Abstract
Background
Skeletal-related events (SREs; pathologic fracture, radiation or surgery to bone, spinal cord compression) frequently occur in patients with advanced cancer with bone metastases/lesions. Limited data on the associated patient and economic burden are available to aid in resource planning and evaluating treatment options.
Methods
Patients with bone metastases/lesions secondary to breast, lung or prostate cancer or multiple myeloma; with at least one SRE within 97 days prior to enrollment; life expectancy of at least 6 months; and Eastern Cooperative Oncology Group performance status 0, 1 or 2 were recruited. Information on health resource utilization (HRU; including number/duration of hospitalizations, outpatient visits, procedures), attributed by investigators to be associated with a SRE, was collected retrospectively for up to 97 days prior to enrollment and prospectively for up to 18–21 months.
Results
A total of 631 patients contributing 1282 SREs, were enrolled across Germany, Italy, Spain and the United Kingdom. Approximately a third of all SREs required an inpatient stay. Mean duration of inpatient stay for patients with SREs requiring one ranged from 8.4 to 41.1 days across all countries and SRE types.
Conclusion
All types of SREs are associated with substantial HRU burden. Preventing SREs by using the best therapeutic options available may help to reduce the burden to patients and healthcare systems.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HRU, health resource utilization; SRE, skeletal-related event
Keywords: Advanced cancer, Bone metastases, Health resource utilization, Observational research, Skeletal-related event
Highlights
-
•
Patients with bone metastases frequently experience skeletal-related events (SREs).
-
•
We used prospective and retrospective data to examine health resource utilization.
-
•
All types of SREs required substantial resource use.
-
•
Therapies that prevent SREs could reduce the burden on healthcare systems.
1. Introduction
Bone is a common site for metastasis in advanced cancer; reports suggest that approximately 65–75% of patients with advanced breast and prostate cancer, and 30–40% of patients with advanced lung, kidney or thyroid cancer, will develop bone metastasis. Almost all patients with multiple myeloma develop lytic bone disease [1], [2]. Owing to the inherent nature of their manifestation, osteolytic lesions (commonly seen in patients who have lung or breast cancer or multiple myeloma) can be related to severe pain, pathologic fractures, life-threatening hypercalcemia, spinal cord compression and other nerve-compression syndromes [3]. In contrast, patients with prostate cancer have predominantly osteoblastic lesions that can also be frequently associated with bone pain, as well as pathologic fractures and spinal cord compression owing to the poor quality of bone formed during the remodeling process [3] and the often osteoporotic state induced by prolonged castration. These skeletal complications are commonly categorized as skeletal-related events (SREs) and defined as pathologic fracture, radiation to bone, spinal cord compression and surgery to bone. History of previous SREs is associated with an increased risk for subsequent events and a poorer prognosis. Previous studies have demonstrated decreased survival rates in those patients who have bone metastases and prior SREs [4], [5]. Although bone metastases/lesions and their associated SREs are predictors for increased mortality [4], [6], [7], [8], patients with advanced cancer today are generally surviving longer as new and more effective treatment options are introduced into the therapeutic armamentarium. Thus, prevention of SREs becomes even more important as patient life-span is extended.
Apart from their association with severe pain and reduced quality of life, SREs require substantial healthcare resource utilization (HRU) in their treatment and management. Previous retrospective studies have attempted to quantify various aspects of the burden of SREs across specific tumor types/countries, with studies in the United States of America (USA), France, Portugal and Spain all reporting high costs associated with SREs [9], [10], [11], [12], [13], [14]. However, these studies did not address the overall HRU burden. With the current cost restraints across European healthcare systems influencing resource reimbursement, this information is important when planning future healthcare requirements and evaluating new treatment options to prevent skeletal complications. Thus, our observational, multinational study was designed to estimate HRU related to each of the defined SRE types in patients with breast, lung or prostate cancer, or multiple myeloma. Owing to the high incidence of these malignancies [15] and the frequency of associated bone metastases [1], [2] these cancers can be considered to be responsible for the majority of the burden associated with bone metastases and their related complications in clinical practice. The study was carried out in Canada, Germany, Italy, Spain, the United Kingdom (UK) and the USA. Herein we report data for a cohort from 4 European countries.
2. Materials and methods
2.1. Patients
Patients were eligible if they were aged at least 18 years, had evidence of one or more bone metastasis secondary to breast, prostate or lung cancer or had multiple myeloma with focal lytic bone disease, and had experienced at least one SRE in the 97 days prior to enrollment. Other eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2 and life expectancy of at least 6 months; these criteria were used because patients with shorter life expectancies or worse performance statuses are more likely to be treated in hospices or managed care facilities, where information is often not transferred to hospital patient charts and thus not accessible to the study investigators. Patients provided signed, informed consent prior to collection of their data. Exclusion criteria included participation in an investigational drug trial for the treatment of bone metastases or SREs.
2.2. Study design
A multicenter, observational study was conducted in centers across four major European countries (Germany, Italy, Spain and the UK) as well as Canada and the USA. Large countries were chosen to help meet our enrollment targets. Planned enrollment was 250 patients per country; annual attrition (dropout and death) was assumed to be 20% for breast cancer and myeloma, 38% for prostate cancer and 55% for lung cancer. Therefore, a country accruing 250 participants had an expected total follow-up of 281 patient-years. Analysis of study results per country was pre-specified in the protocol; these analyses review HRU by country and SRE type.
SREs were classified by the investigators as one of the following: pathologic fracture, radiation to bone, spinal cord compression or surgery to bone. In order to ensure that adequate numbers of each SRE type per tumor group were recorded for the analysis, enrollment targets for the index SRE types were established for each country. Patients were enrolled within 97 days of experiencing the index SRE and patients were planned to be followed for up to 18–21 months. In case of a patient experiencing more than one SRE in the 97 days prior to enrollment, the index SRE was selected using the following hierarchy: (1) spinal cord compression; (2) surgery to bone; (3) pathologic fracture; and (4) radiation to bone.
The study was planned to run for 30 months after enrollment of the first patient and thus not all patients received the full duration of follow-up. Baseline demographics and medical history were collected at enrollment. HRU data were collected retrospectively for up to 97 days before study enrollment and prospectively for up to 18–21 months or until the end of the study. Associated HRU data collected from patient charts included information relating to inpatient stays, outpatient visits, emergency room visits, nursing home/long-term care facility stays, home health visits, procedures and certain medications. HRU was independently attributed to each SRE by the investigators. Data were requested to be collected from the patient׳s chart at least every 90 days during the follow-up observation period to ensure the identification and collection of any prospectively occurring SREs and resource use.
Enrollment began in 2008 and the data cut-off for this primary analysis was 31 May 2010.
2.3. Objectives
The primary study objective was to estimate HRU associated with SREs by type of SRE, tumor type and country. Secondary objectives, not discussed in this paper, included measurement of utility-based health-related quality of life following incident SREs, description of patterns of bisphosphonate use in patients with bone metastases and description of the association between bisphosphonate use and changes in renal function.
2.4. Outcome measures
This paper reports the primary outcome measures for HRU, including inpatient stays, outpatient visits, procedures, emergency room visits, nursing home/long-term care facility stays and home health visits. Details of these outcome measures are listed in Table 1. Secondary outcome measures, not discussed here, included measures of patient-reported outcomes (the 5-domain EuroQol questionnaire), bisphosphonate use (type, date of initiation/discontinuation, dose and frequency) and renal data (serum creatinine level, creatinine clearance rate, glomerular filtration rate, date of renal impairment/failure, and end-stage renal disease and dialysis).
Table 1.
Study health resource utilization outcome measures.
| Outcome measure | Data collected |
|---|---|
| Inpatient stays | Number, duration, reason, type of hospital unit, time spent in each type of hospital unit |
| Outpatient visits | Number, reason, provider type (e.g. medical oncologist, radiation oncologist, urologist, primary care physician, physiotherapist, occupational therapist) |
| Procedures | Type of procedure (e.g. imaging or treatment), reason for procedure, facility type |
| Emergency room visits | Number of visits, reason for visits, disposition (e.g. admitted to hospital, discharged) |
| Nursing home/long-term care facility stays | Type of facility (e.g. hospice, rehabilitation), reason for admission, length of stay |
| Home health visits | Number of visits, provider type, reason for visits |
| Certain medications | Including systemic therapies (e.g. chemotherapy) |
2.5. Statistical methods
All analyses in this observational study were descriptive. Participants who met the eligibility criteria and were enrolled into the study were included in the analysis set. Descriptive analyses of HRU were produced for each SRE type by country.
SREs were planned to be summarized by patient-year. The SRE rate per patient-year was defined as the ratio of total occurrences of SREs divided by the patient-years at risk on study. The patient-years at risk were the sum total duration of follow-up (up to 97 days before enrollment and prospective follow-up after enrollment) among all patients.
The proportion of SREs requiring an inpatient stay was calculated and summarized. Mean duration of an inpatient stay was computed as the total of the number of inpatient stay days attributed to SREs divided by the total number of SREs associated with an inpatient stay. All other primary outcome measures were analyzed in the same way.
If radiation to bone or surgery to bone was carried out as a result of another SRE (i.e. treatment of a primary SRE), the investigator had the option to attribute HRU to the primary SRE. In these instances, the inclusion of radiation to bone or surgery to bone as distinct SREs with no associated HRU in the analysis would result in an underestimation of the mean HRU for these SRE types. Thus, in these cases the SREs were excluded from the HRU analyses.
2.6. Ethics
The protocol and informed consent form were approved by the Ethics Committee/Institutional Review Boards at each participating site. Research was carried out in compliance with the Declaration of Helsinki.
3. Results
3.1. Study population
At the time of the primary data analysis (cut-off date, 31 May 2010), a total of 631 patients were enrolled across 95 sites in Germany, Italy, Spain and the UK, contributing 1282 SREs. By tumor type, 223 patients had a primary diagnosis of breast cancer (35.3%), 135 patients had lung cancer (21.4%), 120 patients had prostate cancer (19%) and 153 patients had multiple myeloma (24.2%). Baseline demographics and disease characteristics were similar across the countries (Table 2), although the proportions of female patients enrolled in Spain (44%) and the UK (42%) were lower than those in Germany (58%) and Italy (60%). Patients enrolled in Spain and the UK generally had a poorer performance status than those enrolled in Germany and Italy, although numerically fewer patients in the UK (versus the other three countries) had experienced an SRE prior to enrollment into the study. With the exception of the UK, over half of patients included in the study had experienced a prior SRE at baseline, despite the relatively short median time since diagnosis of bone metastases.
Table 2.
Baseline demographics and disease characteristics of the study population.
| Germany (N=200) | Italy (N=168) | Spain (N=131) | UK (N=132) | |
|---|---|---|---|---|
| Age, years, mean (SD) | 64 (10) | 63 (11) | 63 (11) | 66 (12) |
| Female, n (%) | 115 (58) | 100 (60) | 58 (44) | 55 (42) |
| Race, n (%) | ||||
| White/Caucasian | 198 (99) | 167 (99) | 128 (98) | 127 (96) |
| Other | 2 (1) | 1 (1) | 3 (2) | 5 (4) |
| Tumor type, n (%) | ||||
| Breast | 85 (43) | 62 (37) | 31 (24) | 45 (34) |
| Lung | 34 (17) | 43 (26) | 41 (31) | 17 (13) |
| Prostate | 30 (15) | 24 (14) | 21 (16) | 45 (34) |
| Multiple myeloma | 51 (26) | 39 (23) | 38 (29) | 25 (19) |
| ECOG performance status, n (%) | ||||
| 0 | 55 (28) | 43 (26) | 24 (18) | 27 (20) |
| 1 | 102 (51) | 79 (47) | 53 (40) | 56 (42) |
| 2 | 43 (22) | 46 (27) | 54 (41) | 49 (37) |
| History of SREs,an (%) | 124 (62) | 95 (57) | 92 (70) | 51 (39) |
| Time since primary cancer diagnosis, months | ||||
| Mean (SD) | 55 (68) | 41 (56) | 46 (61) | 52 (56) |
| Median | 29 | 13 | 17 | 33 |
| Time since bone metastases, months | ||||
| Mean (SD) | 16 (25) | 16 (29) | 16 (29) | 19 (28) |
| Median | 3 | 4 | 3 | 8 |
ECOG, Eastern Cooperative Oncology Group; SD, standard deviation; and SRE, skeletal-related event.
Before the retrospective data collection period.
3.2. Disposition
The median length of prospective follow-up across all countries and tumor types was in the range of 4.6–8.1 months. For patients with breast cancer, median follow-up across the four countries ranged from 6.9 to 10.9 months; for patients with lung cancer, median follow-up was shorter (from 1.5 to 5.6 months); for patients with prostate cancer it was 4.6–9.4 months; and for patients with multiple myeloma it was 5.5–10.8 months.
Across all tumor types, 48% (n=96) of German patients discontinued the study prior to the primary data analysis cut-off date; death was the primary reason for early discontinuation (n=82). In Italy, 39% (n=66) of patients discontinued the study early, with death again the primary reason for early discontinuation (n=59). Similarly in Spain and the UK, 38% (n=50) and 45% (n=59) discontinued the study early, with death cited as the primary reason in 39 and 58 patients, respectively.
3.3. Skeletal-related events
Patients experienced a total of 1282 SREs (Table 3), including 108 SREs that were recorded and subsequently discounted from the HRU analysis (70 events of radiation to bone and 38 events of surgery to bone) because their management was associated with the treatment of a separate primary SRE. The majority of the events recorded (62–78% range across countries) were retrospective, i.e. occurred before study enrollment. Radiation to bone was the most commonly reported SRE reported across all countries (Germany, 269 of 432 events; Italy, 164 of 291 events; Spain, 133 of 243 events; UK, 196 of 316 events).
Table 3.
Summary of skeletal-related events (SREs) by country and SRE type (pooled tumor types).
| Germany (N=432) |
Italy (N=291) |
Spain (N=243) |
UK (N=316) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PF | RB | SCC | SB | PF | RB | SCC | SB | PF | RB | SCC | SB | PF | RB | SCC | SB | |
| Number of SREs, n (%) | 85 (20.0) | 269 (62.0) | 13 (3.0) | 65 (15.0) | 66 (22.7) | 164 (56.4) | 12 (4.1) | 49 (16.8) | 51 (21.0) | 133 (54.7) | 32 (13.2) | 27 (11.1) | 52 (16.5) | 196 (62.0) | 34 (10.8) | 34 (10.8) |
| Patient-year-adjusted SRE rate | 0.4 | 1.4 | 0.1 | 0.3 | 0.4 | 1.1 | 0.1 | 0.3 | 0.5 | 1.3 | 0.3 | 0.3 | 0.5 | 1.8 | 0.3 | 0.3 |
PF, pathologic fracture; RB, radiation to bone; SB, surgery to bone; SCC, spinal cord compression; and SRE, skeletal-related event.
Patient-year-adjusted SRE rates for each SRE type were generally consistent across countries: 2.2 SREs per patient-year in Germany, 1.9 SREs per patient-year in Italy, 2.4 SREs per patient-year in Spain and 2.9 SREs per patient-year in the UK.
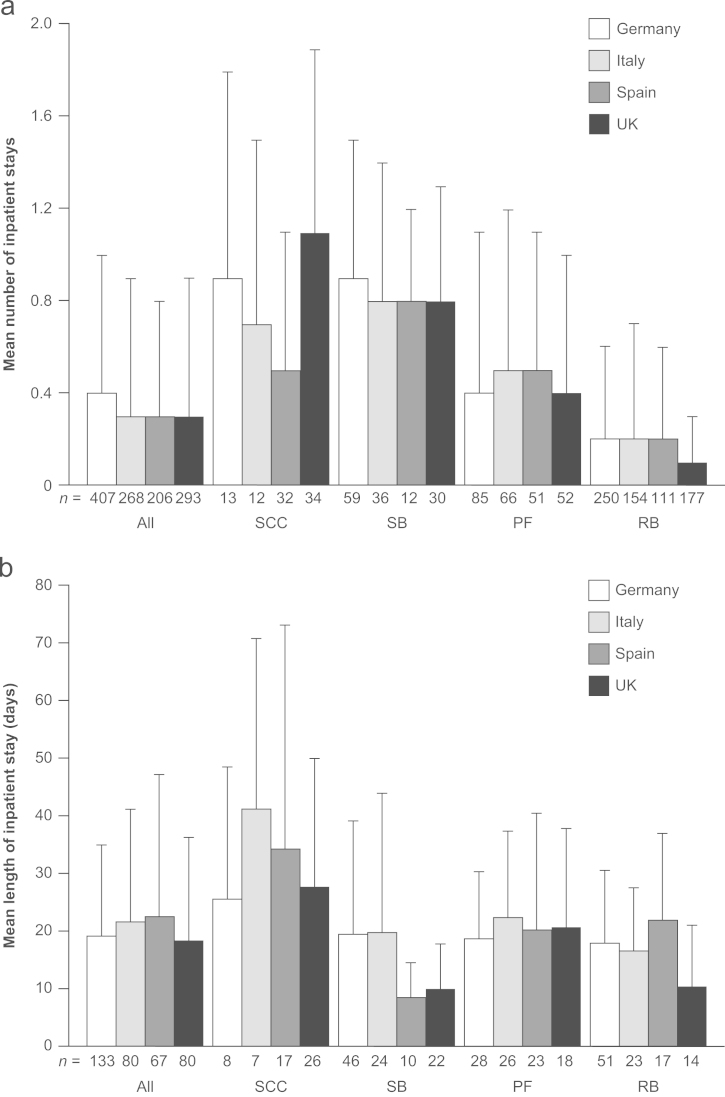
3.4. HRU analyses
Across all countries, approximately one in three SREs required an inpatient stay (mean hospitalizations per SRE ranged from 0.31 to 0.36 across all countries) (Fig. 1A). Spinal cord compression and surgery to bone were consistently associated with a higher requirement for inpatient stays than pathologic fracture and radiation to bone (Table 4; Fig. 1A). Radiation to bone was associated with the lowest mean number of inpatient stays, ranging from 0.1 to 0.2 hospitalizations per event across all countries.
Fig. 1.
(A) Mean+SD number of inpatient stays per SRE and (B) mean+SD length of hospital stays per SRE (excluding SREs without a stay), by country and SRE type. PF, pathologic fracture; RB, radiation to bone; SB, surgery to bone; SCC, spinal cord compression; SD, standard deviation; and SRE, skeletal-related event.
Table 4.
HRU by country and skeletal-related event type (pooled tumor types).
| Germany |
Italy |
Spain |
UK |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PF | RB | SCC | SB | PF | RB | SCC | SB | PF | RB | SCC | SB | PF | RB | SCC | SB | |
| Inpatient hospitalizations, n | ||||||||||||||||
| Mean (SD) | 0.4 (0.7) | 0.2 (0.4) | 0.9 (0.9) | 0.9 (0.6) | 0.5 (0.7) | 0.2 (0.5) | 0.7 (0.8) | 0.8 (0.6) | 0.5 (0.6) | 0.2 (0.4) | 0.5 (0.6) | 0.8 (0.4) | 0.4 (0.6) | 0.1 (0.2) | 1.1 (0.8) | 0.8 (0.5) |
| Median | 0 | 0 | 1.0 | 1.0 | 0 | 0 | 0.7 | 1.0 | 0 | 0 | 0.5 | 1.0 | 0 | 0 | 1.0 | 1.0 |
| Length of stay, daysa | ||||||||||||||||
| Mean (SD) | 18.7 (11.6) | 18.0 (12.5) | 25.6 (23.0) | 19.4 (19.7) | 22.4 (15.0) | 16.6 (11.0) | 41.1 (29.8) | 19.8 (24.2) | 20.2 (20.2) | 21.9 (15.0) | 34.3 (37.9) | 8.4 (6.0) | 20.7 (17.1) | 10.4 (10.7) | 27.7 (22.3) | 10.0 (7.8) |
| Median | 21.5 | 16.0 | 14.0 | 15.0 | 21.5 | 15.0 | 32.0 | 10.0 | 13.0 | 19.0 | 28.0 | 8.0 | 14.5 | 6.5 | 23.0 | 7.5 |
| Outpatient visits, n | ||||||||||||||||
| Mean (SD) | 3.1 (6.3) | 9.3 (7.6) | 5.4 (7.4) | 1.6 (5.1) | 2.9 (4.4) | 6.0 (5.1) | 4.1 (6.8) | 1.4 (2.5) | 1.9 (2.7) | 6.9 (6.7) | 3.3 (5.4) | 1.9 (3.3) | 2.2 (2.6) | 2.7 (2.9) | 3.4 (4.4) | 2.2 (2.5) |
| Median | 0.5 | 8.0 | 1.0 | 0 | 1.0 | 5.0 | 1.0 | 0.5 | 1.0 | 7.0 | 0 | 0.5 | 1.0 | 1.5 | 1.5 | 2.0 |
| Procedures, nb | ||||||||||||||||
| Mean (SD) | 5.2 (7.3) | 13.2 (7.1) | 8.9 (8.7) | 3.0 (4.4) | 4.6 (5.4) | 8.0 (5.0) | 7.8 (6.3) | 2.9 (2.8) | 3.6 (3.4) | 9.0 (5.8) | 7.8 (5.3) | 3.8 (3.3) | 3.1 (2.9) | 3.0 (2.7) | 6.2 (4.7) | 3.7 (4.0) |
| Median | 2.0 | 12.5 | 2.0 | 2.0 | 3.0 | 7.5 | 6.0 | 2.0 | 2.5 | 10.0 | 7.0 | 2.5 | 2.0 | 2.0 | 5.0 | 3.0 |
| Emergency room visits, n | ||||||||||||||||
| Mean (SD) | 0 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0.1 (0.4) | 0 (0) | 0.2 (0.4) | 0 (0.2) | 0.1 (0.3) | 0 (0.2) | 0.2 (0.5) | 0 (0) | 0.2 (0.5) | 0 (0) | 0.2 (0.6) | 0 (0) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nursing home/long-term care facility stays, n | ||||||||||||||||
| Mean (SD) | 0 (0) | 0 (0) | 0 (0) | 0 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.2) | 0 (0) | 0 (0.2) | 0 (0) | 0 (0.2) | 0 (0) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Home health visits, n | ||||||||||||||||
| Mean (SD) | 0 (0) | 0 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.2) | 0 (0) | 0 (0) | 0 (0.1) | 0.1 (0.2) | 0 (0.2) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
HRU, health resource utilization; PF, pathologic fracture; RB, radiation to bone; SB, surgery to bone; SCC, spinal cord compression; SD, standard deviation; and SRE, skeletal-related event.
Based on SREs requiring an inpatient stay.
Including imaging.
In Germany and the UK, mean length of inpatient stays was similar, at 19.1 days and 18.3 days, respectively (among SREs that required an inpatient stay) (Fig. 1B). For Italy and Spain, this figure was slightly higher at 21.6 and 22.5 days, respectively. Spinal cord compressions were associated with the longest inpatient stays, ranging from 25.6 days to 41.1 days across the four countries (Table 4; Fig. 1B). Although radiation to bone was less frequently associated with the requirement for an inpatient stay, those events requiring an inpatient stay were associated with stays of considerable duration (mean, 10.4–21.9 days across the four countries). Mean duration of an inpatient stay varied numerically by facility type, although the sample sizes were small and made interpretation difficult. In Germany, mean duration of inpatient stays across those SREs requiring an inpatient stay ranged from 3.0 days in an urology unit/ward (n=1) to 33.0 days in a gynecology unit/ward (n=1). For Italy, the duration of inpatient stays per SRE was equally widespread across facility types, ranging from 2.0 days in an intensive care unit/ward to 44.0 days in a rehabilitation facility. In Spain, the differences between the duration of stay by facility type were smaller, with a range of 7.0 days in an ‘unknown’ unit (n=1) to 30.6 days in a unit marked as ‘other’ and not further defined (n=14). Mean duration of stay per SRE by facility type in the UK ranged from 2.0 days in an ‘unknown’ unit (n=1) to a notable 58.5 days spent in a nursing facility.
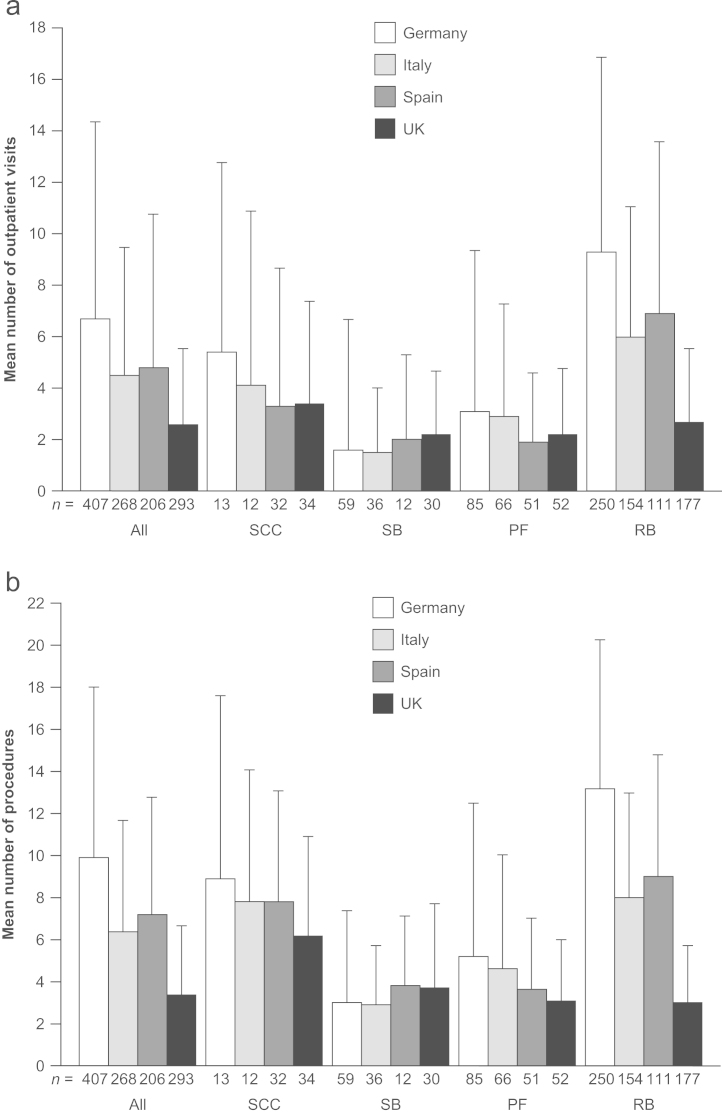
Approximately two-thirds of SREs required at least one outpatient visit (68%, 74%, 66% and 78% of SREs in Germany, Italy, Spain and the UK, respectively). SREs were associated with a mean number of 6.7, 4.5, 4.8 and 2.6 outpatient visits per SRE in each of the countries, respectively (Fig. 2A). Aside from those patients treated in the UK, radiation to bone (versus the other SRE types) was associated with the highest number of outpatient visits (Table 4; Fig. 2A). The SRE type requiring the most outpatient visits in the UK was spinal cord compression; in all other countries, this was the second highest event type requiring outpatient visits.
Fig. 2.
(A) Mean+SD number of outpatient visits per SRE and (B) mean+SD number of procedures performed per SRE, by country and SRE type. (B) Includes both outpatient and procedures (i.e. imaging, surgery, etc.). Radiation procedures with multiple sessions were captured as multiple procedures. PF, pathologic fracture; RB, radiation to bone; SB, surgery to bone; SCC, spinal cord compression; SD, standard deviation; and SRE, skeletal-related event.
The majority (>90%) SREs required at least one procedure to be performed. The mean number of procedures associated with each SRE was 9.9, 6.4, 7.2 and 3.4 for Germany, Italy, Spain and the UK, respectively (Fig. 2B). Reflecting the pattern of outpatient visits, for Germany, Italy and Spain, radiation to bone was associated with the highest mean number of procedures, with 13.2, 8.0 and 9.0 procedures per event, respectively (Table 4). The number of procedures per radiation to bone event was much lower than this in the UK (2.2 procedures per SRE), which was reflected in the lower rates of external beam radiation and intensity modulation radiotherapy procedure used in this country than in Germany, Italy and Spain (Table 5). Types of procedures used per SRE also differed by SRE type: for example, magnetic resonance imaging was frequently used for spinal cord compression, ranging from 0.7 to 1.1 procedures per SRE across the four countries, whereas it was used much less often for the other SREs (Table 5). In Germany, outpatient or emergency room visits were associated with a mean of 7.6 procedures per SRE, while inpatient stays were associated with a mean of 2.3 procedures per SRE. The mean number of procedures associated with outpatient/emergency room visits (4.7 and 5.0 procedures per SRE) versus those carried out during in-patient stays (1.8 and 2.2 procedures per SRE) for Italy and Spain, respectively, was similar. The number of procedures employed per SRE was consistently lower in the UK, where outpatient/emergency room visits were associated with a mean number of 2.6 procedures per SRE versus only 0.9 procedures per SRE in the inpatient setting.
Table 5.
Number of proceduresa performed by country, per SRE by SRE type (pooled tumor types).
| Procedure type | Mean (SD) |
|||
|---|---|---|---|---|
| Germany |
Italy |
Spain |
UK |
|
| Pathologic fracture | N = 85 | N = 66 | N = 51 | N = 52 |
| Chemotherapy | 0 (0.1) | 0.2 (0.9) | 0 (0) | 0.2 (1.1) |
| Computed tomography | 0.4 (0.6) | 0.4 (0.6) | 0.2 (0.5) | 0.3 (0.5) |
| External beam radiation | 2.9 (6.3) | 1.3 (4.8) | 0.7 (2.3) | 0.7 (1.8) |
| Intensity modulation radiotherapy | 0.2 (1.3) | 0 (0) | 0.1 (0.3) | 0 (0) |
| Laboratory assessment | 0.1 (0.2) | 0.4 (1.2) | 0 (0.1) | 0.1 (0.3) |
| Magnetic resonance imaging | 0.2 (0.5) | 0.2 (0.5) | 0.5 (0.6) | 0.2 (0.5) |
| Physical examination | 0.1 (0.3) | 0.4 (0.7) | 0.3 (0.7) | 0.2 (0.5) |
| Surgery to bone – extremities | 0.1 (0.2) | 0.2 (0.4) | 0.2 (0.4) | 0.2 (0.4) |
| X-ray | 0.5 (0.6) | 0.9 (0.9) | 1.2 (1.5) | 0.8 (1.3) |
| Other | 0.6 (1.8) | 0.3 (0.9) | 0.2 (0.6) | 0.4 (0.9) |
| Radiation to bone | N = 250 | N = 154 | N = 111 | N = 177 |
| Computed tomography | 0.2 (0.4) | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.3) |
| External beam radiation | 7.2 (7.8) | 6.8 (5.0) | 6.0 (6.2) | 2.2 (2.4) |
| Intensity modulation radiotherapy | 4.9 (7.7) | 0.3 (1.3) | 2.0 (4.1) | 0 (0) |
| Laboratory assessment | 0.1 (0.7) | 0.2 (0.7) | 0.1 (0.5) | 0 (0.2) |
| Physical examination | 0.1 (0.3) | 0.2 (0.5) | 0.4 (0.8) | 0.1 (0.4) |
| Radionucleotides | 0.2 (1.8) | 0.1 (0.7) | 0 (0.1) | 0 (0.1) |
| X-ray | 0.1 (0.3) | 0.1 (0.3) | 0.1 (0.5) | 0.2 (0.5) |
| Other | 0.2 (0.6) | 0.1 (0.5) | 0.2 (0.5) | 0.2 (0.5) |
| Spinal cord compression | N = 13 | N = 12 | N = 32 | N = 34 |
| Chemotherapy | 0 (0) | 0.2 (0.6) | 0 (0) | 0.2 (0.8) |
| Computed tomography | 0.4 (0.5) | 0.6 (0.5) | 0.3 (0.6) | 0.3 (0.8) |
| External beam radiation | 6.0 (8.1) | 4.0 (4.6) | 3.8 (4.2) | 3.0 (3.4) |
| Intensity modulation radiotherapy | 0.9 (3.3) | 0 (0) | 1.2 (3.1) | 0 (0) |
| Laboratory assessment | 0 (0) | 0.8 (1.8) | 0.1 (0.3) | 0.4 (1.2) |
| Magnetic resonance imaging | 0.7 (0.6) | 1.1 (1.7) | 1.1 (0.8) | 0.9 (0.7) |
| Physical examination | 0.1 (0.3) | 0.3 (0.5) | 0.1 (0.3) | 0.2 (0.6) |
| Radionucleotides | 0 (0) | 0 (0) | 0 (0) | 0.2 (1.0) |
| Surgery to bone – spine | 0.4 (0.5) | 0.2 (0.4) | 0.1 (0.3) | 0.1 (0.4) |
| X-ray | 0.1 (0.3) | 0.4 (0.7) | 0.6 (1.0) | 0.4 (0.9) |
| Other | 0.2 (0.8) | 0 (0) | 0.3 (1.0) | 0.2 (0.5) |
| Surgery to bone | N = 59 | N = 36 | N = 12 | N = 30 |
| Computed tomography | 0.2 (0.6) | 0.3 (0.6) | 0 (0) | 0.1 (0.3) |
| External beam radiation | 0.3 (2.0) | 0.4 (1.8) | 0 (0) | 0.4 (2.0) |
| Laboratory assessment | 0.1 (0.4) | 0.2 (0.8) | 0 (0) | 0.4 (1.0) |
| Magnetic resonance imaging | 0.1 (0.3) | 0.2 (0.5) | 0.1 (0.3) | 0.1 (0.4) |
| Physical examination | 0.1 (0.4) | 0.2 (0.6) | 0.8 (1.7) | 0.2 (0.4) |
| Surgery – other | 0.1 (0.3) | 0.1 (0.2) | 0.5 (0.7) | 0 (0) |
| Surgery to bone – extremities | 0.5 (0.5) | 0.3 (0.5) | 0.3 (0.5) | 0.5 (0.5) |
| Surgery to bone – spine | 0.3 (0.5) | 0.4 (0.5) | 0.3 (0.5) | 0.2 (0.4) |
| Transfusions | 0.1 (0.5) | 0 (0.2) | 0 (0) | 0.2 (0.7) |
| Wound care/debridement | 0.3 (1.1) | 0 (0) | 0.1 (0.3) | 0.1 (0.4) |
| X-ray | 0.4 (0.6) | 0.6 (0.7) | 1.3 (1.6) | 1.0 (1.1) |
| Other | 0.5 (2.6) | 0.1 (0.4) | 0.4 (0.9) | 0.3 (0.8) |
SRE, skeletal-related event.
Procedures performed with a mean of at least 0.2 per SRE in at least one of the countries listed.
Emergency room visits were generally infrequently reported (Table 4). Few nursing home/long-term care facility stays and home health visits were reported for all countries (Table 4).
4. Discussion
This is the first large, multinational study including both prospectively and retrospectively collected data to examine the HRU associated with the different categories of SREs – local, irreversible consequences of bone metastases – in a large sample of patients from four major European countries. Previous studies have either been solely retrospective in nature or have limited their research to a single tumor type. Furthermore, this study captured SRE-specific data that have not previously been extensively estimated, including outpatient and emergency room visits as well as detailed information on types of procedure. In addition, HRU was directly attributed to SREs by the investigators, thus HRU was only assigned when it was deemed to be directly due to the SRE and not to the underlying disease.
Although the distribution of SREs reported in this study is not representative of the real-world distribution of SREs (enrollment was driven by the index SRE recruitment cells), it can be concluded that all SRE types were associated with substantial HRU, and the pattern of HRU was generally consistent within each SRE type across all countries. Notably, approximately a third of all SREs were associated with an inpatient stay. Surgery to bone and spinal cord compression were the SRE types most likely to result in hospitalization. The mean duration of inpatient stays was considerable across all SRE types, ranging from 18 days in the UK to 19 days in Germany and 22 days in Italy and Spain. Although radiation to bone was the SRE type the least likely to require an inpatient stay, those events requiring hospitalization were associated with stays of notable duration (up to 23 days in length). The duration of inpatient stay varied by country and facility type. For example, mean length of stay for surgery to bone was lower in Spain and the UK (8.4 and 10.0 days, respectively) than in Germany and Italy (19.4 and 19.8 days, respectively). This could reflect differences in clinical practice and available resources between countries, such as the frequency of use of less invasive surgical procedures and whether subsequent physiotherapy and rehabilitation is performed in a continued inpatient setting or in an outpatient setting. However, caution is needed when interpreting these data because the numbers of SREs are low when split by both SRE type and country. Across all countries, radiation units/wards were associated with substantial inpatient stays (mean 15.50, 12.00, 18.40 and 8.67 days, respectively, for Germany, Italy, Spain and the UK), suggesting that patients may be required to travel and remain in hospital while receiving treatment.
All SREs were associated with multiple outpatient visits and numerous procedures. Events of radiation to bone were associated with a lower rate of outpatient visits and procedures in the UK (versus Germany, Italy and Spain), which likely reflects differences in clinical practice between the UK and the other three European countries. In the UK, there is a preference to use single-fraction radiotherapy versus the multi-fractionated approach that is favored in the majority of European countries. This preference for single-fraction treatment was also reflected in the lower proportion of radiation to bone events requiring an inpatient stay in the UK and the shorter duration of associated stay, compared with the other three countries.
One of the limitations with the study was that the duration of follow-up was shorter than planned (up to a median of 8.1 months in this European cohort). Slow recruitment (study period was defined as 30 months following enrollment of the first patient) and early withdrawal from the study due to patient death may have been the main contributing factors. Withdrawal from the study was slightly higher than predicted, with death the primary reason for early discontinuation despite the inclusion criteria stating patients should have a life expectancy of at least 6 months. This could reflect the challenges associated with physicians׳ estimation of patients׳ life expectancies [16]. Slow recruitment also contributed to small sample size per SRE in some countries, which limits interpretation of some of the results. This may in part be due to difficulties with engaging patients and their physicians in observational research when no active therapies/interventions are offered as part of the study and participation in a concurrent investigational study is not permitted. However, the data show a generally consistent approach to patient management across countries, which suggests that the outcomes are valid, even though small sample numbers may prevent detailed interpretation. Another potential limitation was that we did not use an independent, blinded review for attributing HRU to SREs; however, there were no external influences on the local investigators, therefore their attribution of HRU should be reflective of clinical practice on a local level.
Despite the robust approach of this study, the HRU observed may underestimate the overall resource burden associated with SREs for patients with bone metastases due to advanced cancer. Patients with low performance status and limited life expectancy (ECOG performance status 3 or 4 or a life expectancy of <6 months) were excluded from this study, although they may arguably utilize substantial healthcare resources during the late stage of their disease. Some data were not accessible to the investigators at all study sites, i.e. information relating to home health visits and/or long-term care facility stays were often not recorded in the hospital patient charts. Emergency room visits were often not reported or required; in case of an emergency, a patient may have contacted the treating specialist department directly for admission or treatment. Furthermore, in case of an emergency, patients may have attended an emergency room in a different center to that of the participating institute and this information may therefore not be transferred to the patient chart. In addition, indirect costs (i.e. transport to and from treatment units and the costs to employers as a result of patients and their care givers taking time off work) that are incurred as a result of SREs were not available to the investigators and not included in the study, Finally, bone pain requiring analgesic use or changes in anticancer therapy to manage tumor burden in the bone are frequent in patients with advanced cancer and bone metastases. These events were not defined as SREs, although they may require additional inpatient stays and/or outpatient visits.
HRU resulting from SREs is of increasing relevance in times of financial and economic restraint because it can help to determine the financial burden to hospital managers and payers. A separate analysis of the costs associated with SREs in this study found that the most costly SRE, spinal cord compression, had a mean cost per SRE of €4884–€12,082, and costs per surgery to bone ranged from €3348 to €9407. Pathologic fracture and radiation to bone had slightly lower costs per SRE (€1015–€6968 and €704–€2461, respectively) but still represented a considerable financial burden [17]. Treatments that delay or prevent SREs could help to reduce the utilization of healthcare resources, thereby lowering the costs associated with metastatic bone disease. Historically, intravenously administered bisphosphonates, such as zoledronic acid, have been the mainstay of treatment for the prevention of SREs in adult patients with advanced malignancies involving the bone [18]. More recently, the RANK ligand inhibitor denosumab has been widely approved for the prevention of SREs in adults with bone metastases from solid tumors [19] after it was demonstrated to be superior to zoledronic acid in an integrated analysis of patients with solid tumors from three identically designed, phase 3, head-to-head clinical trials [20]. The systemic treatments enzalutamide, abiraterone acetate and radium-223 dichloride, currently only available for use in patients with metastatic castration-resistant prostate cancer, have shown a trend towards delayed bone complications compared with placebo in phase 3 trials [21], [22], [23]. In these studies, concomitant use of bone-targeted agents was permitted and preliminary data demonstrated a further trend towards improved outcomes in patients who received additional bone-targeted therapy. As bone outcomes were not a primary focus for these studies, the extent to which these new agents protect against SREs should be further assessed [23], [24], [25].
5. Conclusion
Data from Germany, Italy, Spain and the UK suggest that all types of SRE occurring in patients with bone metastases/lesions secondary to breast, prostate or lung cancer or multiple myeloma are associated with substantial HRU (lengthy inpatient stays, numerous outpatient visits and multiple procedures). Further studies in additional Western European countries will confirm whether these observations reflect the situation across Western Europe. Preventing SREs by using the optimal treatment option available is important to achieve a considerable reduction in patient burden and rate of hospitalization.
Conflict of interest
H. Hoefeler has acted as an advisor to Amgen.
I. Duran has received compensation from Amgen in relation to participation in Advisory Boards related to the topic of this publication.
G. Hechmati, C. Atchison, R. Wei and E. Thomas are employees of Amgen and hold stock.
D. Lüftner has been a speaker for Amgen and participated in Advisory Boards.
J. Ashcroft has recieved honoraria from Amgen and participated in Advisory Boards.
A. Bahl has attended advisory boards and received honoraria from Amgen and Novartis.
C. Garzon Rodriguez and V. Lorusso have no conflicts of interest to declare.
Authors׳ contributions
G. Hechmati and R. Wei were involved in the conception and design of this study. H. Hoefeler, I. Duran, G. Hechmati, C. Garzon Rodriguez, D. Lüftner, J. Ashcroft, A. Bahl, R. Wei and V. Lorusso were involved in the acquisition of data. All authors contributed to the analysis and interpretation of the data, drafted and critically reviewed the manuscript and approved the final version.
Funding
This work was supported by Amgen Inc.
Acknowledgments
The authors would like to acknowledge the contributions of all study 20060392 (STARS) investigators, study teams and patients. Editorial support was provided by Oxford PharmaGenesis™. Funding for this support was provided by Amgen (Europe) GmbH.
References
- 1.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Lipton A. Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol. 2004;2:205–213. ([discussion 213–4, 216–7, 219–20]) [PubMed] [Google Scholar]
- 3.Roodman G.D. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 4.Nørgaard M., Jensen A.Ø., Jacobsen J.B., Cetin K., Fryzek J.P., Sørensen H.T. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999–2007) J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Chia V.M., Cetin K., Jacobsen J.B., Nørgaard M., Jensen A.Ø., Christiansen C.F. The incidence and prognostic significance of bone metastases and skeletal-related events in lung cancer patients: a population-based cohort study in Denmark. J Clin Oncol. 2010;28:e18074. ([Abstract e18074]) [Google Scholar]
- 6.Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res Treat. 2012;131:231–238. doi: 10.1007/s10549-011-1721-x. [DOI] [PubMed] [Google Scholar]
- 7.Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177–183. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura H., Yamada K., Sugiura T., Hida T., Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466:729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delea T., Langer C., McKiernan J., Liss M., Edelsberg J., Brandman J. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–396. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 10.Delea T., McKiernan J., Brandman J., Edelsberg J., Sung J., Raut M. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol. 2006;4:341–347. [PubMed] [Google Scholar]
- 11.Pockett R.D., Castellano D., McEwan P., Oglesby A., Barber B.L., Chung K. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010;19:755–760. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lage M.J., Barber B.L., Harrison D.J., Jun S. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manage Care. 2008;14:317–322. [PubMed] [Google Scholar]
- 13.Decroisette C., Monnet I., Berard H., Quere G., Le Caer H., Bota S. Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601) J Thorac Oncol. 2011;6:576–582. doi: 10.1097/JTO.0b013e318206a1e3. [DOI] [PubMed] [Google Scholar]
- 14.Felix J., Andreozzi V., Soares M., Borrego P., Gervasio H., Moreira A. Hospital resource utilization and treatment cost of skeletal-related events in patients with metastatic breast or prostate cancer: estimation for the Portuguese National Health System. Value Health. 2011;14:499–505. doi: 10.1016/j.jval.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C. International Agency for Research on Cancer; Lyon, France: 2013. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11 [Internet]〈http://globocan.iarc.fr〉 ([accessed 11.03.14]) [Google Scholar]
- 16.Krishnan M., Temel J.S., Wright A.A., Bernacki R., Selvaggi K., Balboni T. Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol. 2013;11:68–74. doi: 10.12788/j.suponc.0004. [DOI] [PubMed] [Google Scholar]
- 17.Hechmati G., Cure S., Gouepo A., Hoefeler H., Lorusso V., Luftner D. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ. 2013;16:691–700. doi: 10.3111/13696998.2013.779921. [DOI] [PubMed] [Google Scholar]
- 18.Novartis Europharm Limited. Zometa (zoledronic acid): summary of product characteristics; 2012.
- 19.European Medicines Agency. Denosumab (XGEVA): summary of product characteristics; 2012.
- 20.Lipton A., Fizazi K., Stopeck A.T., Henry D.H., Brown J.E., Yardley D.A. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Scher H.I., Fizazi K., Saad F., Taplin M.E., Sternberg C.N., Miller K. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 22.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker C., Nilsson S., Heinrich D., Helle S.I., O׳Sullivan J.M., Fossa S.D. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 24.Logothetis C.J., Basch E., Molina A., Fizazi K., North S.A., Chi K. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–1217. doi: 10.1016/S1470-2045(12)70473-4. [DOI] [PubMed] [Google Scholar]
- 25.OncLive. Enzalutamide delays first skeletal-related event, improves pain and QOL in patients with metastatic prostate cancer. Available from: 〈http://www.onclive.com/publications/obtn/2012/November-2012/Enzalutamide-Delays-First-Skeletal-Related-Event-Improves-Pain-and-QOL-in-Patients-With-Metastatic-Prostate-Cancer〉 [accessed 11.03.14].