Abstract
AIM: To study the cell types, localization, distribution density and morphology of APUD cells in the intestinal mucosa of stomachless teleost fishes.
METHOD: By using the peroxidase-antiperoxidase complex ( PAP ) immunocytochemical staining technique the identification, localization and morphology of immunoreactive (IR) endocrine cells seattered in the intestinal mucosa of grass carp (Cyenopharyngodon idellus), black carp (Mylopharyngodon piceu s) and common carp (Cyprinus carpio) were investigated with 20 kinds of an tisera prepared against mammalian peptide hormones of APUD cells, and likewise by using avidin-biotin-peroxidase complex (ABC) method those of silver carp (Hypophthalmichthys molitrix), bighead (Aristichthys nobilis), silver crucian carp (Carassius gibelio) and bluntnose black bream (Megalobrama amblyoce phala) were also studied with 5 different antisera. The replacement of the first antiserum by phosphate buffered saline (PBS) was employed as a control. IR endocrine cells were counted with a square-mesh ocular micrometer from 10 fields selected randomly in every section of each part of the intestine specimen. The average number of IR endocrine cells per mm2 was counted to quantify their dis tribution density.
RESULT: Gastrin (GAS), Gastric inhibitory peptide (GIP), glucagon (GLU), glucagon-like immunoreactants (GLI), bovine pancreatic polype ptide (BPP), leucine-enkephalin (ENK) and substance P (SP)-IR endocrine ce lls were found in the gut of grass carp, black carp and common carp, and somatos tatin (SOM)-IR endocrine cells were only seen in common carp. GAS, GIP and GLU-IR endocrine cells were found in the intestinal mucosa of silver carp, bigh ead, silver crucian carp and bluntnose black bream. Most of IR endocrine cells had the higher distribution density in the foregut and midgut, and were longer in shape. They had a long apical cytoplasmic process extended to the gut lumen and a basal process extended to adjacent cells or basement membrane and touched with it. Sometimes, the basal cytoplasmic process formed an enlarged synapse-like structure in the contiguous part with basement membrane. This phenomenon provide d new morphological evidence for neuroendocrine and paracrine secretory function of these enteroendocrine cells.
CONCLUTION: At least 8 kinds of IR endocrine cells were found in the gut of stomachless teleost species for the first time in China. These IR e ndocrine cells scattering in the gut mucosa belong to the APUD system. Among the m, the hormones secreted by SP-, ENK-, SOM- and GLU-IR endocrine cells belon g to the peptides of dual distribution in the brain and gut. This provided new evidence for the concept of brain-gut peptide. According to the cell types, dist ribution density, morphological characteristics and variety in shape of APUD cells in the gut of stomachless teleost fishes, it is deemed that the digestive tract of fishes is also an endocrine organ of great importance and complexity.
Keywords: stomachless teleost fishes, APUD cells, intestinal mucosa, immunocytochemistry
INTRODUCTION
Gastrointestinal endocrine cells are different from other cells of endocrine gland, are discrete cells spread among the gastrointestinal mucosal epithelial cells. Pearse first proposed that all endocrine cells which produced peptide hormones were called APUD (amine precursor uptake and decarboxylation) cells in 1968[1,2]. APUD cells can be divided two groups: in one are those located in the nervous system, and in the other group are there distributed in the peripheral organs, mainly the digestive system. Many studies of the mammalian gastro-e ntero-pancreatic (GEP) endocrine system have been reported, which, to date, have resulted in the identification of about 18 endocrine cell types and at least as many hormones[3-5]. Enteroendocrine cells of fishes are difficult to demonstrate. For example, most of the identification procedures, which were found to be positive for mammalian enteroendocrine cells, failed on adult fish species. There was only a faint reaction with argyrophilic stains and with lead haematoxylin[6], and also could not identify the types or species of endocrine cells[7]. Although endocrine cell types that exist in the fish gut may differ from those of mammals, the questions of a cross-species specificity of anti sera against mammalian hormones and whether these antisera will show immunoreactivity in the gut of fish are very important. Recent years immunocytochemical studies using antisera against mammalian hormones clearly showed the existence of endocrine cells in the digestive tract of some teleost fishes[8-12]. Our series of studies also showed that antisera against mammalian hormones could be effectively used on teleost species, and identify the types of APUD cells in the GEP of fish species[13-19]. This paper reports the cell types, localization, distribution density and morphology of APUD cells in the gut of 7 kinds of teleost fish species cultivated mainly in China. The studies will provide new informations about neuro-endocrinology (the concept of brain-gut peptide hormones) animals, physiology, pathology and gastroenterology of animals.
MATERIALS AND METHODS
Specimens
Seven kinds of teleost fishes, grass carp ( C. idellus ), black carp ( M. piceus ), common carp (C. carpio ), silver carp ( H. molitrix ), bighead (A. nobilis), silver crucian carp (C. gibelio) and bluntnose black bream (M. amblyocephala), 3-6 fish of each species, were used in this studies. All fishes were reared temporarily and dissected after 2 days fasting. The digestive tract was drawn out, and the gut was divided into five pieces: the anterior segment of foregut, posterior segment of foregut, midgut, anterior segment of hindgut and rectum. All specimens were fixed by immersion in Bouin’s fluid for 24 h, dehydrated and made transparent through ethanol-xylene serial procedures, embedded in paraffin (54 °C) and sectioned (5 μm). The sections were mounted on slide, treated with gelatin and dried 12 h at 45 °C.
Antisera and main reagents
The details of antisera and main reagents used are listed in Table 1.
Table 1.
Details of antisera and main reagents used
| Antisera against | Working dilution | Code | Specificity | Source |
| Synthetic human gastrin | 1:5000 | GP-1304 | No cross reaction with cholecystokinin-8 | Dr. N Yanaihara,Shizuoka |
| Leucine-enkephalin | 1:80000 | 1671 | — | UCB-Bioproducts Bruxelles |
| Bovine pancreatic polypeptide | 1:12000 | 615-R-110 | Cross-react s with human pancreatic polypeptide | Dr. RE Chance, Indianapolis |
| Substance P | 1:2000 | MAS 035B | — | Sera-Lab., Sussex |
| Gastric inhibitory polypeptide | 1:10000 | G/R/34-IIID | No cros s reaction with glucagon | Dr. D Grube, Hannove |
| Porcine glucagon | 1:1000 | RPN1602 | Wholly cross react with pancreatic | Amersham International pl., |
| and intestinal glucagon | Amersham | |||
| Synthetic human cyclic | ||||
| somatostatin | 1:3000 | — | — | Dr. S Ito Niigata |
| Glucagon-like immunoreactants | 1:1000 | RPN1604 | Wholly cross react with pancreatic | Amersham International pl., |
| and intestinal glucagon | Amersham | |||
| Insulin | 1:1000 | 47291 | — | — |
| Avian pancreatic polypeptide | 1:10000 | Iance-10/5/81 | No cross reaction with glucagon | Dr. JR Kimmel, Kansas City |
| 5-Hydroxytriptamine | 1:10000 | Lot.16302 | — | Immunonuclear Corp.Stillwater |
| Synthetic porcine motilin | 1:1000 | R-1104 | Reacts against entire molecules | Dr. N Yanaihara, Shizuoka |
| Natural porcine | ||||
| cholecystokinin-33 | 1:3000 | — | Reacts with cholecystokinin 11-20, | Dr. J Yamada, Shizuoka |
| no cross reaction with gastrin | ||||
| Synthetic porcine secretin | 1:1000 | R-801 | Reacts with the C- and N-terminals | Dr. N Yanaihara, Shizuoka |
| Synthetic bovine neurotensin | 1:1000 | R-3501 | Dr. N Yanaigar a, Shizuoka | |
| Synthetic porcine vasoac tive | ||||
| intestinal polypeptide | 1:2000 | R-502 | Reacts against entire molecules | Dr. N Yanaihara, Shizuoka |
| Bombesin | 1:3000 | 27070 | Cross reaction with GRPImmunonrclear | Corp. Stillwater |
| Neuron specific enolase | 1:1000 | — | — | Dako, Copenharge |
| Prochymosin | 1:2000 | — | — | A. Andren, Uppsala |
| Pepsinogen | 1:2000 | — | — | A. Andren, Uppsala |
| Rabbit PAP | 1:100 | Z - 113 | — | Dako, Copenharge |
| Guineapig PAP | 1:50 | 24699 | — | Dako, Copenharge |
| PAP Kit | 1:100 | 61- 2003 | — | Zymed Lab. Inc., SouthSan |
| Francisco, USA | ||||
| ABC Kit | 1:50 | PK-4001 | — | Vector Lab. Burlingame USA |
Immunocytochemical staining steps and counting
① Put into 3% H2O2-methanol for 10min at 20 °C to block auto-peroxidae; ② to incubate with normal goat serum for 30 min at 20 °C for preventing nonspecific reactivity; ③ drop in 20 different first antisera separately and incubate for 20 h at room temperature (for PAP method); ④ drop in 5 kinds of first a ntisera separately and incubate for 20 h at room temperature (for ABC method); ⑤ for PAP method to see reference 13, and for ABC method to consult reference 19; ⑥ the control was treated in step with adjacent continuous section of immunocytochemical staining slice except replacement of the first antisera with phosphate buffered saline (PBS). IR endocrine cells were counted and photographed with an Olympus photomicroscope (PM-10AD) from 10 fields selected randomly in every section of each part of the gut per specimen with a square-meshed ocular micrometer (0.5 mm). The average number of IR endocrine cells per mm2 was counted to quantify their distribution density.
RESULT
Cell types and distribution characteristics of IR endocrine cells
GAS-, GIP-, GLU-, BPP-, ENK-, GLI-, SP- and SOM-IR endocrine cells were found in the intestinal mucosa of C. carpio; these cells, except SOM-IR end ocrine cells, were seen in C. idellus, and M. piceus. Only GAS, GIP-And GLU-IR endocrine cells were found in the gut of -H. molitrix, A. nobilis, C. gibelio and M. amblyocephala. The location, distribution and density of IR endocrine cells in the gut of 7 teleost fishes are listed in Table 2 & Table 3. Most of IR endocrine cells distributed in the foregut of 7 kinds of fish species. Only SP and GLI-IR endocrine cells were not found in the foregut of C. carpio, but they were most abundant in the midgut. SOM-IR endocrine cells distributed only in the foregut and midgut of C. carpio. In most conditions, the distribution of cell types and density were decreasing along the gut in a distal direction after the foregut. GAS-, GIP- and GLU-IR endocrine cells distributed in the gut of all 7 kinds of fishes. GLU-IR endocrine cells were the most, up to 126 cell s/mm2, in the midgut of H. molitrix. All controls for every IR endocrine cell in the gut of 7 kinds of fish species were negative. Eight kinds of IR endoc rine cells distributed mainly among the epithelium of intestinal mucosa, and only a few of them existed in the lamina propria (Figure 1). Some of the cells were dispersed in the middle and bottom part of the gut fold (Figure 2), but most of them were scattered in the apical part of the gut fold (Figures 3, 4, 5, 6, 7, 8, 9 and 10).
Table 2.
Distribution and density of 8 IR endocrine cells in the gut of three teleost species (-x ± s)
| Fish species | Gut parts | GAS | ENK | B P P | SP | GLI | GIP | GLU | SOM |
| Crass carp | I | 64 ± 13 | 85 ± 17 | 52 ± 12 | 56 ± 14 | 44 ± 19 | 23 ± 19 | 61 ± 7 | — |
| (C.idellus) | II | 26 ± 6b | 57 ± 11a | 31 ± 16c | 76 ± 20a | 45 ± 22c | 11 ± 7 | 44 ± 4b | — |
| III | — | 22 ± 7b | — | 57 ± 10c | 27 ± 9c | — | 31 ± 3b | — | |
| IV | — | 17 ± 10b | — | 17 ± 9b | — | — | 21 ± 5b | — | |
| V | — | 1 ± 2b | — | 18 ± 7b | — | — | 9 ± 5b | — | |
| Black carp | I | 77 ± 8 | 57 ± 6 | 72 ± 6 | 20 ± 5 | 68 ± 17 | 59 ± 13 | 2 ± 3 | — |
| (M. piceus) | II | 63 ± 5b | 51 ± 5c | 58 ± 7a | 47 ± 4b | 88 ± 11c | 34 ± 20a | 1 ± 5c | — |
| III | 32 ± 4b | 38 ± 4b | 22 ± 4b | 38 ± 3b | 92 ± 30c | 31 ± 19a | — | — | |
| IV | 23 ± 5b | 57 ± 9c | — | 60 ± 7b | 16 ± 12b | 17 ± 11b | — | — | |
| V | — | 86 ± 12b | — | 72 ± 9b | 10 ± 6b | 8 ± 6b | — | — | |
| Common carp | I | 25 ± 6 | 38 ± 4 | 12 ± 6 | — | — | 4 ± 3 | 2 ± 2 | 13 ± 12 |
| (C. carpio) | II | 20 ± 5c | 39 ± 5c | 9 ± 8c | 18 ± 4b | 6 ± 1b | 2 ± 2c | 1 ± 5c | 8 ± 5 b |
| III | 12 ± 2b | 28 ± 3b | — | 31 ± 6b | 11 ± 4b | — | — | 10 ± 8 b | |
| IV | 3 ± 4b | 9 ± 5b | — | 19 ± 3b | — | — | — | — | |
| V | — | — | — | — | — | — | — | — |
I = Anterior segment of foregut; II = Posterior segment of foregut; III = M idgut; IV = Anterior segment of hindgut; V = Rectum; —: IR endocrine cell wasn’t found, PAP method was used in the table; n = 5,
P < 0.05,
P < 0.01,
P > 0.05.
Table 3.
Distribution and density of 3 IR endocrine cells in the gu t of four teleost species (-x ± s)
| Fish species | Gut parts | GAS | GIP | GLU |
| Silver carp | I | 17 ± 5 | 2 ± 2 | 43 ± 10 |
| (H. molitrix) | II | 2 ± 4b | 6 ± 3a | 64 ± 35c |
| III | — | — | 126 ± 36b | |
| IV | — | — | 61 ± 26c | |
| V | — | — | 30 ± 28c | |
| Bighead | I | 35 ± 10 | 13 ± 3 | 29 ± 16 |
| (A. nobilis) | II | — | — | 27 ± 7c |
| III | — | — | 79 ± 7b | |
| IV | — | — | 60 ± 62c | |
| V | — | — | 47 ± 46c | |
| Silver crucian | I | 32 ± 17 | 1 ± 2 | 53 ± 18 |
| Carp (C. gibelio) | II | 3 ± 3b | — | 20 ± 11b |
| III | — | — | ||
| IV | — | — | 11 ± 13b | |
| V | — | — | — | |
| Bluntnose black | I | 31 ± 8 | 4 ± 1 | 32 ± 16 |
| Bream (M. | II | — | — | 24 ± 6c |
| Amblyocephala) | III | — | — | 4 ± 3b |
| IV | — | — | 24 ± 11c | |
| V | — | — | 6 ± 8b |
I = Anterior segment of foregut; II = Posterior segment of foregut; III = Midgut; IV=Anterior segment of hindgut; V = Rectum; —: IR endocrine cell wasn’t found, ABC method was used in the table; n = 5,
P < 0.05,
P < 0.01,
P > 0.05.
Figure 1.
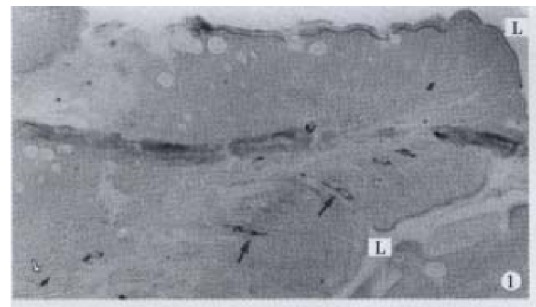
IR endocrine cells in epithelium (↑) and lamina propria (-), × 100
Figure 2.
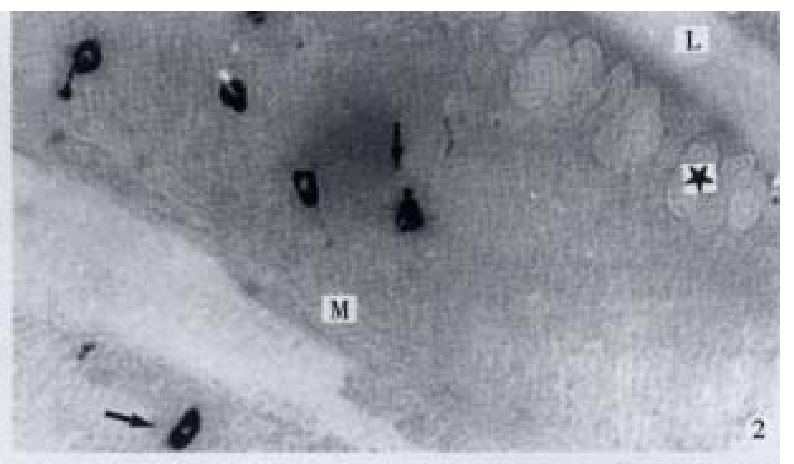
IR endocrine cells (↑) in bottom part of the gut f old, × 200
Figure 3.
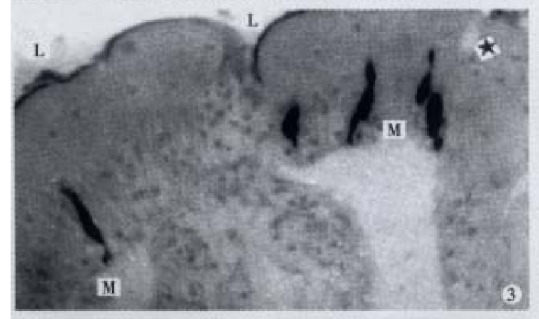
IR endocrine cells in apical part of the gut fold, × 400
Figure 4.
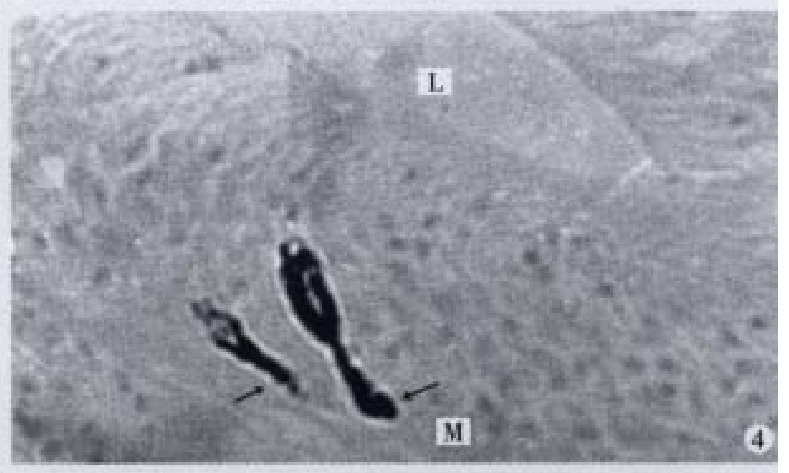
Basal processes (↑) extended to basement membrane, × 600
Figure 5.
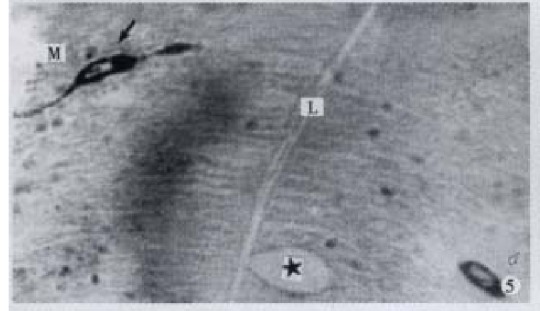
IR endocrine cells of open type (↑) and close type (-), × 600
Figure 6.
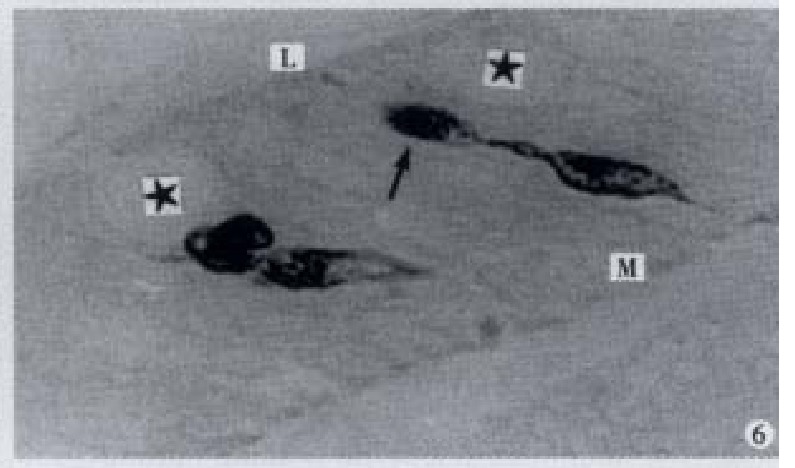
Enlarged process (↑) in sac-shaped, × 1000
Figure 7.
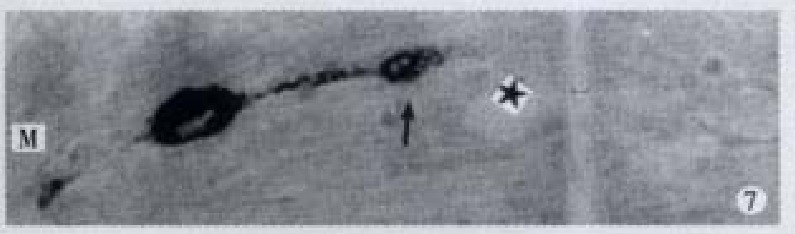
Apical process (↑) as sac in shape, basal process (-) like synapse, × 1000
Figure 8.
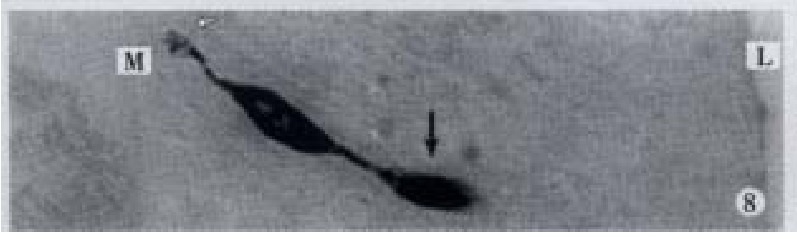
Apical process (↑) as sac in shape, basal process (-) like synapse, × 1000
Figure 9.
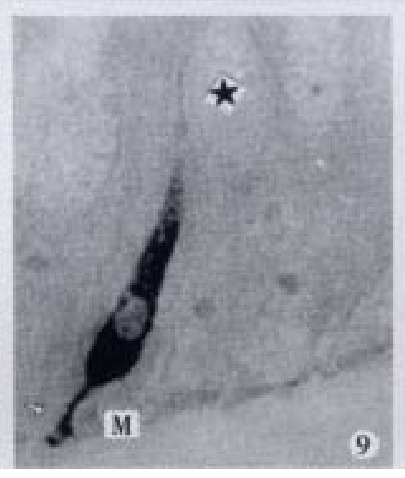
Basal process (-) extended to basement membrane, × 1000
Figure 10.
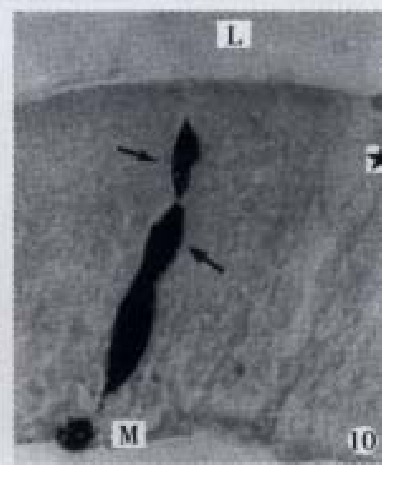
Apical process (↑) as sac in shape, basal process (-) formed an enlarged synapse-like structure, × 1000-In figures, ★: goblet cell; L: gut lumen; M: basement membrane.
Morphological feature of IR endocrine cells
All the IR endocrine cells showed a dark brown colour, and in the cell body and cytoplasmic processes, the secretory granules were seen clearly (Figures 6-9). The shape of IR endocrine cells was distinctive and variform,and quite different from the epithelial and goblet cells. Most of them had one or two long cytoplasmic processes, causing the apical part to be long and thin, the basal part narrow and the middle part of cell body to be broader. The cell nuclei were located in the middle part of body, and showed an empty bubble shape. Sometimes, the cytoplasmic processes showed one or several expanded sacs which were filled with secretory granules (Figures 6-9). The basal cytoplasmic processes emerged an enlarged synapse-like structure in the contiguous place with basement membrane or ad jacent cells (Figures 7-10). A few of IR endocrine cells had only one cytoplasmi c process or none (Figures 2 and 5).
DISCUSSION
The results of the present study revealed that 3-8 kinds of enteroendocrine cells in the gut of 7 stomachless teleost fishes could produce immunoreactive response with antisera which were prepared against mammalian hormone. At the present time, because of the absence of isolates gastro entero pancreatic hormones in fishes antisera against fish hormones are not available, and so the other 12 antisera against mammalian hormones used in the present study did not cause immunoreactive response with enteroendocrine cells of teleost fishes. Therefore, further investigations into the enteroendocrine cells of teleost fishes are necessary.
The description of GAS-, SP-, GLI-, BPP-and ENK-IR endocrine cells in the present study was generally similar to the results of Rombout’s study[6,20] on the enteroendocrine cells in the stomachless fish -Barbus conchonius. The result that the 8 kinds of IR endocrine cells which were more abundant in the first half of the gut of teleost fishes in our study was also similar to that of the study on B. Conchonius[20]. Rombout[6]considered SOM-IR end ocrine cells only existing in the gut of stomach-containing teleosts; this was different from our study in which SOM-IR endocrine cells were found in the gut of a stomachless teleost fish C. carpio.
The distribution density and location of various endocrine cells have some relation to their function. For example, GAS-IR endocrine cells in the stomach of human and other mammals stimulate gastric acid secretion, but these cells distribute mainly in the anterior segment of foregut of 7 stomachless fish species. The anterior segment of the foregut (gut bulb) of stomachless teleosts is like the stomach, and serve a dual function of storing and digesting food-stuff[7,13]. There is a great amount of food in the anterior segment of foregut, and because of the stimulation of undigested particles of food, the activation of endocrine cells there is increased, resulting in the release of greater amount of hormones[15,20]. Substance P can stimulate a contractile function of smooth muscle[1], and SP endocrine cells are more numerous in the rectum; thus these endocrine cells may enhance the contraction of smooth muscle while fish excretes[7]. The differences of cell types and amounts of IR endocrine cells in various fish species relate to their feeding behavior and feeding habits[14-16,21]. Owing to the differences of chemical composition and pH value of the contents in the gut, the amount of hormones released by endocrine cells is also affected[13,14]. Our studies verify that IR endocrine cells of stomachless teleost fishes belong to the open type[1,21], that is, hormones are carried to the gut lumen (lumen-endocrine) by a long apical cytoplasmic process[2,21]. The sac-shaped enlargement of cytoplasmic process has the function of storing. The close type IR endocrine cells lacking the cytoplasmic process seem to be caused by the angle during sectioning[7]. In fact, almost all enteroendocrine cells seem to be the open type[1]. In addition, some IR endocrine cells’ basal cytopoasmic processes extend to the basement membrane or adjacent cells, and form the synapse like structure in the contiguous portions, there by providing morphological evidence for the paracrine and neuroendocrine functions, and furthermore the peptide hormones they secrete may have neurotransmitter function[1,2,21,22]. Previous studies propounded the peptide hormones SP, ENK, SOM and GLU to be present only in the brain[1], but the present study confirmed their existence also in the gut mucosa of stomachless teleost fishes. Wang et al[1] thought that the area of gastrointestinal mucosa was very large; the sum total of gastrointestinal endocrine cells exceeded a lump sum of cells in all other endocrine gland; thus, the digestive tract mucosa was reputed to be the biggest and the most complex endocrine organ in body. Rombout[6] concluded the endocrine system of digestive tract in teleost species was almost as complicated as in mammals. According to the present progress in the studies on the APUD cells in gastro-entero-pancreatic endocrine system of the fish species[21], it is deemed that the digestive tract of fish species is also an endocrine organ of great importance and complexity.
Footnotes
Supported by the National Natural Science Foundation of China. No. 39070 666.
Edited by Lu HM
References
- 1.Wang ZJ, Mei MH, Zhu WY. Gastrointestinal hormones. Beijing: Science Publishing House. 1985:2–372. [Google Scholar]
- 2.Gu J. [The present status of APUD system] Shengli Kexue Jinzhan. 1982;13:120–125. [PubMed] [Google Scholar]
- 3.Calingasan NY, Kitamura N, Yamada J, Oomori Y, Yamashita T. Immunocytochemical study of the gastroenteropancreatic endocrine cells of the sheep. Acta Anat (Basel) 1984;118:171–180. doi: 10.1159/000145840. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura N, Yamada J, Calingasan NY, Yamashita T. Immunocytochemical distribution of endocrine cells in the gastrointestinal tract of the horse. Equine Vet J. 1984;16:103–107. doi: 10.1111/j.2042-3306.1984.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura N, Yamada J, Calingasan NY, Yamashita T. Histologic and immunocytochemical study of endocrine cells in the gastrointestinal tract of the cow and calf. Am J Vet Res. 1985;46:1381–1386. [PubMed] [Google Scholar]
- 6.Rombout JHWM. Function and origin of endocrine cells in gut and pancreas of teleosts. Acta Microsc pica. 1985;8:329–335. [Google Scholar]
- 7.Pan QS, Fang ZP. Preliminary study on endocrine cells in the gut of three cyprinid fishes. Acta Hydrobiologica Sinica. 1989;13:348–352. [Google Scholar]
- 8.Langer M, Van Noorden S, Polak JM, Pearse AG. Peptide hormone-like immunoreactivity in the gastrointestinal tract and endocrine pancreas of eleven teleost species. Cell Tissue Res. 1979;199:493–508. doi: 10.1007/BF00236085. [DOI] [PubMed] [Google Scholar]
- 9.Reifel CW, Linden RD. Endocrine cells in the gastrointestinal tracts of 3 teleostean species. Anat Anz. 1983;154:413–418. [PubMed] [Google Scholar]
- 10.Rombout JH, Lamers CH, Hanstede JG. Enteroendocrine APUD cells in the digestive tract of larval Barbus conchonius (Teleostei, Cyprinidae) J Embryol Exp Morphol. 1978;47:121–135. [PubMed] [Google Scholar]
- 11.Rombout JH, van der Grinten CP, Binkhorst FM, Taverne-Thiele JJ, Schooneveld H. Immunocytochemical identification and localization of peptide hormones in the gastro-entero-pancreatic (GEP) endocrine system of the mouse and a stomachless fish, Barbus conchonius. Histochemistry. 1986;84:471–483. doi: 10.1007/BF00482980. [DOI] [PubMed] [Google Scholar]
- 12.Abad ME, Binkhorst FM, Elbal MT, Rombout JH. A comparative immunocytochemical study of the gastro-entero-pancreatic (GEP) endocrine system in a stomachless and a stomach-containing teleost. Gen Comp Endocrinol. 1987;66:123–136. doi: 10.1016/0016-6480(87)90357-1. [DOI] [PubMed] [Google Scholar]
- 13.Fang ZP, Yamada J, Pan QS. Immunohistochemical identification and localization of endocrine cells in the intestinal mucosa of common carp and black carp. Acta Hydrobiologica Sinica. 1991;15:212–219. [Google Scholar]
- 14.Pan QS, Fang ZP. An immunocytochemical study of endocrine cells in the gut of a stomachless teleost fish, grass carp, Cyprinidae. Cell Transplant. 1993;2:419–427. doi: 10.1177/096368979300200510. [DOI] [PubMed] [Google Scholar]
- 15.Pan QS, Fang ZP, Fan QX, Zhu BK. Immunohistochemical localization and morphological strdy on G cell in the intestine of Hypophthalmichthys molitrix, Aristichthys nobilis, Carassius auratus gibelio and Megalobrama amblyocephala. Acta Zoologica Sinica. 1995;41:167–172. [Google Scholar]
- 16.Pan QS, Fang ZP, Zhao YX, Cheng HL, Bai XM. Immunohis-tochemical studies of 3 kinds of peptide hormones in endocrine cells of the gut of silver carp, bighead, silver crucian carp and blunt snout bream. Acta Hydrobiologica Sinica. 1996;20:311–316. [Google Scholar]
- 17.Fang ZP, Pan QS, Chen SL, Zhao YX. Immunocytochemical localization of nine teleosts using monoclonal antibody of grass carp growth hormone. Acta Hydrobiologica Sinica. 1998;22:355–3 6 0. [Google Scholar]
- 18.Fang ZP, Pan QS, Zhao YX. Distribution of endocrine cells in the GEP system of largemouth bass. In: Advances of histochemistry and cytochemistry (Proceedings of the 4th China-Japan Joint Histochemistry and Cytochemistry Symposium) Chongqing: Chongqing Publishing House. 1996:79–80. [Google Scholar]
- 19.Pan QS, Fang ZP, Liao RL. Study on glucagon-immunoreactive endocrine cells in the gut of four cyprinid fishes. Acta Hydrobiologica Sinica. 1991;15:57–62. [Google Scholar]
- 20.Rombout JH, Taverne-Thiele JJ. An immunocytochemical and electron-microscopical study of endocrine cells in the gut and pancreas of a stomachless teleost fish, Barbus conchonius (Cyprinidae) Cell Tissue Res. 1982;227:577–593. doi: 10.1007/BF00204788. [DOI] [PubMed] [Google Scholar]
- 21.Pan QS, Fang ZP. Present progress in the study of the APUD cells in gastro-entero-pancreatic endocrine system of the fishes. Acta Hydrobiologica Sinica. 1995;19:275–282. [Google Scholar]
- 22.Zhu WY. [The progress of brain-gut peptide research (author's transl)] Shengli Kexue Jinzhan. 1982;13:15–19. [PubMed] [Google Scholar]


