Summary
The ventro-lateral pulvinar is reciprocally connected with the visual areas of the ventral stream important for object recognition. To understand the mechanisms of attentive stimulus processing in this pulvinar-cortex loop, we investigated the interactions between the pulvinar, area V4, and IT cortex in a spatial attention task. Sensory processing and the influence of attention in the pulvinar appeared to reflect its cortical inputs. However, pulvinar deactivation led to a reduction of attentional effects on firing rates and gamma synchrony in V4, a reduction of sensory-evoked responses and overall gamma coherence within V4, and severe behavioral deficits in the affected portion of the visual field. Conversely, pulvinar deactivation caused an increase in low frequency cortical oscillations, often associated with inattention or sleep. Thus, cortical interactions with the ventro-lateral pulvinar are necessary for normal attention and sensory processing, and for maintaining the cortex in an active state.
Introduction
The selection of objects for visual processing and control over behavior is thought to involve the top-down modulation of neuronal responses in ventral stream visual areas, from V1, through areas V2 and V4, to inferior temporal (IT) cortex (Desimone and Duncan, 1995; Kastner and Ungerleider, 2000; Maunsell and Treue, 2006; Squire et al., 2013; Peelen and Kastner, 2014). The frontal eye fields (FEF) is one source of top-down signals for modulating the visual cortex during spatial attention (Squire et al., 2013), but it could not be the only source because lesions of the entire dorsolateral prefrontal cortex, including FEF, reduce but do not completely eliminate either the attentional modulation of responses in area V4, or an animal’s ability to attend to stimuli in the presence of distracters (Gregoriou et al., 2014). Another possible source of signals for the attentional modulation of the ventral stream is the pulvinar, which has long been suggested to play an important role in selective attention (Desimone et al., 1990; Robinson and Petersen, 1992; Olshausen et al., 1993; Shipp, 2004; Saalmann and Kastner, 2011).
The pulvinar is the largest nucleus of the primate thalamus, with several cytoarchitecturally defined sub-nuclei. Ventral stream areas V1, V2, V4, and IT cortex have connections with the ventro-lateral portions of the pulvinar, including the inferior pulvinar and ventral parts of the lateral pulvinar (Soares et al., 2001; Shipp, 2003; Kaas and Lyon, 2007; Gattass et al., 2014). Cells in layers 5 and 6 of these cortical visual areas project topographically to the pulvinar, which in turn projects mainly back to the superficial layers of these areas (Sherman and Guillery, 2002; Shipp, 2003). Such a cortex-thalamus-cortex pathway could be consistent with either a role in attention or in the relay of sensory information from one area to another (Purushothaman et al., 2012). This “ventral-stream” portion of the pulvinar also has connections with other parts of the attentional control network. The inferior pulvinar receives inputs from the superior colliculus (SC) (Benevento and Standage, 1983; Berman and Wurtz, 2010), which has functions in oculomotor control and attention. There are no direct connections between the ventro-lateral pulvinar and parietal or prefrontal cortex (Baizer et al., 1993), but the entire pulvinar receives GABA-ergic inputs from the reticular nucleus of the thalamus, which itself receives converging input from many different cortical areas (Zikopoulos and Barbas, 2006; Jones, 2009; Saalmann and Kastner, 2011; Zikopoulos and Barbas, 2012). Thus, the anatomy raises intriguing but unanswered questions about the functions of the pulvinar-cortex loop in attention and visual processing.
Physiological studies of cells in the pulvinar have found visual responses modulated by attention (Petersen et al., 1985; Robinson and Petersen, 1992; Benevento and Port, 1995; Bender and Youakim, 2001; Wilke et al., 2009), and brain imaging studies have found enhanced activation with attention in the pulvinar (Kastner et al., 2004; Smith et al., 2009). However, it would take more detailed analyses of causal influences to distinguish whether the pulvinar is a cause of attention modulation in visual cortex or simply mirrors its cortical input. Saalman et al (2012) conducted a Granger causality analysis of pulvinar interactions with the visual cortex during the delay period following an attentional cue. They suggested that the pulvinar synchronizes cortical activity at alpha frequencies following the cue, thereby regulating the transmission of information from one cortical area to another according to attentional demands. However, the role of neural synchronization in the processing of the attended stimulus was not clear in that study. Large alpha synchrony has also typically been associated with inattention rather than attention (Bollimunta et al., 2011; Schmid et al., 2012). Thus, the mechanism of the pulvinar-cortex loop in attentive stimulus processing is still unclear.
Finally, pulvinar deactivation or lesions provide a more direct way than the Granger causality analysis to test its causal role in cortical visual and attentive processing, but the results so far have been inconclusive. Numerous studies in humans (Zihl and von Cramon, 1979; Rafal and Posner, 1987; Danziger et al., 2001; Karnath et al., 2002; Ward and Arend, 2007; Arend et al., 2008; Snow et al., 2009) and monkeys (Petersen et al., 1987; Desimone et al., 1990; Wilke et al., 2010; Wilke et al., 2013) have found that pulvinar lesions or deactivation lead to attentional impairments in the contralesional field. However, these studies have all involved the more dorsal portions of the pulvinar with parietal connections, and one lesion study confined to the ventro-lateral pulvinar found no effects on attention (Bender and Butter, 1987). By combining pulvinar deactivation with cortical recording, Purushothamanet al. (2012) found that deactivation of the pulvinar eliminated visual responses in the superficial layers of V1 in anesthetized Galagos, and Soares et al. (2004) found mixed positive and negative effects on responses in V2 following pulvinar deactivation in anesthetized new world Cebus monkeys. Although the differences in results are puzzling, together they suggest a possible role of the pulvinar in cortical visual processing (Sherman and Guillery, 2011). However, the effects of pulvinar deactivation on attentive visual processing in the cortex have never been investigated in awake monkeys.
To further understand the functions of the pulvinar-cortex loop, we recorded simultaneously in the pulvinar and V4 and IT cortex of monkeys performing a spatial attention task. We first tested the synchronous interactions between the pulvinar and visual cortex in normally awake state. We found that cells in the pulvinar had properties similar to those of cells in the cortical areas projecting to them, but the effects of attention were weaker and later than those in V4. Gamma frequency synchrony between V4 and the pulvinar was enhanced by attention, but this synchrony was led by V4. Likewise, we found for the first time that gamma spike-field synchrony between V4 and IT cortex was also enhanced by attention and led by V4. Thus, neuronal properties in the pulvinar seem to reflect its cortical input. Next, we inactivated the ventro-lateral pulvinar and simultaneously recorded in V4 and IT, which allowed us to test for the first time the causal influence of the pulvinar on cortical processing in awake monkeys. The animals were significantly impaired in the task in the affected portion of the visual field. The effects of attention on V4 responses were greatly reduced. However, visual responses in V4 were also reduced even below the level of response normally found without attention, suggesting that the pulvinar is essential for normal visual processing in cortex as well. V4 showed a large increase in low frequency (0.5–20 Hz) power in the LFP following pulvinar deactivation, almost as if the affected part of the cortex went into a state of “sleep”. Thus, the return inputs from pulvinar to the cortex seem necessary to keep the cortex in a normally active state.
RESULTS
We recorded predominantly multi-unit activity and LFPs simultaneously in the ventral portion of the lateral pulvinar (Fig. 1) and areas V4 and IT in two monkeys (Macaca mulatta), but our focus was on its interactions with V4. V4 has widespread connections in the ventro-lateral pulvinar, with a given location in the V4 visual field map having connections with a wide zone extending between the anterior and posterior poles of the pulvinar (Shipp, 2003; Kaas and Lyon, 2007; Gattass et al., 2014). However, the connections seem to be concentrated predominantly in the middle of this range, i.e. the ventral portion of the lateral pulvinar, which was also the portion targeted in the study of Saalman et al. (2012) based on DTI connectivity. Using structural MRI imaging, we first targeted this general region known to have V4 connections, and then we used RF mapping to identify the subregion representing the contralateral lower visual field, overlapping the RFs of the V4 recording sites. For simplicity, we will refer to the region with V4 connections as simply “pulvinar” unless indicated otherwise. Figure 1b shows a representative MRI section through the pulvinar and a track of an electrode. The representative MRI image from another monkey and estimated pulvinar recording sites in the two monkeys are shown in Figure S1. In V4, we targeted areas on the prelunate gyrus where neurons had RFs in the lower contralateral visual field at an eccentricity of about 5–7deg (Fig. S1a). The IT recording sites were located in the mid-portion of IT cortex, in both the lower bank of the STS and the inferior temporal convexity, similar to the sites in a previous study (Zhang et al., 2011; for details see Fig. S1).
Figure 1.
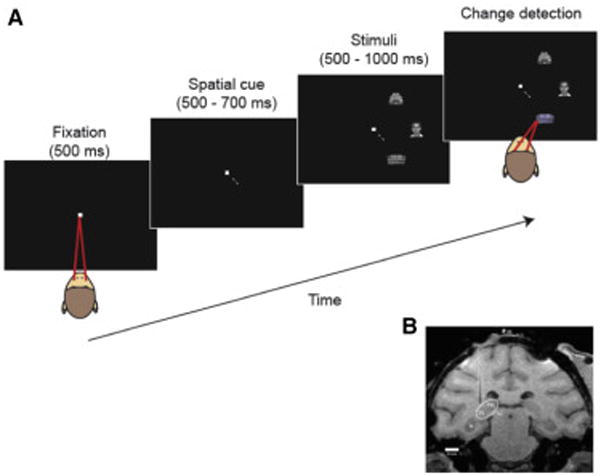
Task and recording sites. (a) Task in the cue-first condition. A spatial cue appeared that pointed to the upcoming target stimulus location, then 3 stimuli (target and 2 distracters) appeared on the contra-recording side of the screen. The monkey was rewarded for making a saccade to the target when it changed slightly in color, which occurred randomly from 500 to 1000ms after stimulus onset. (b) MRI image showing an electrode in place above the recording area in pulvinar.
During recording sessions, the monkeys fixated a central spot and were required to attend to a cued stimulus to detect a brief (50 ms) color change, while ignoring color changes of two other stimuli that served as distracters (Fig. 1a). Stimuli were gray-scale images of complex objects. The cue appeared either 500–700 ms before or after the stimuli appeared on the screen. The target and two distractors were placed at equidistant locations in the contralateral visual field. The precise positions were adjusted to be optimal for V4, but they were normally at an eccentricity of about 5–7 deg.
Pulvinar responses
A total of 339/641(53%) recording sites in the pulvinar were visually responsive (Wilcoxon rank-sum test, P< 0.05), compared to 310/366 (85%) in V4 and 210/422 (50%) in IT cortex, and all further analyses were based on these responsive sites. On average, pulvinar RFs were intermediate in size between those in V4 and IT cortex (Fig. S2a), consistent with anatomical studies showing that separated points in the V4 visual field map may have overlapping connections with a given location in the pulvinar (Gattass et al, 2014).
A total of 120 of 339 pulvinar sites (35%) showed significant stimulus selectivity when tested with the complex objects, compared to 129 sites (42%) in V4 and 68 sites (32 %) in IT cortex, according to an ANOVA calculated on responses to all stimuli, and relative stimulus tuning (object selectivity) for the selective cells was similar across all three areas (Fig. S2b). Thus, to a first approximation, the pulvinar appears to reflect the sensory properties of cells in the cortex, consistent with its role in relaying visual information from one cortical visual area to another.
Effects of attention
We tested for the effects of attention on ‘Attention In’ versus ‘Attention Out’ trials. On the Attention In trials, the stimulus in the RF of the recorded neurons was the target, which was attended by the monkeys. On the Attention Out trials, the stimulus in the RF was an unattended distracter, and attention was directed to a target stimulus outside of the RF. The monkeys performed 72.8% correct on detection of the target color change and rarely (2.6%) responded to a distractor color change.
Figure 2 shows population averages of V4 and pulvinar responses in the Attention In and Attention Out conditions with the cue presented first. Both areas showed larger responses to the attended stimulus compared to the unattended. The latency of attentional effects was assessed in these “cue-first” sessions, because they were not affected by transient inhibitory responses to the cue sometimes found in the “stimulus-first” sessions. Latencies calculated directly from the population histograms showed attentional effects at 135 ms and 115 ms after stimulus onset in the pulvinar and area V4, respectively. However, the latency difference failed to reach significance (two-sided permutation test, P=0.22), possibly because so few cells contributed to early effects in the population responses. We also assessed latencies using the full cumulative distribution of latencies calculated for each recording site separately, which takes into account the full distribution of latencies. The effects of attention were significantly later in pulvinar than in V4 in the cumulative distributions (Wilcoxon rank sum test, P=0.0085; Fig. 2c).
Figure 2.
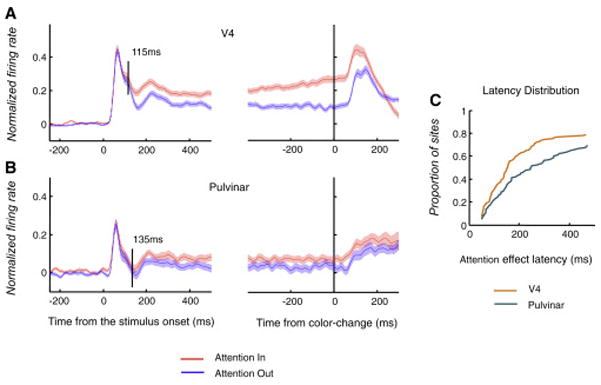
Attentional modulation in V4 and pulvinar. (a) Normalized firing rates averaged across the V4 sites during ‘Attention In’ and ‘Attention Out’ conditions (n=152). Shading around average firing rates indicates the SEM (±). The black vertical line in the left plot marks the time when the Attention In and Attention Out responses reached a significant difference, and it was defined to be the attentional latency. (b) Population averages across pulvinar neurons (n=105). (c) The cumulative distribution of attentional latencies computed individually for V4 and pulvinar sites. The proportions of sites with attentional latencies <=50ms were combined.
The magnitude of the attentional effect was quantified by an attention contrast index (Methods) based on firing rates during the 250 ms window preceding the earliest target or distracter color- change. With the cue presented first or second, the mean attentional index in the pulvinar was 0.04 (cue-first, n=105) or 0.03 (stimulus-first, n=132), respectively (Wilcoxon signed rank test, cue-first, P=1.61e-6; stimulus-first, P=0.0038), which was significantly smaller than the mean index in area V4, which was 0.16 (cue-first, n=152) or 0.13 (stimulus-first, n=136), respectively (Wilcoxon rank sum test, cue-first, P=6.11e-10; stimulus-first, P=2.47e-11). Given that attentional effects in the pulvinar were smaller and occurred later than in V4 in the cumulative distribution of latencies, these pulvinar effects might reflect attentional effects in the cortical areas that project to it.
Effects of attention on spike-LFP coherence within and across areas
To test for synchrony among the three structures, we measured the coherence between spikes and LFPs recorded simultaneously across the three areas. Only those pulvinar and IT neurons with RFs that overlapped the RF of V4 neurons were included in this analysis. We focused on the 256 ms time window immediately before the earliest target or distracter color change so that LFPs would not be contaminated by the transient of the evoked stimulus response. Data from cue-first and stimulus-first sessions were combined in this analysis. We compared trials in which the target was located within a shared RF (Attention In) to those in which a distracter was in the shared RF (Attention Out). To account for spike-field coherence effects that might be influenced by differing firing rates across attentional conditions, we subtracted the coherence computed after shuffling trials (Methods).
Consistent with the results of previous studies (Fries et al., 2008; Mitchell et al., 2009), spike-field coherence within V4 was enhanced by attention at gamma frequencies (Wilcoxon signed rank test, P=4.86e-46; n = 1489; Fig. 3), and coherence below about 15 Hz (theta-alpha) was reduced by attention (Wilcoxon signed rank test, P=7.61e-11; n = 1489). We also found for the first time that spike-field coherence within IT was enhanced in the gamma range (Wilcoxon signed rank test, P=7.58e-13; n =1349) with attention to the preferred location in the RF, despite the fact that the IT neurons had larger RFs and thus less spatial selectivity. Thus, gamma synchrony appears to be enhanced by attention throughout the extrastriate areas of the ventral stream. There was no significant change in gamma coherence within the pulvinar with attention (Wilcoxon signed rank test, P=0.31; n = 1278; Fig. 3).
Figure 3.
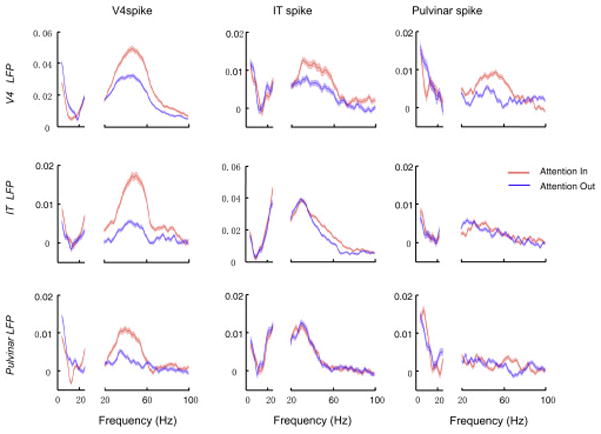
Effects of attention on spike-LFP coherence. Population averages of the coherence in Attention In and Attention Out conditions were calculated between spike and LFP signals within and between V4, IT and pulvinar. The coherence at low frequencies (4–25 Hz) and higher frequencies was calculated and displayed separately. The SEM (±) of the population averages is indicated by the shading around the averages.
Gamma coherence between V4 spikes and IT LFPs was also enhanced by attention (Wilcoxon signed rank test, p=5.12e-36; n = 1720), as was the coherence between IT spikes and V4 LFPs (Wilcoxon signed rank test, p=7.50e-5; n = 917; Fig. 3). The increased gamma coherence between V4 and IT occurred only when attention was directed to a location that fell within the overlap between the V4 RF and IT RF, even though the unattended stimulus location was typically also located within the same large IT RF, similar to what was found between V1 and V4 (Bosman et al., 2012). Thus, the spatial resolution of the attentional modulation of coherence between V4 and IT was finer than the IT RF.
Although there was no significant attentional modulation of gamma coherence within the pulvinar itself, gamma coherence between pulvinar LFPs and V4 spikes was enhanced significantly by attention (Wilcoxon signed rank test, p=1.35e-22; n=1461; Fig. 3), as were pulvinar spikes and V4 LFPs (Wilcoxon signed rank test, p=1.01e-13; n=1625; Fig. 3), and there was a corresponding decrease in coherence at low frequencies (<=15Hz, theta-alpha) in both directions with attention (Wilcoxon signed rank test, V4 spike - Pulvinar LFP, p=6.40e-05; pulvinar spike - V4 LFP, p=1.83e-03). Thus, the effects of attention on gamma synchrony between V4 and pulvinar were similar to what has been found between cortical areas (Buffalo et al., 2011; Grothe et al., 2012; Roberts et al., 2013). Enhanced gamma coherence and firing rates with attention are thought to enhance inter-areal communication (Gregoriou et al., 2009; Bastos et al., 2014). There was very little attentional modulation of gamma coherence between the pulvinar and IT cortex, possibly because the recordings were targeted to the portions of the pulvinar with mainly V4 connections.
Consistent with these interactions in the frequency domain, we also found a correlation between firing rates in the pulvinar and the LFP power within V4 and IT. On a trial-to-trial basis, V4 and IT gamma power in the LFP was positively and significantly correlated with pulvinar firing rates (V4, 40–100Hz, 0.0050, t-test, P=2.66e-4; IT 40–100Hz, 0.0034, t-test, P=1.65e-4; Fig. S3b), whereas alpha-beta frequency power in V4 and IT was negatively correlated with pulvinar firing rates (V4, 16–30Hz, −0.0025, t-test, P=0.0057; IT, 8–30Hz, −0.0035, t-test, P=5.88e-9; Fig. S3b). These results suggest that as pulvinar firing rates increase, gamma power in the cortex is enhanced and alpha-beta frequency power is suppressed, although these results are based on correlations and do not show the direction of causality.
To control for the possibility that the increases in gamma coherence with attention were simply due to increases in firing rates and gamma power, we re-calculated the cross-area coherence after selecting a subset of trials such that overall firing rates and LFP powers were equal (Wilcoxon rank sum test, P>0.05) across different attention conditions. Because all three measures increase with attention, we expected that selecting trials to equate for firing rates and LFP power would reduce the effects of attention on coherence. However, the enhancing effects of attention on gamma coherence across areas were qualitatively maintained (Fig. S3a), suggesting that the effects on coherence could not be attributed solely to the enhancement in firing rates and gamma LFP power.
Directionality of gamma influence across areas
To investigate the direction of interactions between structures, we performed a Granger causality analysis based on the LFPs in the three areas. Fig. 4a shows that the V4 influence on the pulvinar at gamma frequencies increased significantly on Attention In trials compared to Attention Out trials (Wilcoxon signed rank test, P=0; n=1608), consistent with the findings from the coherence analyses. However, the pulvinar influence on V4 was slightly reduced at gamma frequencies (Wilcoxon signed rank test, P=2.80e-45) on Attention In trials. Thus, the major effects of attention were in the direction of V4 to pulvinar at gamma frequencies, consistent with our analysis of the latency of attentional effects on firing rates. At frequencies below 30 Hz, the influence from the pulvinar to V4 was significantly stronger than the influence from V4 to pulvinar in the Granger analyses (Fig. S4a, Wilcoxon signed rank test, P=0), but it was only modestly (12%) reduced by attention (Fig. 4b, Wilcoxon signed rank test, P=2.11e-19). In cue-first sessions, the pulvinar influence on V4 was slightly enhanced at alpha (8–16Hz) frequencies by attention in the last 256 ms period before the onset of the object stimuli (Wilcoxon signed rank test, P=7.48e-35; n=960; Fig. S4d), which is consistent with a previous study (Saalmann et al., 2012).
Figure 4.
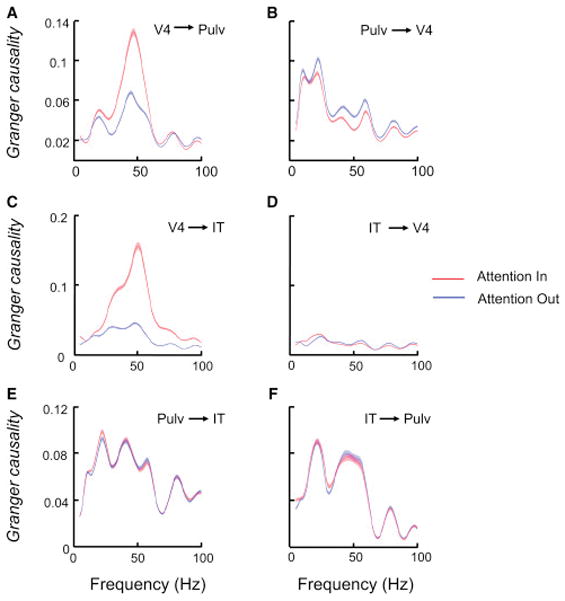
Granger causality between V4, pulvinar and IT LFPs in frequency domain. (a) V4 influence on pulvinar (Pulv). The SEM (±) of the population averages is marked by the shading around the averages. (b) Pulvinar influence on V4. (c) V4 influence on IT. (d) IT influence on V4. (e) Pulvinar influence on IT. (f) IT influence on pulvinar. Format in (b)–(f) is the same as the format in (a).
The same Granger causality analysis also showed a major unidirectional influence from V4 to IT at gamma frequencies (Fig. S4b), which was strongly enhanced by attention (Wilcoxon signed rank test, P=0; n=1072; Fig. 4c–d). This is consistent with the idea that the feedforward pathway from V4 to IT is facilitated by attention. Between the pulvinar and IT, attention did not increase Granger causality in either direction at gamma frequencies (n=1720; Fig. 4e–f), which, again, might be related to the fact that our recordings were targeted to the portion of the pulvinar with mainly V4 connections.
To test the possibility that the gamma Granger causality increases with attention were simply due to increases in V4 gamma power, we re-calculated Granger causality after selecting a subset of trials such that overall V4 gamma power was equal (Wilcoxon rank sum test, P>0.05) across different attention conditions. However, the V4 gamma influences on pulvinar and IT were still enhanced by attention (Fig. S4e–f) on these trials; thus, the V4 gamma power increase alone could not explain the attentional effects on the Granger.
As another measure of the direction of interaction, we also computed the LFP phase shift between areas, across a range of frequency bands centered on the peak gamma coherence (50Hz). A constant transmission delay (the sum of conduction time and synaptic delays) that is invariant to frequency would be evidenced by a linear change of phase as frequency is varied (Schoffelen et al., 2005). The slope of the regression of phase on frequency is determined by the magnitude of time shift and the direction of slope is determined by the direction of interaction. In the large majority of V4-pulvinar LFP recording pairs, the phase of V4 gamma led that in the pulvinar. The gamma phase lag increased linearly and significantly (multiple linear regression, P=2.10e-7) as a function of frequency on Attention In trials, corresponding to a constant 12.2 ms lead of V4 over the pulvinar (see Fig. 5a–c for both phase leads and lags). Again, this is consistent with the idea that V4 plays a leading role in the gamma interactions with the pulvinar. In a minority of recordings between V4 and pulvinar, the regression of phase on frequency was not significant (multiple linear regression, P > 0.05; shown as blue bars in Fig. S5a), and these also had the highest residuals in the regression (Fig. S5b), suggesting that they suffered from shared noise. The phase leads and lags seemed not dependent on the depth of V4 recordings (Fig. S5c). Between V4 and IT, nearly all recorded pairs showed that V4 led IT in gamma, with an average lead of 6.4 ms of V4 over IT, consistent with the feedforward visual hierarchy.
Figure 5.
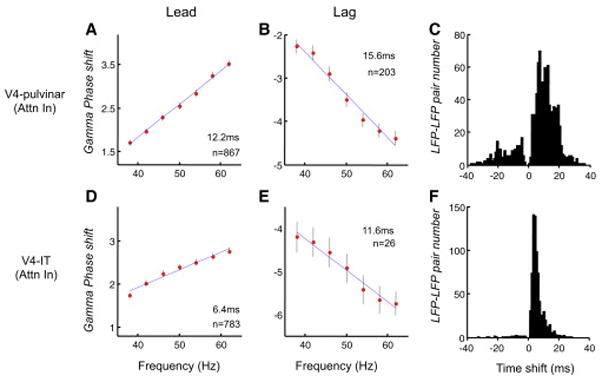
Directionality based on gamma frequency phase-shift across structures. (a) V4 led pulvinar in gamma phase. In 867 of 1608 V4 LFP pulvinar LFP pairs, the phase-lag of pulvinar to V4 increased linearly with the increasing frequency within the gamma range. The thick red dots represent the averaged phase-shifts across the 867 LFP pairs, and the SEM (±) of the averaged phase-shifts are marked by the vertical lines centered on these red dots. The regression line for these averaged phase-shifts is shown in blue, and the slope indicates that V4 led pulvinar by 12.2ms. (b) Pulvinar led V4 gamma phase. In 203 of 1608 V4 LFP – pulvinar LFP pairs, the phase-lag of pulvinar to V4 decreased linearly with the increasing frequency, and the slope indicated that pulvinar led V4 by 15.6ms. (c) Distribution of time shifts between V4 and pulvinar gamma oscillations. The positive time means that V4 gamma led pulvinar gamma. (d)-(e) show the phase-shift and the corresponding time-shift between V4 and IT gamma oscillations (totally 1072 pairs). The formats in (d),(e) and (f) are the same as the formats in (a), (b) and (c), respectively.
Pulvinar deactivation produces visual and attentional deficits
To more directly test for a causal role of the pulvinar in mediating attentional effects in the cortex, we recorded neural activity in V4 and IT before and after reversible deactivation of the pulvinar with muscimol, a GABAA agonist. After baseline data were collected in each deactivation session, muscimol was injected at 2 to 4 pulvinar sites (1ul/site) representing the lower, contralateral visual quadrant containing the V4 RFs. We modified the task such that one of the three visual stimuli was placed in the visual hemifield ipsilateral to the injection sites (Fig. S6a), which was expected to be unaffected by the pulvinar deactivation.
Pulvinar deactivation caused profound, spatially localized behavioral deficits in the task (Fig. 6a). In the attention-demanding conditions with distracters, the monkeys’ performance in the lower contralateral field fell from 74.3% correct pre-injection to 10.8% correct post-injection (Wilcoxon rank sum test, P=1.55e-4; n=8). There was no significant effect at the location ipsilateral to the injection site (76.6% correct pre-injection versus 72.8% correct post-injection; Wilcoxon rank sum test, P=0.57). When the target was in the upper contralateral field, the monkeys’ performance decreased only slightly from 69.2% correct pre-injection to 59.1% correct post-injection (Wilcoxon rank sum test, P=0.13). The much larger effects in the lower field indicated that the deactivation was largely confined to the intended visual field representation of the pulvinar. Without distractors, the effect of the deactivation was reduced but still substantial (Fig. S6b), suggesting that the pulvinar deactivation caused either sensory deficits or a profound neglect. Performance at the V4 RF location decreased from 81.0% correct pre-injection to 19.5% correct post-injection. At the other two locations, performance decreased only slightly from pre-injection 82.8% and 82.7% correct to post-injection 74.4% and 76.3% correct, respectively.
Figure 6.
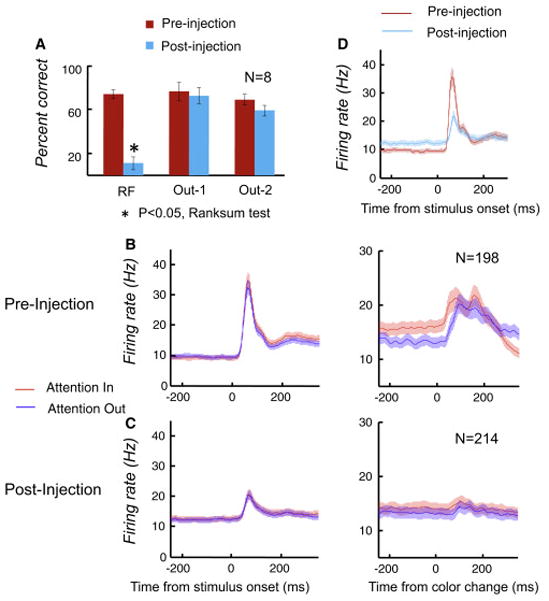
Effects of pulvinar deactivation on behavior and neuronal activity in V4. (a) Muscimol injection in pulvinar selectively lowered monkeys’ performance when the target was located in the RF of recorded V4 neurons, while performances at the other two locations outside of the RF (Out-1 and Out-2) were not affected. The averaged performance and SEM (±) of the 8 sessions are shown. (b) and (c) Population averages of firing rates during Attention In and Attention Out condition before and after muscimol injections(n=198 pre-injection; n=214 post-injection). During the last 250ms before the color-change, V4 neurons showed enhanced responses with attention before pulvinar injection (b), but this enhancement with attention was reduced after the injection (c). (d) Pulvinar deactivation reduced visual responses, but increased baseline activity before stimulus onset in V4. Attention Out responses before and after the injection are shown here.
Following deactivation, V4 firing rates in the baseline period (250 ms period before the stimulus onset) increased significantly from 9.54Hz pre-injection to 12.20 Hz post-injection (Wilcoxon rank sum test, P=0.0515; pre-injection, n= 198; post-injection, n=214; Fig. 6d). The visually evoked response, computed from the average firing rates in a period from 50 to 150 ms after stimulus onset after subtracting activity averaged in the baseline period, showed a 59.7% decrease in V4, from 11.65 Hz pre-injection to 4.69 Hz post-injection (Wilcoxon rank sum test, P=2.92e-8; Fig. 6d). This reduction in the evoked response to levels well below those to the unattended stimulus prior to the deactivation suggested that the cortical effects were due at least in part to a loss of sensory responsiveness and not just a reduction in attention. There was also a 90.7% reduction in response to the target color change, from 4.76 spikes/sec to 0.44 spikes/sec (response during 50 to 200 ms after target color change subtracting activity in the last 250 ms period before the target color change).
The reduction in the evoked response in V4 with pulvinar deactivation was so substantial, we considered whether the muscimol might have spread to the LGN, located approximately 4–5 mms from the pulvinar injection sites. We therefore simultaneously recorded from V4 and portions of the LGN located closest to the injection sites, in two deactivation sessions. Fig. S6g–h shows that the pulvinar deactivation nearly eliminated the evoked visual response in V4 in those sessions, but there was no effect on the visual response of the LGN neurons. We also recorded from a few responsive IT cells (n = 28 pre-injection; n=31 post-injection) during four deactivation sessions. Their responses were also not reduced by the deactivation (Wilcoxon rank sum test, P=0.28; Fig. S6i). Thus, the effect of the deactivation appeared to be specific to V4, the cortical area connected with the pulvinar sites targeted by the injection.
In addition to the reduction in evoked response, the attention modulation index in V4 was reduced from 0.106 before pulvinar deactivation to 0.053 after deactivation (Wilcoxon rank sum test, P=0.0015; Fig. 6b and c), a 50% reduction Thus, the pulvinar deactivation reduced both the evoked response and the effects of attention in V4 to about the same degree, but did not eliminate either.
We were not able to localize the V4 recording sites to specific layers, but we made a crude division between the superficial sites and deep sites by separating the upper half of recording sites from the lower half. Both the superficial and deep sites showed similar effects of deactivation on evoked responses and effects of attention (Fig. S6c–f).
Effects of pulvinar deactivation on oscillations in cortex
Pulvinar deactivation caused robust reductions in spike-LFP coherence within V4 (Fig. 7a). The overall gamma coherence averaged across Attention In and Attention Out conditions decreased from 0.051 pre-deactivation to 0.031 post-deactivation, or by 39% (Wilcoxon rank sum test, P=4.92e-28; pre-injection, n=2177; post-injection, n=2306). As was true with firing rates, pulvinar deactivation reduced V4 gamma coherence with attention even below the level of coherence in the unattended condition before deactivation. Likewise, Fig. 7a shows that deactivation also significantly reduced the effects of attention on V4 gamma coherence from 0.123 before deactivation to 0.062 after deactivation by 49.6% (Wilcoxon signed rank test, P=0.0016). Similar effects of the deactivation were observed on the coherence in the roughly estimated superficial and deep layers of V4 (Fig. S7a–b).
Figure 7.
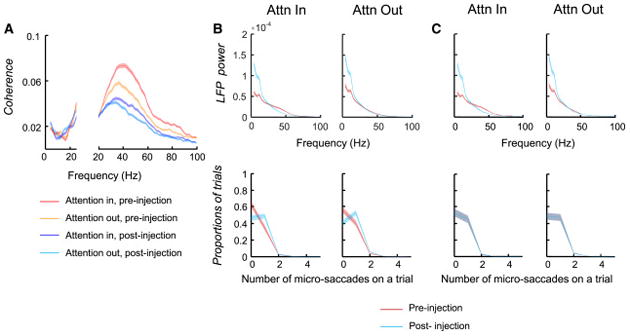
Effects of pulvinar deactivation on coherence and LFP power in V4. (a) Population averages of V4 spike V4 LFP coherence in Attention In and Attention Out conditions before and after the muscimol injection (pre-injection, n=2177; post injection, n=2306). (b) Effects of the pulvinar deactivation on V4 LFP power without matching the number of microsaccades. The power spectrums were calculated in the last 256ms period prior to the color change. The upper plots show population averages of V4 LFP power spectrum before and after pulvinar deactivation. In both Attention In and Attention Out conditions, the low frequency LFP power after injection was significantly stronger than the power before injection (Wilcoxon signed rank test, P<0.05; n=153). The lower two plots show the distribution of proportions of trials with different number of microsaccades within the 256 ms period. There were slightly more microsaccades in the post-injection than in the pre-injection period. (c) Effects of the pulvinar deactivation on V4 LFP powers after matching the number of microsaccades. The effects on low frequency power were almost the same as those shown in (b).
Interestingly, the deactivation caused a large increase in LFP power below 20 Hz in V4 (Fig. 7b, Wilcoxon signed rank test, Attention In, P=1.10e-22; Attention Out, P=2.22e-23; also see Fig. S8a) and a smaller increase in IT cortex (Fig. S8b–c). The effects of deactivation on low frequency power were similar in the superficial and deep layers of V4 (Fig. S7e–f). This increase in low frequency (< 20Hz) power did not appear to be a reflection of volume conduction from a common source, since the low frequency LFPs in V4 and IT differed in phase for most recorded pairs (Fig. S7c). We also found similar increases in low frequency power in V4 current-source-density signals (Fig. S7d), which discounts common sources and further suggested that the low frequency power increase in V4 was not due to volume conduction from other areas. To rule out any lingering influence from the stimulus evoked response transient, we calculated the power on trials with long delays (> mean delay) and short delays (< mean delay) between the stimulus onset and the color change. Fig. S8g–h shows that V4 power and the effects of the deactivation were almost the same on the short and long delay trials, suggesting that the stimulus response transient had little or no effect on the V4 LFP power.
We considered whether the large increase in power from 4–20 Hz could be due to any change in eye blinks or eye movements following deactivation. All trials with eye blinks were excluded from the analyses, but we did find a small increase in microsaccades within the fixation window (Fig. 7b) after deactivation. To control for any influence of this microsaccade increase, we recalculated LFP power after selecting trials to match the microsaccade frequency before and after deactivation. After equating microsaccades, the increase in low frequency power was maintained (Fig. 7c). Because an increase in low frequency power, particularly below 2 Hz, is found in sleep, we also tested whether the increase in low frequency power extended to very low frequencies. Our standard analysis window of 256 ms before the color change was too short to measure frequencies below 4 Hz, so we extended the analysis window to 2 sec (from 1500ms before to 500 ms after the color change). This window necessarily included different trial events and also extended into the intertrial interval. However, the power measured in this longer window showed a very striking increase after deactivation that extended to at least 0.5 Hz in V4 (Fig. S8d).
To test for effects on oscillatory couplings across cortical areas, we calculated field-field coherence between V4 and IT before and after the deactivation (Fig. 8). Attention enhanced gamma coherence both before and after deactivation (Wilcoxon signed rank test, P=0; n=2048), but the magnitude of enhancement was reduced significantly following deactivation from a mean index value of 0.33 to 0.23, or by 30% (Wilcoxon signed rank test, P=2.29e-15). The overall gamma coherence between areas averaged across Attention In and Attention Out conditions decreased from 0.17 pre-deactivation to 0.11 post-deactivation, or by 35% (Wilcoxon signed rank test, P=0). Thus, pulvinar deactivation reduced both the effects of attention and the overall gamma coherence within and across areas irrespective of attention. Similar effects of the deactivation were observed on the coherence between superficial V4 and IT, and on the coherence between deep V4 and IT (Fig. S8e–f).
Figure 8.
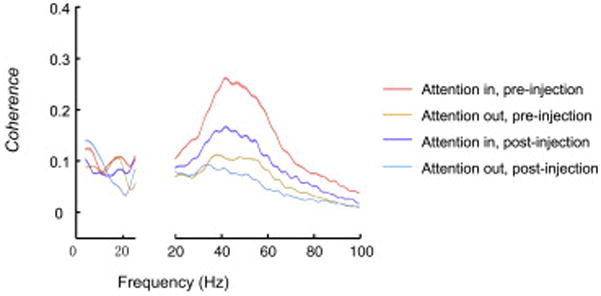
Effects of pulvinar deactivation on coherence between V4 and IT. Population averages of V4 LFP – IT LFP coherence in Attention In and Attention Out conditions before and after the muscimol injection are shown (n=2048).
Discussion
By recording simultaneously in the ventro-lateral pulvinar and areas V4 and IT cortex, we were able to compare sensory properties in the pulvinar with those in the connected cortical areas for the first time. Pulvinar cells had a comparable degree of stimulus selectivity as in V4 and IT cortex, and their RFs were also intermediate in size between those in V4 and IT, on average, consistent with the pulvinar’s anatomical connectivity with V4 (Gattass et al., 2014). Thus, the properties of pulvinar cells seem to reflect the properties of cortical cells that project to it. The pulvinar also engaged in gamma frequency synchrony with the cortex during attentive stimulus processing, which resembled the gamma synchrony often found between cortical visual areas (Gregoriou et al., 2009; Bosman et al., 2012).
We also found for the first time that area V4 also has gamma frequency spike-field interactions with IT, predominantly in the feedforward direction, and this interaction is strongly enhanced by attention. Interestingly, this occurred even when both attended and ignored stimuli were both located within the large IT RFs, but only when the attended stimulus was located in the joint IT-V4 RF, which was much smaller. Thus, the spatial resolution of gamma enhancement with attention is much finer than the IT RF, thereby increasing the drive from V4 to IT only at the attended stimulus location.
The responses of pulvinar cells were also modulated by spatial attention, but these effects of attention were smaller in the pulvinar than in V4. The latency of attentional effects on the population firing rate histogram was later, but not significantly so, in pulvinar compared to V4. However, the latency of the attentional effects on the cumulative distribution of latencies in the pulvinar, which takes into account the full distribution of latencies, was significantly later than in V4. Certainly the pulvinar attentional latencies are not earlier than in V4, which would have been expected if the pulvinar modulated cortical responses with attention. Neuronal properties in the pulvinar seem in contrast to the FEF, a major source of feedback in attention. Unlike in the pulvinar, FEF cells have little to no intrinsic stimulus selectivity, but their responses are strongly modulated by attention, and the effects of attention on firing rates occur significantly earlier than in V4 (Gregoriou et al., 2009; Zhou and Desimone, 2011). Measures of gamma synchrony, phase shifts and Granger casualty analysis of LFPs in V4 and pulvinar all suggested that the cortex led the pulvinar in synchronous interactions at gamma frequencies, which were modulated by attention. Taken together, the evidence suggests that, unlike FEF, the portion of the pulvinar connected with V4 is not a major source of feedback that directly mediates the effects of attention in the cortex.
Our conclusion that cells in the ventro-lateral pulvinar resemble those in V4 parallels that of Berman and Wurtz (Berman and Wurtz, 2011) who recorded in the portion of the pulvinar with area MT and SC connections. They found that the directional properties of the pulvinar cells were derived from cortical area MT, and that the responses of the pulvinar cells with a direct input from the SC were not modulated by attention.
In contrast to the ventro-lateral pulvinar studied here, there is strong evidence that the dorsal portion of the pulvinar with parietal connections (Baizer et al., 1993) has a role in attention similar to that of the posterior parietal cortex. Cells in this portion of the pulvinar have properties that resemble those in parietal cortex (Petersen et al., 1985), and deactivation of this portion of the pulvinar leaves sensory processing intact but impairs attention in a manner similar to parietal deactivation (Robinson and Petersen, 1992; Wilke et al., 2010). Taken together with the present results, it seems likely that each subdivision of the pulvinar shares the properties and functions of its anatomically connected cortical areas.
A previous study of the ventro-lateral pulvinar and areas V4 and TEO reported a causal relationship between the pulvinar and the cortex in the alpha frequency range, with nearly a unidirectional influence of the pulvinar on the cortex in the delay period before the appearance of the attended stimulus (Saalman et al, 2012). The authors suggested that the pulvinar regulates the transmission of information across cortical areas by synchronizing cortical activity at alpha frequencies. Indeed, alpha is a prominent feature of thalamo-cortical interactions (Vijayan and Kopell, 2012), although it is typically associated with inattention rather than attention (Bollimunta et al., 2011; Schmid et al., 2012). We also observed an attentional enhancement of alpha frequencies in the pulvinar influence on V4 before the appearance of the visual stimulus (Fig. S4d). However, during the processing of the attended stimulus in our task, the Granger causality from pulvinar to V4 at alpha frequencies was actually slightly reduced with attention. Likewise, we found that higher pulvinar firing rates were associated with suppression of alpha-beta (16–30 Hz) frequency LFPs in the cortex. Thus, our results suggest that the pulvinar input suppresses alpha frequency cortical LFPs during attentive stimulus processing.
By inactivating the pulvinar and recording in V4 and IT, we were able to test the influence of the pulvinar on cortical responses directly. Irrespective of the modest effects of attention on pulvinar responses in the normal state, the deactivation results revealed that the pathway from the pulvinar to the cortex plays a major role in maintaining visual responsiveness and high-frequency synchronous activity in area V4. The animals were substantially impaired when the target appeared in the lower contralateral quadrant affected by the pulvinar deactivation. The effects were only modestly larger in the attentionally demanding conditions with distracters than in the conditions without distracters. This suggests impaired sensory processing, although we cannot rule out a profound neglect. Consistent with the behavioral results, the deactivations reduced the effects of attention on cells in V4, but also substantially reduced V4 sensory-evoked responses to the onset of target stimuli and to the target color change. The reductions in sensory responses and the effects of attention were comparable (59.7% vs 50%). However, the reduced sensory responses following deactivation were well below the magnitude of response to the unattended stimulus prior to the injections and cannot be accounted for solely by a loss of normal attention to the target. Rather, the pulvinar seems to have a broader role in relaying sensory information and maintaining the cortex in an active state.
A study of the pulvinar in anesthetized Galagos found that deactivation of the pulvinar eliminated visual responses in the superficial layers of V1 (Purushothaman et al., 2012). It seems unlikely that our deactivation results in V4 were due to a loss of visual responsiveness in V1, as we targeted the more posterior portion of the pulvinar with V4 connections. Furthermore, visual responsiveness in IT cortex is known to be dependent of V1 (Rocha-Miranda et al., 1975), and we found no significant decrement in IT responses following pulvinar deactivation. The IT responses were presumably driven by inputs that bypassed V4. Soares et al. (2004) found that pulvinar deactivation in the anesthetized new world Cebus monkey had mixed effects on responses in V2. Purushothaman et al. (2012) also found that raising pulvinar sustained activity with the GABA antagonist bicuculine increased the responsiveness of V1 cells to visual input. They suggested that the pulvinar plays an enabling or facilitating role for sensory processing in V1, either mediated by the direct pulvinar-V1 projection or indirectly by pulvinar inputs to extrastriate cortex and subsequent feedback to V1 from these extrastriate areas. The present results are consistent with this idea of a general enabling role of the pulvinar, e.g. the inputs from the pulvinar might be necessary for V4 to respond normally to sensory inputs from areas such as V2. By maintaining the cortex in an active state, the pulvinar could also play a role in enabling top-down signals from other areas to modulate cortical responses with attention.
Consistent with the idea that the input from the pulvinar maintains the cortex in an active state, there was a large increase in low frequency LFP power in the affected cortex following pulvinar deactivation, and this low frequency power persisted even during baseline periods without visual stimulation. The increase in low frequency power extended from 20 Hz to at least 0.5 Hz, i.e. into the range of slow wave sleep. Indeed, with the pulvinar intact, we found that decreased firing rates in the pulvinar were associated with reduced high frequency LFP power and greater low frequency power in the cortex. With the pulvinar inputs eliminated, the affected region of cortex might enter a state similar to slow-wave sleep, with slow oscillations, reduced sensory responsiveness and a reduced influence of attention. Baseline firing rates in V4 and IT were also higher following pulvinar deactivation, as has been reported in visual cortex during sleep (Livingstone and Hubel, 1981). Whatever the mechanisms of the pulvinar’s influence, the pulvinar contributions to the functioning of the cortex are profound.
Experimental Procedures
General procedures
Two male rhesus monkeys weighing 11–13 kg were used. Monkeys were implanted under aseptic conditions with a post to fix the head and recording chambers over areas V4, IT and pulvinar. Localization of the areas was based on MRI scans obtained before surgery. We targeted V4 area with RFs in low contralateral visual field at an eccentricity of about 5–7 deg, then mapped the pulvinar to find regions with RFs overlapping those of the V4 sites. Visual stimulation and behavioral control were carried out using Cortex software (http://dally.nimh.nih.gov/index.html). All procedures and animal care were in accordance with the NIH guidelines.
Tasks and visual stimuli
Visual stimuli were displayed on a CRT screen with a refresh rate of 100 Hz. The stimuli consisted of 7 monochrome objects (hand, flower, face, couch, car, guitar and kiwi) (Zhang et al., 2011), 2.3° × 2.3° in size. On the cue-first trials, a dim spatial cue (line segment) appeared near a center spot after monkeys had fixated on the center spot for 500 ms, which “pointed” to a location to be attended. At 500 – 700 ms after the cue onset, three object stimuli appeared on the screen. The object cued by the line segment was the target, and the other two objects served as irrelevant distracters. Monkeys were rewarded for making a saccade to the target when it changed slightly in color, which occurred randomly from 500 to 1000 ms after stimulus onset. On half the trials, one of the distracters changed color before the target color-change, and the monkeys were required to keep central fixation until the target changed color. Except for the deactivation sessions, the three object stimuli were placed at equi-distant locations in the contralateral visual field. The precise positions were adjusted to be optimal for the recorded cells in V4, but they were normally at an eccentricity of ~ 5 – 7 deg. In the stimulus-first sessions, the three object stimuli appeared after the initial 500 ms center fixation and 500–700 ms before the appearance of the spatial cue, but the other parts of the task were the same in cue-first and stimulus-first sessions. In the sessions with pulvinar deactivation, one of the three stimuli was placed in the ipsilateral visual field to ensure that its representation in the pulvinar was not directly affected by the muscimol.
A separate fixation task was used to map a cell’s RF. On each trial, a mapping stimulus flashed for 200 ms, 5 times at 5 different locations every 300ms. The flash locations were selected randomly from a total of 80 locations covering both sides of the visual field, at eccentricities of 1, 3, 6, 10 and 15 deg, with 16 different polar angles. Monkeys were required to keep central fixation while the stimuli were flashing, and were rewarded after the last (5th) flash.
Recording
Single units and multi-unit spikes and local field potentials (LFPs) were recorded from V4, IT and pulvinar simultaneously using an OmniPlex system (Plexon Inc, Dallas, USA). On a given day, one to three 16-contact linear electrodes (U-Probe, Plexon Inc) were advanced to each area. Neural signals were filtered between 250 Hz-8 kHz, amplified and digitized at 40 kHz to obtain spike data, and between 0.7 – 170 Hz to obtain the LFP signals. Eye movements were recorded by an infrared eye tracking system (Eye Link II, SR Research Ltd. Ontario, Canada) at a sampling rate of 500Hz.
Pulvinar deactivation combined with cortical recording
Behavioral and neural data before and after pulvinar deactivation were collected in the same session, and the targeted sites were locations where we had previously collected recording-only data. Thus, the visual field representation at those pulvinar sites was in the lower contralateral field, overlapping the locations of the V4 RFs. Following the collection of control data, we injected muscimol (5ug/ul), a GABAA agonist. In each session, we made 2–3 1ul injections at two to three depths by one steel cannula (30 gauge, BD Needles), or four 1ul injections at two depths by two cannulas at two locations. The distance between two adjacent injections depths was ~700 to 1000 um, and the injections started at the deepest location. The injection rate was 0.05 ul/min with a 10-minute delay between injections. About 35 min after the last injection, we began to collect neural and behavioral data.
Data Analysis
Data analyses including firing rate, LFP power, coherence, granger causality, LFP phase shift analysis etc., are described in details in the Supplemental Information.
Supplementary Material
Acknowledgments
We thank Ellen DeGennaro and Matt Heard for help with the monkeys. This work was supported by the National Institutes of Health grant R01 EY017292 (R.D.), Shenzhen Peacock plan grant KQTD20140630180249366 (R.D., and H.Z.), CAS Hundred Talent program (H.Z.), and National Natural Science Foundation of China grant No. 31540023 (H.Z.).
Footnotes
Author Contributions
R.S. and R.D. designed the experiment, R.S. and H.Z. collected the data, H.Z. and R.S. analyzed the data, and H.Z. and R.D. wrote the paper with contributions from R.S.
Supplemental Information includes 8 figures and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arend I, Rafal R, Ward R. Spatial and temporal deficits are regionally dissociable in patients with pulvinar lesions. Brain. 2008;131:2140–2152. doi: 10.1093/brain/awn135. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Desimone R, Ungerleider LG. Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Vis Neurosci. 1993;10:59–72. doi: 10.1017/s0952523800003229. [DOI] [PubMed] [Google Scholar]
- Baldauf D, Desimone R. Neural mechanisms of object-based attention. Science. 2014;344:424–427. doi: 10.1126/science.1247003. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Fries P. Communication through coherence with inter-areal delays. Curr Opin Neurobiol. 2014;31C:173–180. doi: 10.1016/j.conb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Bender DB, Butter CM. Comparison of the effects of superior colliculus and pulvinar lesions on visual search and tachistoscopic pattern discrimination in monkeys. Exp Brain Res. 1987;69:140–154. doi: 10.1007/BF00247037. [DOI] [PubMed] [Google Scholar]
- Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. J Neurophysiol. 2001;85:219–234. doi: 10.1152/jn.2001.85.1.219. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Port JD. Single neurons with both form/color differential responses and saccade-related responses in the nonretinotopic pulvinar of the behaving macaque monkey. Vis Neurosci. 1995;12:523–544. doi: 10.1017/s0952523800008439. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Standage GP. The organization of projections of the retinorecipient and nonretinorecipient nuclei of the pretectal complex and layers of the superior colliculus to the lateral pulvinar and medial pulvinar in the macaque monkey. J Comp Neurol. 1983;217:307–336. doi: 10.1002/cne.902170307. [DOI] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J Neurosci. 2011;31:373–384. doi: 10.1523/JNEUROSCI.4738-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci U S A. 2011;108:11262–11267. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger S, Ward R, Owen V, Rafal R. The effects of unilateral pulvinar damage in humans on reflexive orienting and filtering of irrelevant information. Behav Neurol. 2001;13:95–104. doi: 10.1155/2002/917570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Desimone R, Wessinger M, Thomas L, Schneider W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harb Symp Quant Biol. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Galkin TW, Desimone R, Ungerleider LG. Subcortical connections of area V4 in the macaque. J Comp Neurol. 2014;522:1941–1965. doi: 10.1002/cne.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Rossi AF, Ungerleider LG, Desimone R. Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat Neurosci. 2014;17:1003–1011. doi: 10.1038/nn.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe I, Neitzel SD, Mandon S, Kreiter AK. Switching neuronal inputs by differential modulations of gamma-band phase-coherence. J Neurosci. 2012;32:16172–16180. doi: 10.1523/JNEUROSCI.0890-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kastner S, O’Connor DH, Fukui MM, Fehd HM, Herwig U, Pinsk MA. Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol. 2004;91:438–448. doi: 10.1152/jn.00553.2003. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–61. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Anderson CH, Van Essen DC. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J Neurosci. 1993;13:4700–4719. doi: 10.1523/JNEUROSCI.13-11-04700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Kastner S. Attention in the real world: toward understanding its neural basis. Trends Cogn Sci. 2014;18:242–250. doi: 10.1016/j.tics.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Purushothaman G, Marion R, Li K, Casagrande VA. Gating and control of primary visual cortex by pulvinar. Nat Neurosci. 2012;15:905–912. doi: 10.1038/nn.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci U S A. 1987;84:7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MJ, Lowet E, Brunet NM, Ter Wal M, Tiesinga P, Fries P, De Weerd P. Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron. 2013;78:523–536. doi: 10.1016/j.neuron.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- Rocha-Miranda CE, Bender DB, Gross CG, Mishkin M. Visual activation of neurons in inferotemporal cortex depends on striate cortex and forebrain commissures. J Neurophysiol. 1975;38:475–91. doi: 10.1152/jn.1975.38.3.475. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Singer W, Fries P. Thalamic coordination of cortical communication. Neuron. 2012;75:551–552. doi: 10.1016/j.neuron.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Seth AK. A MATLAB toolbox for Granger causal connectivity analysis. J Neurosci Methods. 2010;186:262–273. doi: 10.1016/j.jneumeth.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol. 2011;106:1068–1077. doi: 10.1152/jn.00429.2011. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Smith AT, Cotton PL, Bruno A, Moutsiana C. Dissociating vision and visual attention in the human pulvinar. J Neurophysiol. 2009;101:917–925. doi: 10.1152/jn.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc Natl Acad Sci U S A. 2009;106:4054–4059. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JG, Gattass R, Souza AP, Rosa MG, Fiorani M, Jr, Brandao BL. Connectional and neurochemical subdivisions of the pulvinar in Cebus monkeys. Vis Neurosci. 2001;18:25–41. doi: 10.1017/s0952523801181034. [DOI] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T. Prefrontal contributions to visual selective attention. Annu Rev Neurosci. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan S, Kopell NJ. Thalamic model of awake alpha oscillations and implications for stimulus processing. Proc Natl Acad Sci U S A. 2012;109:18553–18558. doi: 10.1073/pnas.1215385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Arend I. An object-based frame of reference within the human pulvinar. Brain. 2007;130:2462–2469. doi: 10.1093/brain/awm176. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kagan I, Andersen RA. Effects of pulvinar inactivation on spatial decision-making between equal and asymmetric reward options. J Cogn Neurosci. 2013;25:1270–1283. doi: 10.1162/jocn_a_00399. [DOI] [PubMed] [Google Scholar]
- Wilke M, Mueller KM, Leopold DA. Neural activity in the visual thalamus reflects perceptual suppression. Proc Natl Acad Sci U S A. 2009;106:9465–9470. doi: 10.1073/pnas.0900714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Turchi J, Smith K, Mishkin M, Leopold DA. Pulvinar inactivation disrupts selection of movement plans. J Neurosci. 2010;30:8650–8659. doi: 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meyers EM, Bichot NP, Serre T, Poggio TA, Desimone R. Object decoding with attention in inferior temporal cortex. Proc Natl Acad Sci U S A. 2011;108:8850–8855. doi: 10.1073/pnas.1100999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Desimone R. Feature-based attention in the frontal eye field and area V4 during visual search. Neuron. 2011;70:1205–1217. doi: 10.1016/j.neuron.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihl J, von Cramon D. The contribution of the ‘second’ visual system to directed visual attention in man. Brain. 1979;102:835–856. doi: 10.1093/brain/102.4.835. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal Projections to the Thalamic Reticular Nucleus form a Unique Circuit for Attentional Mechanisms. The Journal of Neuroscience. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci. 2012;32:5338–5350. doi: 10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


