Abstract
Background
Terlipressin with albumin is recommended in hepatorenal syndrome (HRS). Terlipressin is expensive and not licensed in many countries. Alternative therapy is necessary. We compared the efficacy of terlipressin and albumin with concurrent low-dose dopamine, furosemide, and albumin in HRS.
Methods
In an open-label, randomized trial, forty consecutive patients each with HRS type I and HRS type II received either concurrent infusion of terlipressin 0.5 mg for every 6 hr and albumin 20 g/day for 5 days (n = 20) or a combination of dopamine 2 μg/kg/min, furosemide 0.01 mg/kg/hr, and albumin 20 g/day (triple therapy), in one of two therapeutic arms. Twenty-four-hour urine output, urinary sodium, and plasma renin activity (PRA) were assessed before and after treatment.
Results
The two groups were comparable at baseline in both HRS-I and II. In HRS-I, 24 hr urine output and urine sodium at the end of 5 days increased in both treatment groups (terlipressin, urine output 278 ± 136 to 765 ± 699 ml/day, P < 0.01; urine sodium 28 ± 25.1 to 39 ± 32.1 meq/l, P = 0.05. Triple therapy: urine output 219 ± 134 to 706 ± 595 ml/day, P < 0.01; urine sodium 25 ± 18.3 to 41 ± 27.5 meq/l, P < 0.01). PRA (ng/ml/hr) decreased from 28.1 ± 9.76 to 24.2 ± 9.5 (P = 0.01) and from 29.5 ± 15.8 to 27.3 ± 17.1 (P = 0.02) in the terlipressin and triple therapy groups, respectively. In HRS-II, similar significant improvement (P < 0.01) was seen in 24 hr urine output and urine sodium; decrease in PRA (P < 0.05) was documented after treatment in both the arms. Post-treatment changes in parameters were comparable between the two arms, in both HRS-I and HRS-II cases.
Conclusions
Concurrent triple therapy improved renal function in HRS and was less expensive than terlipressin (Registration: CTRI/2011/07/001860; www.ctri.nic.in).
Abbreviations: HRS, hepatorenal syndrome; MARS, Molecular Absorbent Recirculating System; PRA, plasma renin activity; TIPS, transjugular intrahepatic portosystemic shunts
Keywords: liver cirrhosis, ascites, hepatorenal syndrome, terlipressin, PRA
Annually, hepatorenal syndrome (HRS) occurs in 18% of the patients with decompensated cirrhosis and is associated with high mortality.1 Spontaneous regression of the syndrome is very rare.1 The treatment of choice for HRS is liver transplantation, with which a 5-year survival of 65% has been achieved in type 1 HRS.2 However, access to liver transplantation is limited, and due to short survival in HRS and organ shortage, only a proportion of such patients are transplanted. Therefore, medical treatments to improve survival in HRS are of interest.
Transjugular intrahepatic portosystemic shunts (TIPS) are effective in both type 1 and 2 HRS; however, its use is limited due to concurrent presence of contraindications to TIPS in a substantial proportion of such patients.3 Extracorporeal albumin dialysis using Molecular Absorbent Recirculating System (MARS) has improved survival in one controlled trial.4 However, this is not yet widely available and has been tried in a very small number of patients. Medical therapies, which have been shown to be promising, include catecholamines along with octreotride and albumin infusion,5 noradrenaline,6 midodrine,7 ornipressin, and terlipressin.7, 8, 9 The most widely used drug in HRS till date is terlipressin with albumin infusion. Terlipressin has been documented to improve renal function in both type 1 and type 2 HRS with survival benefit.10 Terlipressin is a vasopressin analog which acts on the V1 receptors. The rationale for administration of a vasoconstrictor is to counteract the splanchnic vasodilatation in patients with cirrhosis and HRS. This improves the effective arterial volume, and subsequently, renal perfusion and glomerular filtration.10 However, terlipressin is expensive (costing about $340 for 5 days) and not licensed to be used in a few countries, like USA, for HRS.
The pathogenesis of HRS consists of peripheral vasodilation2, 11 with consequent renal vasoconstriction12 and poor natriuresis.2 Preliminary observation in our center using low-dose dopamine to reverse renal vasoconstriction, low-dose furosemide to improve natriuresis, and intravenous albumin to reduce relative hypovolemia due to vasodilatation (triple therapy) demonstrated that this combination is also effective in HRS.13 The constituents of triple therapy are less expensive than terlipressin, and are safe in HRS.13 Further, terlipressin has been reported to have side effects in up to 30% of patients.8 Therefore, the present prospective, open-labeled, randomized trial was performed to assess the efficacy of triple therapy as against the established therapy with terlipressin and albumin.
Patients and Methods
Patients
Between February 2005 and June 2010, all consecutive patients with cirrhosis of the liver and clinically detectable ascites having oliguria (≤500 ml urine/24 hr) admitted to the department of Gastroenterology at the All India Institute of Medical Sciences, New Delhi, a tertiary care center, were evaluated for the presence of HRS. Patients satisfying the diagnostic criteria for HRS14 were included in the trial after obtaining an informed consent from the patient, or, if the patient was not in a sound mental condition, from the nearest relative present. Patients were excluded from the study if they had any of the following: (1) active gastrointestinal bleeding, (2) hypotension requiring ionotropic support with or without sepsis, (3) pre-existing underlying primary renal disease, (4) myocardial dysfunction due to ischemic or any other cardiac disease, (5) associated systemic diseases like diabetes mellitus and systemic hypertension with evidence of nephropathy, (6) hepatocellular carcinoma, (7) portal vein thrombosis, (8) hepatic venous outflow tract obstruction, or (9) tubercular peritonitis. The study was approved by the Institutional ethics committee.
Consecutive patients of cirrhosis with HRS satisfying the inclusion and exclusion criteria were randomized, either to the group receiving iv dopamine, furosemide, and albumin, or terlipressin and albumin.
Diagnostic Criteria
Cirrhosis of liver with ascites was diagnosed by clinical, biochemical, and imaging features. HRS was defined using the International ascites club definition.14 Clinically, HRS was categorized into 2 types according to intensity and form of progression of renal failure. Type I HRS was characterized by a severe and rapidly progressive renal failure, which was defined as a doubling of serum creatinine concentration reaching a level greater than 2.5 mg/dl in less than 2 weeks. Type II HRS was characterized by a moderate and steady decrease in renal function (serum creatinine <2.5 mg/dl).
Study Design, Randomization, and Implementation
A stratified, open-label, randomized trial was conducted. HRS was stratified as HRS type I or HRS type II, and within each type of HRS, patients were randomly allocated to receive triple therapy or terlipressin plus albumin. The simple randomization sequence was generated by a computerized random number generator, by the Biostatistician (VS) in blocks of 10 for each type of HRS. The programmer who generated the sequence had no role in recruitment, treatment, or assessment of patients. There was no restriction, blocking, or further stratification of the randomization sequence. Because it was an open-labeled, randomized controlled trial, concealment of allocation and blinding were not features of the study design. Enrollment of participants, assessing eligibility, obtaining informed consent, and allocation to the specific therapeutic arm were carried out by one of the 2 authors (SS and SKA). Assessment of outcome measures, management, and monitoring were performed by one of the 2 authors (S and HS). The latter authors were not informed about the treatment allocation and were blinded to the treatment regimen.
Treatment Regimen
A dose of 0.5 mg intravenous terlipressin was administered every 6 hr along with albumin 20% (100 ml) per day for 5 days in the terlipressin arm. Patients under the triple therapy arm received, in addition to albumin 20% (100 ml), concurrent intravenous dopamine infusion in the dose of 2 μg/kg/min, and furosemide in the dose of 0.01 mg/kg/hr for 5 days.
Monitoring of Patients
All patients had volume restitution by infusion of colloids and crystalloids as clinically appropriate to maintain central venous pressure at 10–12 cm of water. Empirical antibiotics (cefotaxime 2 g iv 8 hourly) were given to all; these were subsequently modified according to culture sensitivity reports. All received lactulose in doses adjusted to achieve 2 to 3 semi-formed stools daily. All patients were fed enterally whenever possible, with a target intake of 2000 kcal/day. Ascitic fluid paracentesis was done when required.
Patients were monitored closely for clinical parameters, such as heart rate, mean arterial pressure, urine output, and grade of hepatic encephalopathy, and for biochemical parameters, namely hemoglobin, blood urea, serum creatinine, sodium, potassium, urine sodium, and plasma renin activity (PRA). Clinical parameters were assessed twice daily for first 5 days and then daily till discharge. Biochemical parameters were recorded daily till discharge. PRA was measured before therapy and at the end of 5 days of therapy, for all patients. All patients had their ECG recorded at baseline. Surviving patients were discharged after 15 days and followed up as outpatients for another 15 days. Urine output and biochemical parameters were assessed at each weekly follow-up visit.
PRA Estimation
Blood for PRA estimation was collected from the antecubital vein of patients, in supine position. It was collected in EDTA vial kept in icebox and was transferred immediately to the laboratory in an icebox. In the laboratory, the blood was immediately centrifuged at 3000 rpm for 15 min to separate plasma, and then immediately stored at −20 °C. PRA was estimated by using radioimmunoassay (RIA) kit from Immunotech (Prague, Czech Republic).15 The detection limit for PRA was 0.1 ng/ml/hr. Intra-assay and inter-assay coefficient of variation were 10.4% and 10.5%, respectively, and physiological range was 0.50–1.90 ng/ml/hr.
Outcome Measures
Primary outcome measures were (i) survival at end of therapy, day 15, and at one month and (ii) change in urine output, serum creatinine, serum sodium, serum potassium, and PRA at end of therapy. Secondary outcome measures were side effects of therapy and cost of therapy.
End Point
The end point of the study was death or completion of 1 month of follow-up.
Sample Size
The sample size was based on noninferiority assumptions for the efficacy of two trial drugs. We assumed a SD (of change in urine output) of 100 ml between the 2 trial drugs. Sample sizes of 20 from the experimental group (triple therapy) and 20 from the standard group (terlipressin therapy) for each type of HRS (HRS I and HRS II) were estimated to achieve 80% power at a 5% significance level using t-test for equivalence of means.
Statistical Analysis
Patients were analyzed as per intention to treat principle. Quantitative variables were summarized as mean ± SD and qualitative variables as proportions. Chi-square/Fisher's Exact test and Student's t-test were used to compare the qualitative and quantitative variables between the two treatment groups. When the normality of a variable was suspect, Wilcoxon Ranksum test was used instead of Student's t-test. Paired t-test was used to compare pre- and post-treatment levels of quantitative variables within a treatment group. For the comparison of improvement in urine output between the two treatment groups, a one-sided t-test for noninferiority was applied. All analyses were carried out using STATA version 11.2. All authors had access to the study data and had reviewed and approved the final manuscript. Trial is registered with Clinical trial registry India (CTRI/2011/07/001860; www.ctri.nic.in).
Results
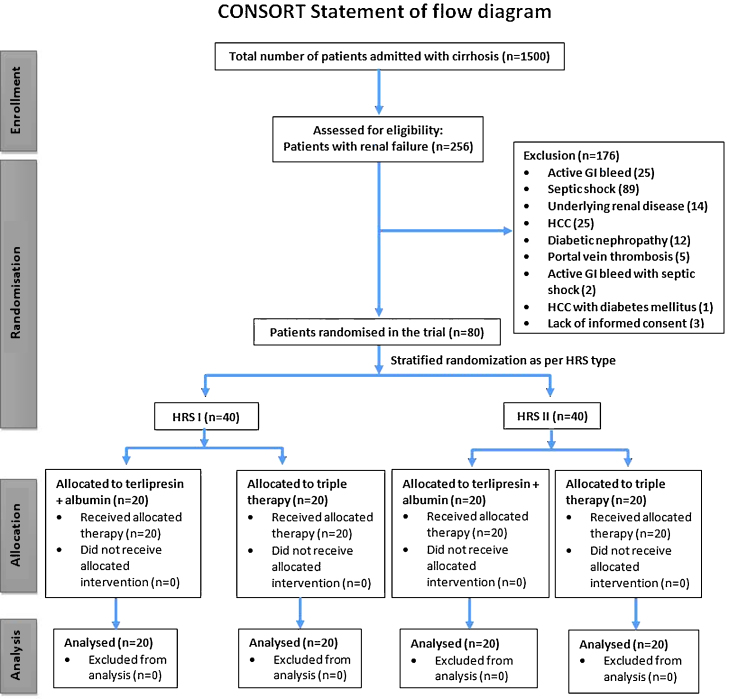
During the study period from February 2005 to June 2010, a total of 1500 patients (328 deaths; 21.9%) of cirrhosis of the liver were admitted to the Gastroenterology ward of the All India Institute of Medical Sciences. Of these, 256 patients (17.6%) were admitted with, or developed, renal failure during the course of hospital stay. Eighty patients were included in present trial. CONSORT checklist illustrating the progress of patients through the trial, including recruitment, enrollment, randomization, withdrawal, and completion, and description of the randomization procedure has been depicted in Figure 1.
Fig. 1.
CONSORT chart of patients randomized in the trial.
The etiology of cirrhosis, clinical, demographic, and biochemical parameters of the patients randomized to receive the two treatment regimens in each type of HRS are shown in Table 1 and were similar (P > 0.05). Of the 40 patients included in each type of HRS (type I and type II), 20 each were randomized to receive terlipressin plus albumin or triple therapy.
Table 1.
Baseline Characteristics of Patients with HRS I and HRS II.
| Characteristics | HRS I |
P | HRS II |
P | ||
|---|---|---|---|---|---|---|
| Terlipressin (N = 20) | Triple therapy (N = 20) | Terlipressin (N = 20) | Triple therapy (N = 20) | |||
| Etiology: no. (%) | ||||||
| Alcohol | 20 (50) | 21 (52.5) | 0.97 | |||
| HBV | 6 (15) | 7 (17.5) | ||||
| HCV | 3 (7.5) | 4 (10) | ||||
| HBV and HCV | 1 (2.5) | 1 (2.5) | ||||
| Wilson's disease | 1 (2.5) | 0 | ||||
| Others | 9 (22.5) | 7 (17.5) | ||||
| Gender (M:F) | 19:1 | 17:3 | 0.60 | 17:3 | 16:4 | 0.99 |
| Age (years) | 45.8 ± 13.86 | 39.2 ± 9.64 | 0.09 | 44.6 ± 13.45 | 42.6 ± 11.76 | 0.62 |
| Serum creatinine (mg/dl) | 3.8 ± 1.38 | 4.1 ± 1.50 | 0.53 | 1.9 ± 0.36 | 2.0 ± 0.33 | 0.62 |
| 24 hr urine output (ml) | 278 ± 135.8 | 220 ± 134.3 | 0.44 | 268 ± 131.8 | 276 ± 127.4 | 0.95 |
| Median (range) | 265 (100–500) | 200 (100–500) | 250 (100–500) | 250 (100–500) | ||
| Urine sodium (meq/24 hr) | 28.3 ± 25.15 | 24.7 ± 18.32 | 0.84 | 14.9 ± 10.17 | 16.8 ± 20.76 | 0.57 |
| Median (range) | 17.5 (3–81) | 24 (2–66) | 12 (2–31) | 12.9 (1–82) | ||
| Heart rate (per min) | 92.1 ± 15.87 | 89.6 ± 14.66 | 0.62 | 91.2 ± 18.42 | 91.4 ± 16.66 | 0.96 |
| MAP (mmHg) | 82.2 ± 13.20 | 75.4 ± 12.84 | 0.11 | 77.9 ± 7.59 | 81.6 ± 10.05 | 0.20 |
| Hemoglobin (g/dl) | 9.1 ± 2.57 | 8.8 ± 2.10 | 0.71 | 8.7 ± 2.04 | 9.0 ± 2.45 | 0.69 |
| PT prolongation (s) | 14.4 ± 11.84 | 18.1 ± 10.87 | 0.14 | 20.7 ± 15.88 | 17.0 ± 11.75 | 0.77 |
| Median (range) | 11 (2–50) | 16 (6–46) | 14 (4–58) | 14 (2–48) | ||
| Urea (mg/dl) | 129.5 ± 43.72 | 137.1 ± 48.99 | 0.62 | 85.1 ± 34.27 | 105.9 ± 40.18 | 0.09 |
| Serum sodium (meq/l) | 136.0 ± 7.55 | 135.3 ± 8.08 | 0.79 | 131.4 ± 9.47 | 134.8 ± 8.63 | 0.26 |
| Potassium (meq/l) | 4.4 ± 0.81 | 4.2 ± 0.83 | 0.39 | 4.0 ± 1.00 | 4.2 ± 0.67 | 0.45 |
| Child's Score | 11.9 ± 1.88 | 12.8 ± 1.61 | 0.13 | 12.4 ± 1.70 | 11.9 ± 1.93 | 0.44 |
| Bilirubin (mg/dl) | 20.3 ± 17.94 | 19.8 ± 19.15 | 0.89 | 13.2 ± 12.48 | 10.6 ± 13.91 | 0.23 |
| Median (range) | 19.9 (0.7–69.6) | 15.2 (0.5–71.7) | 8.0 (0.8–41.0) | 5.2 (0.6–51.4) | ||
| ALT (IU/L) | 76.5 ± 51.83 | 101.6 ± 119.63 | 0.88 | 68.6 ± 73.85 | 88.9 ± 90.16 | 0.46 |
| Median (range) | 72 (12–162) | 50 (28–526) | 45 (13–329) | 47 (20–344) | ||
| Albumin (g/dl) | 2.5 ± 0.48 | 2.4 ± 0.62 | 0.56 | 2.3 ± 0.50 | 2.6 ± 0.75 | 0.13 |
| PRA (ng/ml/hr) | 28.1 ± 9.76 | 29.5 ± 15.80 | 0.74 | 27.7 ± 10.15 | 31.5 ± 9.20 | 0.22 |
| Median (range) | 28.8 (8.1–52.9) | 28.8 (4.7–79.0) | 29.6 (6.4–51.4) | 30.9 (13.4–47.0) | ||
Note: All data presented as mean ± SD (unless specified). HBV: hepatitis B virus; HCV: hepatitis C virus; MAP: mean arterial pressure; PT: prothrombin time; ALT: alanine aminotransferase; PRA: plasma renin activity.
Outcomes in HRS I
At the end of 5 days of treatment, 24 hr urine output significantly improved in both the treatment arms (P < 0.01) (Table 2). Significant improvement in natriuresis was documented in terlipressin and triple therapy groups (P = 0.05 and <0.01 respectively). Mean PRA also significantly decreased in both the treatment groups (28.1 ± 9.8 to 24.2 ± 9.5 ng/ml/hr, P = 0.01 in terlipressin group and from 29.5 ± 15.8 to 27.3 ± 17.1 ng/ml/hr, P = 0.02 in triple therapy group). At the end of 5 days of treatment, even though trend toward decrease in serum creatinine and blood urea was documented in both the treatment groups, the reduction was not significant. We compared the absolute increase in urinary output, 24 hr urinary sodium, decrease in PRA, and alteration in the serum electrolytes between the two treatment arms. Comparison of these delta values of the parameters pre- and post-treatment, between terlipressin and triple therapy groups, was similar (P > 0.05) as seen in Table 4. Average improvement in the urine output, though appearing similar, did not achieve statistical significance for noninferiority of the triple therapy (P = 0.31).
Table 2.
Outcomes of Terlipressin and Triple Therapy in Type I HRS.
| Characteristics | Terlipressin (N = 20) |
P | Triple therapy (N = 20) |
P | ||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |||
| Urine output (ml/day) | ||||||
| Mean ± SD | 277.8 ± 135.85 | 765.0 ± 699.27 | <0.01 | 219.5 ± 134.30 | 706.5 ± 595.45 | <0.01 |
| Median (range) | 265 (100–500) | 325 (100–2000) | 200 (100–500) | 575 (100–2000) | ||
| Creatinine (mg/dl) | ||||||
| Mean ± SD | 3.8 ± 1.38 | 3.6 ± 1.83 | 0.43 | 4.1 ± 1.50 | 3.7 ± 2.25 | 0.19 |
| Median (range) | 3.9 (1.6–6.3) | 3.8 (0.7–6.5) | 4.1 (2.0–7.4) | 3.3 (1.2–11.0) | ||
| Blood Urea (mg/dl) | ||||||
| Mean ± SD | 129.7 ± 43.72 | 127.0 ± 66.10 | 0.64 | 137.1 ± 48.99 | 138.8 ± 59.88 | 0.79 |
| Median (range) | 123 (70–237) | 135.5 (23–300) | 131 (62–245) | 129 (41–289) | ||
| Serum sodium (meq/l) | ||||||
| Mean ± SD | 136.0 ± 7.55 | 137.2 ± 9.13 | 0.43 | 135.3 ± 8.08 | 135.5 ± 10.50 | 0.91 |
| Median (range) | 137.5 (115–148) | 138 (115–149) | 135 (112–152) | 136.5 (112–154) | ||
| Serum potassium (meq/l) | ||||||
| Mean ± SD | 4.4 ± 0.81 | 4.1 ± 0.82 | 0.15 | 4.2 ± 0.83 | 4.3 ± 0.88 | 0.66 |
| Median (range) | 4.3 (3.1–6.3) | 4.2 (3.0–5.7) | 4.2 (3.0–6.0) | 4.3 (3.0–5.8) | ||
| Urine sodium (meq/24 hr) | ||||||
| Mean ± SD | 28.3 ± 25.15 | 38.7 ± 32.09 | 0.05 | 24.7 ± 18.32 | 40.5 ± 27.54 | <0.01 |
| Median (range) | 17.5 (3–81) | 29.8 (3–126) | 24 (2–66) | 37 (2–86) | ||
| PRA (ng/ml/hr) | ||||||
| Mean ± SD | 28.1 ± 9.76 | 24.2 ± 9.54 | 0.01 | 29.5 ± 15.80 | 27.3 ± 17.12 | 0.02 |
| Median (range) | 28.8 (8.1–52.9) | 27.1 (8.0–41.1) | 28.8 (4.7–79.0) | 27.6 (4.7–79.0) | ||
Note: PRA: plasma renin activity.
Table 4.
Comparison of Pre-Treatment to Post-Treatment Changes in Outcome Parameters Between the Two Treatment Groups in Both Types of HRS.
| Characteristic | HRS I |
P | HRS II |
P | ||||
|---|---|---|---|---|---|---|---|---|
| Terlipressin (N = 20) | Triple therapy (N = 20) | Difference (95% CI) | Terlipressin (N = 20) | Triple therapy (N = 20) | Difference | |||
| Increase in urine output (ml/day) | ||||||||
| Mean ± SD | 487.2 ± 665.6 | 487.0 ± 604.7 | 0.25 (−406.8 to 407.3) | 0.31* | 979.0 ± 908.0 | 864.0 ± 613.1 | 115.0 (−380.9 to 610.9) | 0.47a |
| Median (range) | 62 (−150 to 1800) | 250 (−300 to 1650) | 1100 (−300 to 250) | 800 (0 to 2300) | ||||
| Reduction in serum creatinine (mg/dl) | ||||||||
| Mean ± SD | 0.2 ± 1.15 | 0.4 ± 1.45 | −0.2 (−1.07 to 0.60) | 0.30 | 0.4 ± 0.55 | 0.5 ± 0.75 | −0.1 (−0.57 to 0.28) | 0.30 |
| Median (range) | 0.05 (−1.7 to 2.5) | 0.4 (−3.6 to 2.6) | 0.3 (−0.6 to 1.4) | 0.8 (−1.3 to 1.4) | ||||
| Reduction in blood urea (mg/dl) | ||||||||
| Mean ± SD | 2.7 ± 51.17 | −1.6 ± 36.81 | 4.4 (−24.16 to 32.91) | 0.59 | 10.8 ± 14.40 | 23.2 ± 51.73 | −12.5 (−36.8 to 11.81) | 0.51 |
| Median (range) | 0 (−114.0 to 110.4) | 0 (−86.0 to 50.0) | 4 (−6.0 to 38.0) | 21 (−62.0 to 114.0) | ||||
| Increase in serum sodium (meq/l) | ||||||||
| Mean ± SD | 1.2 ± 6.65 | 0.2 ± 8.58 | 1.0 (−3.91 to 5.91) | 0.52 | 3.8 ±8.31 | 2.7 ± 12.60 | 1.2 (−5.68 to 7.98) | 0.42 |
| Median (range) | 0 (−9.0 to 16.0) | −0.5 (−17.0 to 18.0) | 2 (−11.0 to 25.0) | −0.5 (−17.0 to 3.0) | ||||
| Reduction in serum potassium (meq/l) | ||||||||
| Mean ± SD | 0.3 ± 0.89 | −0.1 ± 0.94 | 0.4 (−0.19 to 0.98) | 0.14 | −0.1 ± 1.09 | 0.4 ± 0.90 | −0.5 (−1.19 to 0.09) | 0.09 |
| Median (range) | 0.3 (−1.8 to 1.7) | 0 (−1.7 to 1.9) | 0 (−2.7 to 1.3) | 0.6 (−1.9 to 1.5) | ||||
| Increase in urine sodium (meq/24 hr) | ||||||||
| Mean ± SD | 10.4 ± 25.17 | 15.8 ± 21.46 | −5.4 (−20.37 to 9.57) | 0.24 | 24.2 ± 32.59 | 35.2 ± 30.23 | −10.9 (−31.07 to 9.17) | 0.13 |
| Median (range) | 0 (−13.0 to 100.0) | 0.5 (−3.0 to 56.0) | 0.5 (−3.0 to 100.0) | 28.0 (0.0–109.0) | ||||
| Reduction in PRA (ng/ml/hr) | ||||||||
| Mean ± SD | 3.9 ± 6.48 | 2.2 ± 3.93 | 1.7 (−1.73 to 5.13) | 0.52 | 4.0 ± 7.69 | 4.6 ± 6.39 | −0.6 (−5.16 to 3.89) | 0.70 |
| Median (range) | 0 (0.0–17.45) | 0 (0.0–12.2) | 0 (0.0–28.1) | 0 (−0.8 to 15.2) | ||||
Note: PRA: plasma renin activity.
P for noninferiority of triple therapy.
The proportion of patients surviving till the end of therapy [15 (75%) vs. 16 (80%)], at the end of 15 days [4 (21%) vs. 4 (20%)], and at 1 month after start of therapy [3 (15.7%) vs. 3 (15%)] were similar in terlipressin and triple therapy groups, respectively. Progressive liver failure was the commonest cause of death in both the groups. The patients who survived were followed up at weekly intervals as outpatients. None of the patients who showed improvement after completion of therapy had recurrence of renal dysfunction.
Outcomes in HRS-II
After completion of 5 days of therapy, 24 hr urinary output, 24 hr urinary sodium, serum creatinine, and blood urea improved in terlipressin therapy group (P < 0.05), while in triple therapy group all parameters except blood urea showed significant improvement (P < 0.01). PRA decreased significantly in both the treatment groups (P < 0.05). Blood urea also showed improvement, but the significance was marginal (P = 0.06). The details are shown in Table 3.
Table 3.
Outcomes of Terlipressin and Triple Therapy in Type II HRS.
| Characteristic | Terlipressin |
P | Triple therapy |
P | ||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |||
| Urine output (ml/day) | ||||||
| Mean ± SD | 268.5 ± 131.76 | 1247.5 ± 921.59 | <0.001 | 275.5 ± 127.38 | 1139.5 ± 627.02 | <0.001 |
| Median (range) | 250 (100–500) | 1275 (100–3500) | 250 (100–500) | 1150 (200–2500) | ||
| Serum creatinine (mg/dl) | ||||||
| Mean ± SD | 1.9 ± 0.36 | 1.6 ± 0.58 | <0.01 | 2.0 ± 0.33 | 1.5 ± 0.71 | <0.01 |
| Median (range) | 1.8 (1.3–2.5) | 1.6 (0.4–2.7) | 1.9 (1.5–2.5) | 1.2 (0.7–3.3) | ||
| Blood urea (mg/dl) | ||||||
| Mean ± SD | 85.1 ± 34.27 | 74.4 ± 38.88 | <0.01 | 105.9 ± 40.18 | 82.6 ± 47.78 | 0.06 |
| Median (range) | 77.8 (29–184) | 64.5 (22–184) | 96.5 (18–172) | 64 (27–182) | ||
| Serum sodium (meq/l) | ||||||
| Mean ± SD | 131.4 ± 9.47 | 135.3 ± 12.14 | 0.05 | 134.8 ± 8.63 | 137.4 ± 9.25 | 0.35 |
| Median (range) | 132 (111–145) | 137 (107–152) | 133 (111–155) | 136.5 (124–158) | ||
| Serum potassium (meq/l) | ||||||
| Mean ± SD | 4.0 ± 1.00 | 4.1 ± 0.98 | 0.62 | 4.2 ± 0.67 | 3.8 ± 0.75 | 0.05 |
| Median (range) | 4.4 (2.3–5.2) | 4.0 (1.9–5.8) | 4.3 (3.0–5.4) | 3.7 (2.2–5.1) | ||
| Urine sodium | ||||||
| Mean ± SD | 14.9 ± 10.17 | 39.1 ± 33.28 | <0.01 | 16.8 ± 20.76 | 52.0 ± 30.60 | <0.001 |
| Median (range) | 12 (2–31) | 26.5 (7–129) | 12.9 (1–82) | 50.5 (10–126) | ||
| PRA (ng/ml/hr) | ||||||
| Mean ± SD | 27.7 ± 10.15 | 23.7 ± 7.81 | 0.03 | 31.5 ± 9.20 | 26.9 ± 10.78 | <0.01 |
| Median (range) | 29.6 (6.4–51.4) | 24.7 (6.4–39.4) | 30.9 (13.4–47.0) | 25.4 (9.2–47.0) | ||
Note: PRA: plasma renin activity.
As seen in HRS-I, the delta values did not differ significantly (P value ranged from 0.09 to 0.73) between terlipressin and triple therapy groups (Table 4). The average difference in the improvement of urine output was more by 115 ml/day in the terlipressin group and triple therapy could not be observed to be noninferior to terlipressin (P = 0.47).
Survival was also similar between the two treatment groups in HRS-II, the proportions being 16 (80%) vs. 18 (90%) at the end of therapy, 9 (47%) vs. 13 (65%) at 15 days, and 6 (35%) vs. 5 (31%) at one month (P > 0.2). In HRS-II also, progressive liver failure was the commonest cause of death in either group.
Cost of Triple Therapy and Terlipressin
Triple therapy was less expensive than terlipressin for management of HRS. Albumin is included in both the arms; hence, if cost of albumin is not taken into consideration for comparison, then the cost of 5-day therapy with terlipressin was Rs. 15,000 (approximately $250), whereas cost of triple therapy was Rs. 400 ($6.5).
Adverse Events
No major side effects (requiring dose reduction or drug withdrawal) were reported from either terlipressin or triple therapy arm. Sinus tachycardia was reported in 4 (10%) patients receiving terlipressin (3 with type I HRS and 1 with type II HRS) and 2 (5%) in triple therapy group.
Discussion
The results of this prospective, randomized controlled trial indicate that short-term regimen of continuous volume expansion using intravenous albumin, renal vasodilatation using low-dose dopamine, and diuresis induced by continuous low-dose furosemide is as beneficial as short-term terlipressin plus albumin in patients with HRS.
The pre-treatment parameters were similar in the two therapeutic groups between both Type I and II HRS included in the present study (Table 1). Post-therapy, improvement in renal parameters (urine output and urinary sodium) was significant in both groups, and in either type of HRS (Table 2, Table 3). Their delta values, which indicate the numeric improvement in various renal parameters, electrolytes, and in PRA levels between pre-treatment and post-treatment values, were similar in the two treatment groups between type I and II HRS (Table 4). These findings support the contention that the triple therapy is as effective as terlipressin treatment, between both the types of HRS.
Documentation in significant improvement in PRA at the end of 5 days of triple therapy would suggest that the triple therapy is able to ameliorate the pathogenetic hemodynamic alterations responsible for HRS (decrease in systemic vascular resistance), and these events are similar to alteration documented by terlipressin treatment. Further, the survival was similar in both treatment arms between both the types of HRS. Furthermore, once these patients recovered from HRS, subsequent follow-up over a period of one month showed no subsequent deterioration in renal function. This supports earlier observations that the kidneys are essentially normal in HRS and once recovery occurs, renal function remains normal.
Dopamine infusion alone or along with intermittent bolus of furosemide has not been shown to be effective in HRS.16 However, in the present study, simultaneous reversal of intense renal vasoconstriction by dopamine, enhanced or maintained intravascular volume with albumin, and continuous low-dose furosemide possibly resulted in improved renal function evident by marked and significant improvement in urine output and natriuresis. The hemodynamic benefit of the triple therapy was documented by the improvement in PRA activity in both types of HRS. Conceptually, the multiple mechanisms involved in the pathogenesis of HRS may require multimodality therapy as used in this study.
Vasoconstrictor therapy for HRS is an attractive option even though it has not been evaluated in randomized controlled trials.17 Though some toxicity has been reported with vasoconstrictors,17 the present study did not show significant toxicity or adverse effects of the triple therapy among patients receiving terlipressin. Importantly, even with a short period of treatment, response to therapy was rapidly evident. Triple therapy has the advantage of low cost. If patients can be reversed from HRS using this therapy, elective liver transplantation can be offered. This is important because it has been shown that the outcome of liver transplantation in patients with HRS is worse than those without HRS and reversal of HRS would therefore be highly desirable.18, 19
We looked for methodology errors to explain the results. Randomization was successful as both groups in either type of HRS were comparable before treatment (P > 0.05). We used albumin for volume expansion in patients with HRS, similar to protocol followed by Guevara et al.20 In this latter study, prolonged administration of albumin was used to keep a pulmonary capillary wedge pressure of 14–19 mmHg, while in our study, albumin infusions were given to keep the central venous pressure (an indirect indicator of pulmonary capillary wedge pressure) at 10–12 cm of water.
Dose and duration of terlipressin therapy is controversial. We used 2 mg terlipressin per day, which is similar to the dose used by Hadengue et al.21 In this double-blind, randomized cross-over study, 6 of 9 patients experienced HRS reversal. Similarly, Solanki et al.8 reported findings from a small, single-blind, randomized, placebo-controlled trial in which 5 of 12 patients treated with terlipressin at a dose of 2 mg/day experienced HRS reversal compared with 0 of 12 patients treated with placebo. Using lower dose of terlipressin possibly safeguards against complications related to terlipressin. In our study, only 4 (10%) patients had sinus tachycardia, which was self-limited and did not require dose modification. This rate is much lower than reported with higher drug dose. Duration of using terlipressin is also controversial, with various trials using terlipressin from 2 to 15 days. We used terlipressin for 5 days.
Dose of terlipressin in two recently published studies was much higher than what was used in the present study. Sanyal and the Terlipressin Study Group22 evaluated the safety and efficacy of terlipressin in a multinational cohort of 112 patients with HRS type 1 from 35 centers across the United States, Germany, and Russia. The authors used a dose of 1 mg iv every 6 hr initially, then 2 mg iv every 6 hr if the serum creatinine failed to decrease by 30% by day 4 of the study, up to 14 days total. While, significantly more patients in the terlipressin group achieved HRS reversal than the placebo group (33.9% vs. 12.5%; P = 0.008), only a trend toward increased treatment success rates was observed in the terlipressin group when compared to the placebo group (25.0% vs. 12.5%; P = 0.093). Overall 6-month survival between the terlipressin and placebo groups was not significantly different (42.9% vs. 37.5%, P = 0.84). Martin-Llahi and the TAHRS (Terlipressin and Albumin for Hepatorenal Syndrome) investigators23 evaluated the safety and efficacy of terlipressin plus albumin in patients with HRS type 1 or type 2. The terlipressin was initially dosed as 1 mg iv every 4 hr, and then 2 mg iv every 4 hr if the serum creatinine failed to decrease by 25% after the first 3 days. The terlipressin plus albumin group experienced a significantly higher rate of improvement in renal function than the albumin group (43.5% vs. 8.7%, P = 0.017), and a trend toward improved 3-month survival was also documented (27% vs. 19%, P = 0.7).
In the present study, the use of terlipressin or triple therapy for 5 days provided similar efficacy. The present study was designed to assess whether triple therapy can be used for treatment in HRS and whether its efficacy will match the conventional treatment of terlipressin and albumin. Therefore, the study was designed to provide an adequate dose of therapy for a presumably adequate duration. The study was not designed to assess whether increasing duration of treatment improves therapeutic response.
The study has a number of limitations. Firstly, the sample size was estimated based on change in urine output expected, whereas the observed urine output was significantly different. While calculating sample size, we assumed an SD (of change in urine output) of 100 ml, but results showed SD of >300 ml. With this SD, calculated power of the study became 14% instead of 80%. With an SD as high as we observed, 446 cases in each arm would be required to demonstrate the noninferiority of triple therapy. Secondly, as the study was performed in a tertiary care center, referral bias for sick patients being recruited could have affected the overall results. Thirdly, it took 5 years to recruit patients, though a uniform protocol was followed in the diagnosis and management of HRS.
In conclusion, our observations based on a prospective, randomized controlled trial suggest that a combination of continuous volume expansion, subpressor doses of dopamine, and continuous low-dose furosemide infusion improves the outcome in patients with cirrhosis of the liver and HRS, and is as good as terlipressin and albumin with respect to improvement in renal parameters and survival, with considerable difference in cost. A larger study is required to look into equivalence of the results. Triple therapy, being a less expensive option, holds promise as a bridge between HRS and liver transplant in a resource poor country like India.
Contributions
Siddharth Srivastava (SS): Implementation of study design (enrollment and assignment to the therapy group), data acquisition, patient management, review of literature, and write up for the study; V. Sreenivas (VS): Generation of random allocation, analysis of data, and biostatistical inputs; Shyam Prakash (SP): Laboratory investigations; Shalimar (S): Data acquisition, analysis of data, and intellectual inputs; Hanish Sharma (HS): Data acquisition, patient management, and analysis; Bhaskar Thakur (BT): Analysis and biostatistics; Subrat K. Acharya (SKA): Conceptualization, study design, intellectual input, laboratory work, and write up.
Funding
The study was supported by the Indian Council of Medical Research – Sanction number: 5/8/726/99-ECD-I.
Conflicts of Interest
The authors have none to declare.
Ethics Approval
Ethical approval was obtained from the Ethics committee of the All India Institute of Medical Sciences, India.
References
- 1.Gines A., Escorsell A., Gines P. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105(1):229–236. doi: 10.1016/0016-5085(93)90031-7. [Epub 1993/07/01] [DOI] [PubMed] [Google Scholar]
- 2.Arroyo V., Terra C., Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46(5):935–946. doi: 10.1016/j.jhep.2007.02.001. [Epub 2007/03/30] [DOI] [PubMed] [Google Scholar]
- 3.Anderson C.L., Saad W.E., Kalagher S.D. Effect of transjugular intrahepatic portosystemic shunt placement on renal function: a 7-year, single-center experience. J Vasc Interv Radiol. 2010;21(9):1370–1376. doi: 10.1016/j.jvir.2010.05.009. [Epub 2010/08/10] [DOI] [PubMed] [Google Scholar]
- 4.Mitzner S.R., Stange J., Klammt S. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6(3):277–286. doi: 10.1002/lt.500060326. [Epub 2000/05/29] [DOI] [PubMed] [Google Scholar]
- 5.Angeli P., Volpin R., Gerunda G. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29(6):1690–1697. doi: 10.1002/hep.510290629. [Epub 1999/05/29] [DOI] [PubMed] [Google Scholar]
- 6.Singh V., Ghosh S., Singh B. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56(6):1293–1298. doi: 10.1016/j.jhep.2012.01.012. [Epub 2012/02/11] [DOI] [PubMed] [Google Scholar]
- 7.Cavallin M., Kamath P.S., Merli M. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62(2):567–574. doi: 10.1002/hep.27709. [Epub 2015/02/04] [DOI] [PubMed] [Google Scholar]
- 8.Solanki P., Chawla A., Garg R., Gupta R., Jain M., Sarin S.K. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18(2):152–156. doi: 10.1046/j.1440-1746.2003.02934.x. [Epub 2003/01/25] [DOI] [PubMed] [Google Scholar]
- 9.Uriz J., Gines P., Cardenas A. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol. 2000;33(1):43–48. doi: 10.1016/s0168-8278(00)80158-0. [Epub 2000/07/25] [DOI] [PubMed] [Google Scholar]
- 10.Moller S., Hansen E.F., Becker U., Brinch K., Henriksen J.H., Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver. 2000;20(1):51–59. doi: 10.1034/j.1600-0676.2000.020001051.x. [Epub 2000/03/22] [DOI] [PubMed] [Google Scholar]
- 11.Cazzaniga M., Salerno F., Visentin S., Cirello I., Donarini C., Cugno M. Increased flow-mediated vasodilation in cirrhotic patients with ascites: relationship with renal resistive index. Liver Int. 2008;28(10):1396–1401. doi: 10.1111/j.1478-3231.2008.01847.x. [Epub 2008/08/05] [DOI] [PubMed] [Google Scholar]
- 12.Laffi G., La Villa G., Pinzani M., Marra F., Gentilini P. Arachidonic acid derivatives and renal function in liver cirrhosis. Semin Nephrol. 1997;17(6):530–548. [Epub 1997/11/14] [PubMed] [Google Scholar]
- 13.ASMKBYDSS A. Prospective randomized controlled trial of concurrent triple therapy for hepatorenal syndrome. J Gastroenterol Hepatol. 2004;19(S7):A119. [Google Scholar]
- 14.Salerno F., Gerbes A., Gines P., Wong F., Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–1318. doi: 10.1136/gut.2006.107789. [Epub 2007/03/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasqualetti P., Festuccia V., Collacciani A., Di Lauro G., Casale R. Circadian rhythms of plasma atriopeptin, plasma renin activity and plasma aldosterone in patients with hepatorenal syndrome. Life Sci. 1997;60(4–5):289–297. doi: 10.1016/s0024-3205(96)00629-7. [Epub 1997/01/01] [DOI] [PubMed] [Google Scholar]
- 16.Badalamenti S., Graziani G., Salerno F., Ponticelli C. Hepatorenal syndrome. New perspectives in pathogenesis and treatment. Arch Internal Med. 1993;153(17):1957–1967. doi: 10.1001/archinte.153.17.1957. [Epub 1993/09/13] [DOI] [PubMed] [Google Scholar]
- 17.Leung W., Wong F. Hepatorenal syndrome: do the vasoconstrictors work? Gastroenterol Clin North Am. 2011;40(3):581–598. doi: 10.1016/j.gtc.2011.06.011. [Epub 2011/09/07] [DOI] [PubMed] [Google Scholar]
- 18.Chok K.S., Fung J.Y., Chan S.C. Outcomes of living donor liver transplantation for patients with preoperative type 1 hepatorenal syndrome and acute hepatic decompensation. Liver Transpl. 2012;18(7):779–785. doi: 10.1002/lt.23401. [Epub 2012/02/01] [DOI] [PubMed] [Google Scholar]
- 19.Restuccia T., Ortega R., Guevara M. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40(1):140–146. doi: 10.1016/j.jhep.2003.09.019. [Epub 2003/12/16] [DOI] [PubMed] [Google Scholar]
- 20.Guevara M., Gines P., Fernandez-Esparrach G. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology. 1998;27(1):35–41. doi: 10.1002/hep.510270107. [Epub 1998/01/13] [DOI] [PubMed] [Google Scholar]
- 21.Hadengue A., Gadano A., Moreau R. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29(4):565–570. doi: 10.1016/s0168-8278(98)80151-7. [Epub 1998/11/21] [DOI] [PubMed] [Google Scholar]
- 22.Boyer T.D., Sanyal A.J., Garcia-Tsao G. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55(2):315–321. doi: 10.1016/j.jhep.2010.11.020. [Epub 2010/12/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Llahi M., Guevara M., Torre A. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140(2) doi: 10.1053/j.gastro.2010.07.043. 488–496.e4 [Epub 2010/08/05] [DOI] [PubMed] [Google Scholar]