ampulla of SO signif icantly decreased the phasic amplitude in the experimental animals. The cineradi ography showed that the low-pressure ampulla retained a fixed spasm state, thus, the diastolic and peristaltic functions were impaired. According to the hydrodynamic laws, Qout = -dv/dt, i.e. excretion volume of liquid is equal to the shrunken volume of the elastic vessel. This suggested that the effective excretion of each pumping (peristaltic wave) per unit time of low-pressure am pulla was impaired in groups I and II.
Additionally, all experimental animals characterized by a more distinctly elevated basal pressure in HPZ than SO proximal segment, and the manometric curve appeared almost like a plateau, on this basis, the phasic amplitude significantly decreased. The HPZ is not merely a passive “valve”, but also an intrinsic conducting tube of bile, so that the increased basal pressure may enhance the excreting resistance of the bile significantly. This study demonstrated that the volume of gallbladder increased in experiment animals, suggesting that the tonus alteration in ampulla and HPZ of experimental rabbits may play an important role in the bladder bile stasis, subsequently that may be the pathologic motility function of cholithiasis formation.
There were many reports about nitric oxide (NO) regulation of SO smooth muscle. Some authors confirmed that NO may be the terminal neuron transmitter of SO smooth muscle[2]. Several studies showed that there were abundant NO synthetase (NOS) in SO, which had tissue-specific characteristics. The results may demo nstrate that relaxation of SO is related to the quality and quantity of NOS directly. But our data showed no difference in quantities of NOS between the experimental and control animals, indicating that the decreased diastolic activity of the sphincter may not be the result of reduced quantities of NOS in sphincter tissues.
Gaasch WH et al[10] reported that diastolic deficiency is the early change of heart dysfunction. This may be related to malfunctioning energy metabolism of the heart muscle cell. This study showed that the cell of SO underwent changes of plasmosome swelling which may affect the energy metabolism of sphincter cell, while the swelling of myofilament may indicate the intracellular edema of sphincter. Zhang JS et al[11] reported that during sphincter cell culture in vitro in high cholesterol solution, the cell underwent similar alteration. The sensitivity of cell membrane to digestive synthetase was increased. According to these data, the changes of sphincter cells of experimental animals may relate to the alteration of cell membrane biological characteristics due to hypercholesterolemia. Since the sphincter functional ampulla (low pressure segment) acts like kinetic heart pump during bile excretion, we conclude that diastole of functional ampulla may be an active movement and energy consumption process, the swelling of plasmosome may result in decrease of energy supply during diastole, leading to diastole deficiency eventually.
Footnotes
Edited by Ma JY
References
- 1.Shu JD, Li SJ, Zhao HL. Prevention of cholelithiasis formation with caffeine in rabbits. Zhonghua Shiyan Waike Zazhi. 1994;11:27–28. [Google Scholar]
- 2.Thune A, Delbro DS, Nilsson B, Friman S, Svanvik J. Role of nitric oxide in motility and secretion of the feline hepatobiliary tract. Scand J Gastroenterol. 1995;30:715–720. doi: 10.3109/00365529509096318. [DOI] [PubMed] [Google Scholar]
- 3.Toouli J, Dodds WJ, Honda R, Sarna S, Hogan WJ, Komarowski RA, Linehan JH, Arndorfer RC. Motor function of the opossum sphincter of Oddi. J Clin Invest. 1983;71:208–220. doi: 10.1172/JCI110761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodds WJ. Biliary tract motility and its relationship to clinical disorders. AJR Am J Roentgenol. 1990;155:247–258. doi: 10.2214/ajr.155.2.2115247. [DOI] [PubMed] [Google Scholar]
- 5.Velanovich V. Biliary dyskinesia and biliary crystals: a prospective study. Am Surg. 1997;63:69–74. [PubMed] [Google Scholar]
- 6.Lange K, Gottschalk M. [Gallbladder contractility in early stages of lithogenesis in the lithogenic fed guinea pig] Z Gastroenterol. 1995;33:333–339. [PubMed] [Google Scholar]
- 7.Tierney S, Pitt HA, Lillemoe KD. Physiology and pathophysiology of gallbladder motility. Surg Clin North Am. 1993;73:1267–1290. doi: 10.1016/s0039-6109(16)46191-8. [DOI] [PubMed] [Google Scholar]
- 8.Li YF, Weisbrodt NW, Moody FG. Effect of bile diversion and sphincterotomy on gallbladder muscle contractility and gallstone formation. Am J Surg. 1991;162:31–35. doi: 10.1016/0002-9610(91)90197-l. [DOI] [PubMed] [Google Scholar]
- 9.Wei JG, Che SH, Wang YC, He HD, Wang XH. The physiological anatomy of the sphincter of Oddi in dog and the role for biliary excretion. Dan Dao J Japan. 1994;8:336–344. [Google Scholar]
- 10.Gaasch WH, Levine HJ, Quinones MA, Alexander JK. Left ventricular compliance: mechanisms and clinical implications. Am J Cardiol. 1976;38:645–653. doi: 10.1016/s0002-9149(76)80015-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JS, Wei JG, Zhang ML, Wang D, Shi YH, Ji ZL. Effect of cholesterol liposome on cytoskeleton of rabbit sphincter of Oddi cells in culture. Disi Junyi Daxue Xuebao. 1997;18:528–531. [Google Scholar]


