Abstract
Purpose: Radiation therapy is the standard treatment for symptomatic bone metastases. Several randomized control trials and meta-analyses have concluded a similar efficacy in pain relief when comparing single versus multiple fraction regimes. However, there continues to be reluctance to conform to published guidelines that recommend a single treatment for the palliation of painful bone metastases. The purpose of this literature review is to summarize international patterns of practice, and to determine if guidelines recommending single fraction treatment have been implemented in clinical care. Methods: A literature search was conducted in Ovid Medline, Embase, and Cochrane Central. Search words included, ‘bone metastases’, ‘radiation therapy’, ‘radiotherapy’, ‘patterns of practice’, and ‘dose fractionation’. Both prospective and retrospective studies that investigated the prescription of radiotherapy to bone metastases using actual patient databases were included. Articles were excluded if they investigated hypothetical scenarios. Results: Six hundred and thirteen results were generated from the literature search. Twenty-six articles met the inclusion criteria. Of these, 11 were Canadian, 8 were European, 6 were American, and 1 was Australian. The use of single fraction radiotherapy (SFRT) ranged from 3% to 75%, but was generally lower in American studies. Choice of fractionation depended on a variety of factors, including patient age, prognosis, site of irradiation, and physician experience. Conclusion: Despite the publication of robust randomized control trials, meta-analyses, and clinical practice guidelines recommending the use of a single treatment to palliate uncomplicated bone metastasis, SFRT is internationally underutilized.
Keywords: Bone metastases, Radiation, Pattern of practice, Dose fractionation
1. Introduction
Bone metastases are a common event in metastatic cancer and are a significant cause of morbidity that can lead to pain, hypercalcaemia, pathologic fracture, spinal cord compression, and reduction in quality of life (QOL) [1]. The prevalence in metastatic breast and prostate cancer patients is as high as 70–90% at autopsy [1], [2]. In patients with bone metastases, goals of care often surround pain management and maintenance or improvement of QOL [3]. A variety of treatment modalities are utilized in a multidisciplinary fashion in order to achieve this goal. This includes analgesia, bone modifying agents such as bisphosphonates, and radiation therapy (RT) [3], [4]. Where analgesic medications frequently result in side effects like constipation and dry mouth, radiation therapy is well tolerated with fewer side effects and provides significant pain relief in approximately 70% of patients [5]. Furthermore, RT has been shown to help prevent subsequent skeletal related events associated with bone metastases and improve overall QOL [6].
Many dose fractionation options exist in the palliation of symptomatic bone metastases, with the most common fractionation schedules being a single 8 Gy in one fraction, 20 Gy in five fractions, and 30 Gy in ten fractions [7]. Numerous randomized control trials (RCTs) have concluded that both single fraction radiation therapy (SFRT) and multiple fraction radiation therapy (MFRT) are efficacious in providing pain relief caused by uncomplicated bone metastases [4], [7]. MFRT may be indicated in the treatment of complicated bone metastases, such as those causing neuropathic pain, pathologic fractures, or spinal cord compression [8], [9]. As such, guidelines from the American Society for Radiation Oncology (ASTRO) and the American College of Radiology (ACR) recommend SFRT as the preferred treatment for uncomplicated bone metastases [10], [11].
Despite these evidence based guidelines, studies have shown that there is a reluctance to implement them into current practice [7], [12]. Many of these studies are survey-based asking physicians about fractionation schedules of choice regarding hypothetical scenarios [12], [13]. Conclusions from these studies may overestimate the prevalence of SFRT due to their survey based methodologies. Our review focussed on studies that have investigated actual patient databases with the goal being to present a more accurate assessment of international patterns of practice over the past twenty years, particularly with respect to the use of SFRT.
2. Methods
A literature search was conducted using OvidSP Medline (1946 – Week 4 2014), Embase (1947 – Week 5 2014), and the Cochrane Central Register of Controlled Trials (Dec 2013) databases. A search was also conducted in the bibliographies of chosen articles as well as in the ASTRO 2014 book of published abstracts. The search was limited to English and restricted to those published after 1994. Subject headings and keywords used included ‘bone metastases’, ‘radiation therapy’, ‘radiotherapy’, ‘patterns of practice’, and ‘dose fractionation’. Titles and abstracts were screened independently by two authors (RM, LR) to determine relevant articles to be obtained for full-text review.
Articles met inclusion criteria if they were primary research studies with either a primary or secondary goal of investigating different palliative radiation therapy dose fractionation schedules for treatment courses to bone metastases. The data must have been collected from verified patient databases. Abstracts were also included if they were deemed relevant and provided sufficient information regarding RT regime. Although the focus was patterns of practice in the past twenty years (1994–2014), articles that analyzed data beginning before this time period but continuing past 1994 were included. Exclusion criteria were articles that investigated patient preference or those that surveyed physicians using hypothetical scenarios. Full text articles were again screened independently by two authors (RM, LR). If disagreement existed, a discussion ensued until a consensus was obtained.
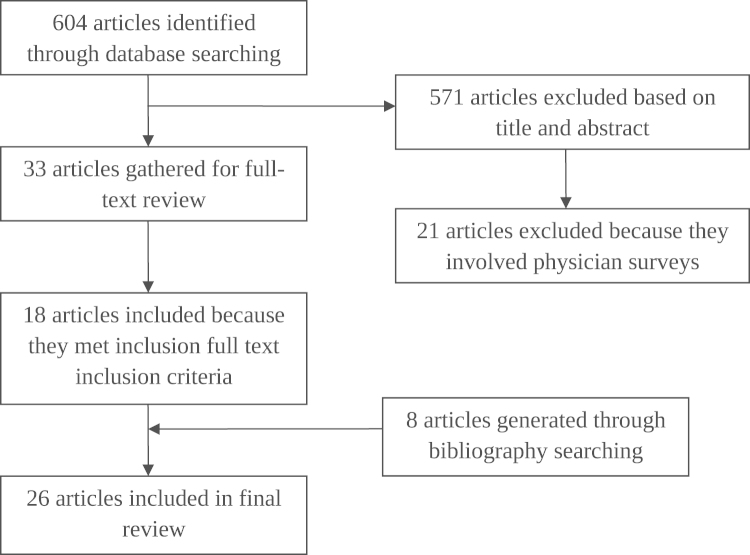
The prevalence of various fractionation schedules was extracted from each of the final articles included in the review. Other relevant information, such as significant predictive factors for the use of single fraction radiation therapy, changes in the prevalence of fractionation schedules over time, and types of bone metastases irradiated, was also included. The flow chart describing the inclusion process for the articles can be found in Fig. 1.
Fig. 1.
Flow of article inclusion and exclusion process.
3. Results
Of a total of 613 articles generated by the search, 26 met the inclusion criteria. The articles were published from 2003 to 2014: 11 were Canadian [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], 8 were European [25], [26], [27], [28], [29], [30], [31], [32], 6 were American [33], [34], [35], [36], [37], [38], and 1 was Australian [39]. The use of SFRT to treat bone metastases was widely variable among the studies examined, ranging from as low as 3.3% to as high as 75% [36], [39]. Table 1 features the dose fractionation schedules prescribed in all studies, arranged by region and study period.
Table 1.
Pattern of practice in radiation doses in the treatment of bone metastases.
| First author (year) | Region | Time period | Number of RT courses delivered | Fractionation schedule | Ref. |
|---|---|---|---|---|---|
| Kong (2007) | Canada | 1984–2001 | 44,223 RT courses to bone | 34.4% of RT courses were SFRT | [14] |
| Sutton (2006) | Canada | 1984–2004 | Patients who died of cancer between 1984–2004; 236,078 RT courses to bone delivered with palliative intent | In last 2 years of life: 30% were SFRT, 44% were five fractions, 11% were 10 fractions | [15] |
| Ashworth (2014) | Canada | 1984–2012 | 186,694 Palliative RT courses to bone | 41.3% of RT courses were SFRT | [24] |
| Danjoux (2006) | Canada | 1996–2003 | 2989 RT courses to bone | Single 8-Gy fraction in 45%, 20 Gy in five fractions in 42%, 30 Gy in 10 fractions in 4% | [16] |
| Haddad (2005) | Canada | 1998–2002 | 882 RT courses to bone | 32% of courses were SFRT | [17] |
| Bradley (2008) | Canada | 1999–2005 | 965 RT courses to bone | 65% of patients received SFRT | [18] |
| Naidoo (2011) | Canada | 2000–2010 | 17,682 Patients, 42% of which were referred for bone metastases | 20 Gy/5 fractions was most common, used to treat 59% of bone metastases SFRT used to treat bone metastases in 35% of patients | [19] |
| Wu (2010) | Canada | 2003–2005 | 1354 RT courses to bone | SFRT was prescribed in 57% of patients who went to rapid access clinic and 33% of patients who went to regular care | [20] |
| Thavarajah (2013) | Canada | 2005–2012 | 2549 Courses of RT to patients with bone metastases | 65% of RT courses were SFRT | [21] |
| Potter (2009) | Canada | 2007–2008 | 422 RT courses to bone metastases in 389 patients | Of 137 patients with uncomplicated bone pain, 48.9% were treated with SFRT | [22] |
| Olson (2014) | Canada | 2007–2011 | 16,898 Total courses of RT to 8601 patients | 49.2% SFRT; More than 10 fractions were prescribed to only 0.2% of patients | [23] |
| Foro Arnalot (2010) | Spain | 1990–2009 | 3042 Palliative treatments, 78.14% of which were to bone | 46.85% of courses were SFRT from 1990 to 1996, 49.25% were SFRT from 1999 to 2009 | [25] |
| Szostakiewicz (2004) | Poland | 1995–2002 | 1165 Patients irradiated to 1754 bone metastases | 19% of patients treated with SFRT | [26] |
| Laughsand (2013) | Norway | 1997–2007 | Patients treated with either 8 Gy/1 fraction or 30 Gy/10 fractions to bone metastases; 14,380 RT courses to bone metastases total | SFRT delivered to 31.3% of patients | [27] |
| Santacaterina (2004) | Italy | 2000 | 325 Patients treated to 458 bone metastases | 257 (79%) of patients were treated with MFRT (30 Gy/10 fractions) | [28] |
| Bhalla (2012) | United Kingdom | 2000 and 2006 | 120 Patients per year who received palliative radiation therapy to bone metastases | SFRT was prescribed in 42% of patients in 2000 and 40% in 2006 | [29] |
| Moller (2003) | Sweden | Sept–Dec 2001 | A total of 1144 irradiated sites of bone metastases | SFRT used in 37% of treatment sites | [30] |
| Williams (2006) | United Kingdom | One week, starting Sept 29, 2003 | Palliative radiotherapy given to 43% of 2498 patients (exact number for bone metastases not provided) | SFRT was the most common prescription, followed by 20 Gy in 5 fractions | [32] |
| van Oorschot (2011) | Germany | 1 month in 2008 | 62 Patients were treated to bone metastases | Of 18 patients who were treated to bone metastases with the intent of pain relief, only 2 received SFRT. Most common fractionation was 30 Gy/10 fractions for bone metastases | [31] |
| Chen (2013) | United States | 2003–2005 | 194 Patients with lung cancer who received radiation treatment to bone | 50% Received 6–10 fractions, 20% received five fractions or lower, 6% received a single fraction | [33] |
| Beriwal (2012) | United States | 2003–2010 | 7905 Sites of bone metastases treated with RT | 37.8% of patients received 1–5 fractions. Single-fraction was chosen for palliation in 13.5% of cases at academic centers, and 3.9% at community centers | [34] |
| Bekelman (2013) | United States | 2006–2009 | 3050 Patients treated with palliative RT to bone | 3.3% were treated with SFRT. When previously documented complicated bone metastases were excluded, 3.8% of 2028 patients were treated with SFRT | [36] |
| Bekelman (2014) | United States | 2006–2012 | 5160 Patients treated with palliative RT to bone | 4.0% were treated with SFRT. When previously documented complicated bone metastases were excluded, 4.1% of 4006 patients were treated with SFRT | [38] |
| Ellsworth (2014) | United States | 2007–2012 | 339 Patients whose final RT was for bone metastases | 8% Received SFRT 83% of patients were prescribed less than or equal to 10 fractions | [37] |
| Hess (2012) | United States | 2008–2009 | 207 and 213 breast and prostate cancer patients treated with palliative RT to bone | Majority of patients received at least 10 fractions | [35] |
| Holt (2010) | Australia | May–October 2005 | 77 and 207 RT courses for bone metastases at the rapid response clinic and regular care, respectively | 75% and 58% of patients were treated with SFRT to bone at the rapid response clinic and regular care, respectively | [39] |
3.1. Canada
Eleven Canadian studies that reported on dose fractionation in the treatment of bone metastases were published between 1994 and 2014, with data ranging from 1984 to 2011. The study with the earliest cohort included 44,223 irradiated bone metastases treated between 1984 and 2001 [14]. Thirty four percent of the RT courses were SFRT. The authors also reported a general increase in the prescription of SFRT over time, with 27.2% of patients treated with SFRT in 1984–1986 increasing to 35.4% of patients treated with SFRT from 1999 to 2001 [14]. A Canadian study published by Sutton et al. [15] investigated a similar cohort of irradiated bone metastases (1984–2004), but rather looked at RT schedules prescribed within the last two years of life. Of the 236,078 bone metastases irradiated, 30% were treated with SFRT. The authors did not report on specific changes in SFRT, but did mention that it was more frequently prescribed in the latter half of the time period [15]. Another recent abstract was published by Ashworth et al. [24], which updated the previous cohorts to include 97,150 patients who received 186,694 palliative RT courses to bone from 1984 to 2012. Over the total study period, SFRT was prescribed for 41.3% of RT courses. The prevalence of SFRT was found to increase from 39% in 1999–2003 to 58% in 2006, then decrease to 42% in 2009–2012.
Danjoux et al. [16] investigated a cohort of patients treated between 1996 and 2003. Of a total 2989 RT courses to bone, 45% were treated with SFRT. These authors did not comment on any change in prescription over time. Similar cohorts were investigated by Haddad et al. [17] and Bradley et al. [18], who investigated RT courses to bone delivered from 1998 to 2002 and 1999 to 2005, respectively. Haddad et al. [17] reported that an average 32% of 882 RT courses to bone were prescribed SFRT over the study period. The authors also reported a general decrease in the prescription of SFRT over time, from 37% in 1998 to 28% in 2002. A much higher rate was prescribed to patients included in the study by Bradley et al. [18], with 65% of 965 RT courses to bone treated with SFRT. Contrary to Haddad et al., Bradley et al. [18] found an increase in the prescription of SFRT, from 51% in 1999 to 66% in 2005. Of note, these authors specified that the cohort only included uncomplicated bone metastases, whereas Haddad et al. [17] did not specify the type of bone metastases treated. Wu et al. [20] investigated 1354 bone metastases irradiated between 2003 and 2005. Single fraction radiation therapy was prescribed in 57% and 33% of patients who were treated in rapid access clinics and regular clinics, respectively. These authors did not report on a change in SFRT prescription over time.
Naidoo et al. [19] investigated a group of 7426 patients referred for RT to bone metastases from 2000 to 2010. Of these patients, 35% were prescribed SFRT. Again, these authors did not specify whether there was any change in the prescription over time. Potter et al. [22] conducted a study with 422 RT to bone metastases treated from 2007 to 2008. The authors differentiated intent to treat and reported that, of 137 patients with uncomplicated bone pain, 48.9% were treated with SFRT.
The most recent cohorts investigated were those by Thavarajah et al. [21] and Olson et al. [23], from 2005 to 2012 and 2007 to 2011, respectively. Thavarajah et al. [21] included 2549 RT to bone metastases, of which 65% were prescribed SFRT. These authors stated that there was no significant change in the prescription of SFRT over the study period. Olson et al. [23] examined 16,898 total courses of RT to bone metastases. They reported that an average of 49.2% of irradiated bone metastases received SFRT and that the prescription of SFRT declined slightly over the study period.
3.2. Europe
European studies were very heterogeneous in the prescription of SFRT, with Central and Southern European countries [26], [28], [31] generally prescribing a lower proportion of SFRT and Northern and Western European countries [25], [27], [29], [30], [32] prescribing a greater proportion. A Polish study examined 1754 irradiated bone metastases from 1995 to 2002, of which 19% were treated with SFRT [26]. These authors also reported that the proportion of patients treated with SFRT was greater in 2001–2002 when compared to earlier in the study period. A study conducted in Italian cancer centers by Santacaterina et al. [28] reported a comparable prevalence of SFRT, prescribed for 21% of 458 bone metastases treated in 2000. The lowest rate of SFRT prescription was reported by German authors who conducted a one month audit of a cancer center in 2008 [31]. Of the 18 patients treated for painful bone metastases, 2 (11%) were prescribed SFRT. Both the Italian and German studies did not report on a change in prescription of SFRT due to the narrow time period examined.
Western and Northern European countries generally reported a much greater proportion of bone metastases irradiated with SFRT compared to other European countries, with rates ranging from 31% to 49%. A study conducted in Spain explored a cohort of patients treated to 2377 bone metastases from 1990 to 1996 and 1999 to 2009 [25]. SFRT was prescribed to 46.9% and 49.3% of patients, respectively. A Norwegian study investigated a similar cohort of patients treated to 14,380 bone metastases between 1997 and 2007 [27]. An average of 31.3% of RT courses delivered over this time period were SFRT. A very large increase in the proportion of SFRT prescribed was observed in this study, rising from 16.1% of patients in 1997 to 40.5% of patients in 2007. A comparable proportion of SFRT was observed in a Swedish cohort of patients, treated to 1144 bone metastases between September and December 2001 [30]. In this population, SFRT was prescribed to 37% of bone metastases. The prescription of SFRT was slightly greater in a United Kingdom study, which examined 120 patients per year between 2000 and 2006 who were treated for bone metastases [29]. SFRT was prescribed in 35% of RT courses. The authors specified that no significant change in SFRT prevalence was observed over the study period. Another study was conducted in the United Kingdom with data collected for one week, beginning September 29, 2003 [32]. Although exact data is not available, the authors state that the most common prescription in the palliation of bone metastases was a single 8 Gy, followed by 20 Gy in five fractions. The least common schedule of use was 30 Gy delivered in 10 fractions.
3.3. United States
SFRT was the least commonly used in the United States. An American study with a small cohort of patients (n=194) with a lung primary and treated to bone metastases from 2003 to 2005 reported that 6% of patients received SFRT [33]. These authors did not specify whether or not a change in the prescription of SFRT was observed. A much larger cohort of patients with over 7000 bone metastases irradiated between 2003 and 2010 was investigated by Beriwal et al. [34]. The prevalence of SFRT in this population was 3.9% and 13.5% at community and academic centers, respectively. Although the authors did not include specific numerical values, they stated that the total number of delivered fractions decreased gradually over the study period.
Bekelman et al. [36] investigated prostate cancer patients who were treated with palliative RT to bone metastases between January 2006 and December 2009. A total of 3050 patients were included, of which 3.3% were prescribed SFRT and 50.3% were prescribed greater than 10 fractions. When adjusted to exclude patients with previously complicating events (i.e., pathologic fractures, spinal cord compression, etc.), the prevalence of SFRT increased slightly to 3.8% [36]. Bekelman et al. [38] also published an abstract in 2014 that expanded on the previous study and included patients with breast, prostate, lung, kidney, colon and bladder cancer who were treated with palliative RT to bone from 2006 to 2012. A similar prevalence of SFRT was prescribed for this cohort of patients, at 4.0%. Again, when restricted to patients who experienced no prior complicating event, the prevalence increased by only 0.1% [38]. A study conducted by Hess et al. [35] investigated patients with breast and prostate cancer primaries, who were treated to bone metastases in the United States between July 2008 and December 2009. Similar to the previous American studies, these authors reported that the majority of patients received at least 10 fractions. Contrastingly, Ellsworth et al. [37] studied 339 patients who received RT for bone metastases between April 2007 and July 2012, of which the majority of patients received equal to or less than 10 fractions. The prevalence of SFRT was also approximately double that which has been reported in previous US studies (8%) [37].
3.4. Australia
Holt et al. [39] published the only Australian study investigating patterns of practice in the delivery of RT to bone metastases. These authors examined RT courses delivered to bone metastases between May and October 2005 at a rapid response clinic and regular care center. They reported the greatest prevalence of SFRT among all studies; 75% and 58% of patients treated were prescribed SFRT in the rapid response clinic (n=77) and regular care center (n=207), respectively [39].
3.5. Factors associated with SFRT
Sixteen of the 26 included studies reported on predictive factors for the use of SFRT [14], [17], [18], [21], [22], [23], [24], [26], [27], [29], [33], [34], [36], [37], [38], [39]. The most common significant predictive factors were increased age [14], [17], [18], [21], [24], [27], poor patient prognosis [14], [17], [18], [23], [24], [27], [36], [38], irradiation to non-spine locations [14], [18], [23], [24], [26], [34], and patients with primary prostate cancer [18], [21], [23], [27]. SFRT was also more likely to be prescribed to those living a greater distance from the treatment center [14], [18], [24], [27], those with a lung primary [26], [27], [29], when the prescribing physicians had greater experience [18], [21], [23], and when the treatment center was an academic as opposed to community practice [20], [34]. The significance of some factors, such as the age of patients, treatment site, and physician experience, was contradictory as they were reported in two studies to not be significant [29], [34], [37]. Table 2 provides a detailed summary of the predictive factors of SFRT found in each article.
Table 2.
Changes of SFRT and its associations.
| First author (year) | Region | Time period | Change in SFRT prescription practice over study period | Associations with SFRT | Ref. |
|---|---|---|---|---|---|
| Kong (2007) | Canada | 1984–2001 | 1984–1986: 27.2% | Increased age, poorer prognosis, non-spine locations, greater distance to cancer center | [14] |
| 1987–1992: 40.3% | |||||
| 1993–1998: 31.6% | |||||
| 1999–2001: 35.4% | |||||
| Sutton (2006) | Canada | 1984–2004 | SFRT was used more frequently in latter half of study period | NA | [15] |
| Ashworth (2014) | Canada | 1984–2012 | 1999–2003: 39% | Non-spine locations, increased age, poorer prognosis, greater distance to cancer center | [24] |
| 2006: 58% | |||||
| 2009–2012: 42% | |||||
| Haddad (2005) | Canada | 1998–2002 | 1998: 37% | Increased age, greater weight loss, poorer prognosis | [17] |
| 1990: 30% | |||||
| 2000: 43% | |||||
| 2001: 26% | |||||
| 2002: 28% | |||||
| Bradley (2008) | Canada | 1999–2005 | 51% in 1999, to 70% in 2001, to 71% in 2004 and 66% in 2005 | Increased age, prostate primary, poor performance status, greater distance from cancer center, treatment to limbs, hips, pelvis, and ribs, and increasing physician experience | [18] |
| Potter (2009) | Canada | 2007–2008 | NA | Uncomplicated bone metastases | [22] |
| Thavarajah (2013) | Canada | 2005–2012 | No significant change over the study period | Increased age, prostate cancer, patients receiving re-irradiation, physicians who were trained prior to 1990; most common reasons for MFRT included spinal cord compression, postoperative RT, and impending fracture | [21] |
| Olson (2014) | Canada | 2007–2011 | Declined from 50.5% to 48.0% | Those with hematological and prostate cancer, treatment to ribs and extremity, poor prognosis, increasing physician experience and site of training, and re-irradiation | [23] |
| Szostakiewicz (2004) | Poland | 1995–2002 | The proportion of patients treated with SFRT was greater in 2001–2002 compared to previous periods | Used most commonly for ribs, long bones, and from lung and breast cancer | [26] |
| Foro Arnalot (2010) | Spain | 1990–2009 | Increase in number of SFRT and number of fractions with 20 Gy/5 Similar instances of 30 Gy/10 | NA | [25] |
| Laughsand (2013) | Norway | 1997–2007 | Patients treated with SFRT rose from 16.1% in 1997 to 40.5% in 2007 | Lung and prostate cancer, increased age, poorer prognosis, greater living distance from treatment center | [27] |
| Bhalla (2012) | United Kingdom | 2000 and 2006 | No significant change over the study period | Age and treatment site were not significant predictors of fractionation choice; patients with lung cancer were more likely to receive SFRT | [29] |
| Chen (2013) | United States | 2003–2005 | NA | Patients treated in integrated networks received an average 3.4 fewer fractions and 4.0 Gy less | [33] |
| Beriwal (2012) | United States | 2003–2010 | Number of fractions decreased gradually over time | Academic practices were more likely to treat with fewer fractions. Treatment of spine and extremity metastatic sites were associated with greater fractions Experience of oncologist was not predictive of RT regime | [34] |
| Bekelman (2013) | United States | 2006–2009 | NA | Poorer prognosis | [36] |
| Bekelman (2014) | United States | 2006–2012 | No significant difference by year | No significant difference by diagnosis | [38] |
| Ellsworth (2014) | United States | 2007–2012 | No significant difference in fractions prescribed before and after publication of 2011 ASTRO guidelines | Irradiated site not predictive of SFRT | [37] |
| Holt (2010) | Australia | May–Oct 2005 | NA | Treatment in the rapid response clinic | [39] |
4. Discussion
The majority of advanced cancer patients will develop bone metastases at some point in their illness [1], [2]. As such, optimal management of bone metastases is central to patient care. Optimal management involves considering all risks and costs of treatment with appropriate goals of care in mind. In advanced cancer patients, these goals of care are palliative in intent and should be centered on improving pain and QOL [3].
A recent systematic review on randomized control trials investigating the efficacy of various dose fractionation schedules was published by Chow et al. in 2012 [40]. The authors identified 25 RCTs and concluded that both SFRT and MFRT were equally efficacious in providing pain relief. Retreatment rates were greater in patients treated with a single fraction; however, this may be a product of the increased likelihood for physicians to offer re-irradiation to patients who have been treated with lower overall doses [40]. Other meta-analyses [41], [42] have been previously published, concluding similar results to Chow et al. Different trials have been conducted to evaluate dose fractionation schedules in complicated bone metastases, such as those presenting with neuropathic pain, at risk of fracture, and spinal cord compression. In these circumstances, multiple fractions may have increased efficacy, especially in those with greater prognosis [8], [9], [43].
As expected, treatments of a single fraction are more cost effective compared to multiple treatments. Konski et al. [44] conducted an economic analysis on the Radiation Therapy and Oncology Group (RTOG) 97-14 trial, which investigated the efficacy of single versus multiple fractions in pain relief caused by bone metastases from breast and prostate cancer. The authors concluded that, even with the potential for greater re-treatments with SFRT, a single treatment is still more cost effective when compared to multiple. This study did not factor in patient cost of travel and lost productivity, which would further confirm SFRT as cost effective as less patient visits are required [44]. Findings were similar in a study by van den Hout et al. [45], who found that the cost of SFRT and MFRT was $2438 and $3311, respectively. These figures include potential re-treatments in both groups. When the authors included societal costs, the estimated expenses increased to $4700 for SFRT and $6453 for MFRT [45]. Again, this study clearly favours single fraction treatments as more cost effective when compared to protracted radiotherapy courses.
In light of the various published randomized control trials and meta-analyses, guidelines regarding appropriate dose fractionation schedules have been released by several cancer organizations. Cancer Care Ontario (CCO) is a predominant cancer organization in Ontario, Canada, and went through an extensive process with radiation oncologists across the province to develop a guideline for appropriate dose fractionation schedules. The ultimate recommendation of this 2004 guideline was the use of a single 8 Gy RT course to palliate symptomatic uncomplicated bone metastases [46]. The American College of Radiology (ACR) most recently in 2009, on recommendation of an expert panel, stated that a single fraction was just as efficacious as multiple fractions in the relief of uncomplicated bone pain. Furthermore, they concluded that SFRT is likely more desirable in patients with poor prognosis and that SFRT is more cost effective when compared to MFRT [11]. A similar evidence based guideline was released in 2011 by ASTRO and reinforced the recommendation that a single 8 Gy be used to palliate uncomplicated bone metastases [10]. Overall, although there has been some increase in the prescription of SFRT seen in this review, the guidelines and meta-analyses have not had a significant impact on international patterns of practice to date.
Our review found that American radiation oncologists demonstrated the most unwillingness to adhere to guidelines, with the lowest prescription of SFRT compared to all other countries worldwide. The prevalence of SFRT was as low as 3.3% in this country. European and Canadian physicians also demonstrated reluctance, although generally less-so than their American counterparts. Single fraction RT courses were prescribed to approximately 20–50% of patients within these regions. The single study published in Australia in 2005 showed that Australian radiation oncologists, particularly those in the rapid access clinic, were the most consistent with recommendations and had the highest prevalence of SFRT prescription (up to 75%).
It has been speculated that the geographical variation among radiotherapy prescription is a function of the respective healthcare systems within each region. If physicians receive greater reimbursement for multiple fractions compared to a single fraction, they will likely have greater willingness to ignore guidelines and prescribe a longer course. Lievens et al. [47] confirmed this hypothesis in a study published in 2000. The authors investigated reimbursement methods and their relation to the delivery of different radiotherapy dose fractionation schedules in Western Europe. Payment methods included case payment, where a department is reimbursed per patient or per case, and fee-for-service, where each individual service performed (i.e., planning, fixation, treatment) is reimbursed separately [47]. The authors found that countries that employ a case payment method of remuneration (Spain, Netherlands, and the United Kingdom) also more frequently prescribe SFRT. In contrast, countries such as Germany that reimburse physicians using a fee-for-service method have a greater prescription of MFRT [47]. These results are consistent with those found in our review. The privately funded health care system most prevalent in the United States might also explain the increased prescription of MFRT; radiation oncologists will be paid more by private insurance companies for prescribing protracted courses of treatment.
The international under-prescription of SFRT may also be related to inadequate knowledge translation and communication; if physicians are unaware of the most recent evidence for various dose fractionation schedules, patients will not be receiving those recommended treatments [48]. In this way, it is crucial that the dissemination of knowledge is a priority among all physicians and cancer organizations. It is only through this that all patients will be able to receive the best standard of care.
Many of the studies included in this literature review are limited in that they do not differentiate the fractionation schedules or bone metastases by the intent of treatment. This poses difficulty because SFRT is not indicated in all cases of bone metastases, but rather only in the majority of uncomplicated cases. In patients with neuropathic pain, pathologic fractures, and spinal cord compression for example, more protracted courses of radiotherapy may be more effective. Therefore, the studies likely do not represent the true amount of physicians who are prescribing to evidence-based guidelines; regions where the prescription of SFRT is low may just be those with a greater incidence of patients presenting with complicated bone metastases. Future trials should incorporate intent to treat as an evaluable factor in RT. This will allow for the adequate assessment of clinician adherence to guidelines.
Furthermore, this review focuses only on conventional radiation therapy. With the advent of new technology, guidelines and practice must be continuously revisited and adjusted appropriately. Stereotactic body radiation therapy (SBRT), which allows for the delivery of radical doses of radiation to oligometastatic disease with great precision [49], is particularly useful for patients with prolonged survival, radioresistant tumors, or those receiving re-irradiation [50], [51], [52]. Guidelines for practice for SBRT are much different than those for conventional radiotherapy. Future studies investigating patterns of practice in this setting would be beneficial.
5. Conclusions
Despite the publication of many randomized control trials, meta-analyses, and evidence-based practice guidelines, all recommending the prescription of SFRT for pain relief of uncomplicated bone metastases, there remains an international unwillingness to conform to this practice. This may be related to the method of remuneration for radiation oncologists or perhaps a lack of informed decision making on part of the physician. Knowledge dissemination is a critical component of research to ensure that patients are receiving the most recent best standard of care. Future trials should ensure that a differentiation is made between uncomplicated and complicated bone metastases, as these classifications have significant impact on dose fractionation prescribed.
Conflict of Interest
The authors report no conflicts of interest.
Acknowledgments
We thank the generous support of the Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
References
- 1.Coleman R.E., Rubens R.D. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubendorf L., Schopfer A., Wagner U., Sauter G., Moch H., Willi N. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Cai B., Nickman N.A., Gaffney D.K. The role of palliative external beam radiation therapy in boney metastases pain management. J Pain Palliat Care Pharmacother. 2013;27(1):28–34. doi: 10.3109/15360288.2012.757267. [DOI] [PubMed] [Google Scholar]
- 4.Smith H.S. Painful osseous metastases. Pain Physician. 2011;14(4):E373–E403. [PubMed] [Google Scholar]
- 5.Steenland E., Leer J.W., van Houwelingen H., Post W.J., van den Hout W.B., Kievit J. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 6.Falkmer U., Jarhult J., Wersall P., Cavallin-Stahl E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol. 2003;42(5–6):620–633. doi: 10.1080/02841860310014895. [DOI] [PubMed] [Google Scholar]
- 7.Culleton S., Kwok S., Chow E. Radiotherapy for pain. Clin Oncol (R Coll Radiol) 2011;23(6):399–406. doi: 10.1016/j.clon.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Roos D.E., Turner S.L., O’Brien P.C., Smith J.G., Spry N.A., Burmeister B.H. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05) Radiother Oncol. 2005;75(1):54–63. doi: 10.1016/j.radonc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Loblaw D.A., Mitera G., Ford M., Laperriere N.J. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84(2):312–317. doi: 10.1016/j.ijrobp.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Lutz S., Berk L., Chang E., Chow E., Hahn C., Hoskin P. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Janjan N., Lutz S.T., Bedwinek J.M., Hartsell W.F., Ng A., Pieters R.S., Jr. Therapeutic guidelines for the treatment of bone metastasis: a report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. J Palliat Med. 2009;12(5):417–426. doi: 10.1089/jpm.2009.9633. [DOI] [PubMed] [Google Scholar]
- 12.Fairchild A., Barnes E., Ghosh S., Ben-Josef E., Roos D., Hartsell W. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501–1510. doi: 10.1016/j.ijrobp.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 13.Popovic M., den Hartogh M., Zhang L., Poon M., Lam H., Bedard G. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111(1):11–17. doi: 10.1016/j.radonc.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Kong W., Zhang-Salomons J., Hanna T.P., Mackillop W.J. A population-based study of the fractionation of palliative radiotherapy for bone metastasis in Ontario. Int J Radiat Oncol Biol Phys. 2007;69(4):1209–1217. doi: 10.1016/j.ijrobp.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Sutton D.S., Kong W., Ding K., Mackillop W.J. The use of palliative radiotherapy for bone metastasis. Radiother Oncol. 2010;97(3):548–553. doi: 10.1016/j.radonc.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Danjoux C., Chow E., Drossos A., Holden L., Hayter C., Tsao M. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38–43. doi: 10.1007/s00520-005-0822-7. [DOI] [PubMed] [Google Scholar]
- 17.Haddad P., Wong R.K., Pond G.R., Soban F., Williams D., McLean M. Factors influencing the use of single vs multiple fractions of palliative radiotherapy for bone metastases: a 5-year review. Clin Oncol (R Coll Radiol) 2005;17(6):430–434. doi: 10.1016/j.clon.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Bradley N.M., Husted J., Sey M.S., Sinclair E., Li K.K., Husain A.F. Did the pattern of practice in the prescription of palliative radiotherapy for the treatment of uncomplicated bone metastases change between 1999 and 2005 at the rapid response radiotherapy program? Clin Oncol (R Coll Radiol) 2008;20(5):327–336. doi: 10.1016/j.clon.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naidoo N., Zurawel-Balaura L., Cheung A., Lau M., Garraway C., Panzarella T. The Role of Specialized Palliative Radiotherapy (RT) Programs: a decade of experience in a Tertiary Oncology Center. Int J Radiat Oncol. 2011;81(2):S112. [Google Scholar]
- 20.Wu J.S., Kerba M., Wong R.K., Mckimmon E., Eigl B., Hagen N.A. Patterns of practice in palliative radiotherapy for painful bone metastases: impact of a regional rapid access clinic on access to care. Int J Radiat Oncol Biol Phys. 2010;78(2):533–538. doi: 10.1016/j.ijrobp.2009.07.1716. [DOI] [PubMed] [Google Scholar]
- 21.Thavarajah N., Zhang L., Wong K., Bedard G., Wong E., Tsao M. Patterns of practice in the prescription of palliative radiotherapy for the treatment of bone metastases at the Rapid Response Radiotherapy Program between 2005 and 2012. Curr Oncol. 2013;20(5):e396–e405. doi: 10.3747/co.20.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter A., Bezjak A., Levin W., McLean M., Zurawel-Balaura L., Wong R. single fraction radiotherapy for bone metastases: reasons for physician non-compliance with evidence-based practice guidelines. Support Care Cancer. 2009;17(7):972–973. [Google Scholar]
- 23.Olson R.A., Tiwana M.S., Barnes M., Kiraly A., Beecham K., Miller S. Use of single- versus multiple-fraction palliative radiation therapy for bone metastases: population-based analysis of 16,898 courses in a Canadian province. Int J Radiat Oncol Biol Phys. 2014;89(5):1092–1099. doi: 10.1016/j.ijrobp.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 24.Ashworth A., Kong W., Chow E.L., Mackillop W. The fractionation of palliative radiation therapy for bone metastases in Ontario: Do guidelines guide practice? Int J Radiat Oncol. 2014;90(1S):S63–S64. doi: 10.1016/j.ijrobp.2015.07.2291. [DOI] [PubMed] [Google Scholar]
- 25.Foro Arnalot P., Jiminez R., Sanz X., Rodriguez De Dios N., Reig A., Membrive Conejo I. Palliative radiotherapy evolution in the last 20 years at the Institut d’Oncologia Radioterapica, Parc de Salut MAR. Radiother Oncol. 2010;96:s375–s376. [Google Scholar]
- 26.Szostakiewicz B., Dziadziuszko R., Welnicka-Jaskiewicz M., Jassem J. Palliative irradiation of bone metastases: patterns of care with focus on single fraction treatment. Rep Pract Oncol Radiother. 2004;9:9–12. [Google Scholar]
- 27.Laugsand T.S., Kaasa S., Romundstad P., Johannesen T.B., Lund J.A. Radiotherapy for bone metastases: practice in Norway 1997–2007. A national registry-based study. Acta Oncol. 2013;52(6):1129–1136. doi: 10.3109/0284186X.2012.747697. [DOI] [PubMed] [Google Scholar]
- 28.Santacaterina A., Pontoriero A., Pergolizzi S., Delia P. External beam irradiation in the palliation of bone metastases: a practice analysis among Sicilian Departments of Radiation Oncology. Tumori. 2004;90:86–90. doi: 10.1177/030089160409000118. [DOI] [PubMed] [Google Scholar]
- 29.Bhalla N., Wong H., Ibrahim A., Green J.A. Palliative radiotherapy for bone metastases: assessment of factors influencing dose-fractionation schedules at a UK cancer centre. J Radiother Pract. 2013;12(3):208–217. [Google Scholar]
- 30.Moller T.R., Brorsson B., Ceberg J., Frodin J.E., Lindholm C., Nylen U. A prospective survey of radiotherapy practice 2001 in Sweden. Acta Oncol. 2003;42(5–6):387–410. doi: 10.1080/02841860310011131. [DOI] [PubMed] [Google Scholar]
- 31.van Oorschot B., Schuler M., Simon A., Schleicher U., Geinitz H. Patterns of care and course of symptoms in palliative radiotherapy: a multicenter pilot study analysis. Strahlenther Onkol. 2011;187(8):461–466. doi: 10.1007/s00066-011-2231-9. [DOI] [PubMed] [Google Scholar]
- 32.Williams M.V., James N.D., Summers E.T., Barrett A., Ash D.V., Sub-Committe Audit. National survey of radiotherapy fractionation practice in 2003. Clin Oncol (R Coll Radiol) 2006;18(1):3–14. doi: 10.1016/j.clon.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Chen A.B., Cronin A., Weeks J.C., Chrischilles E.A., Malin J., Hayman J.A. Palliative radiation therapy practice in patients with metastatic non-small-cell lung cancer: a Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) Study. J Clin Oncol. 2013;31(5):558–564. doi: 10.1200/JCO.2012.43.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beriwal S., Rajagopalan M.S., Flickinger J.C., Rakfal S.M., Rodgers E., Heron D.E. How effective are clinical pathways with and without online peer-review? An analysis of bone metastases pathway in a large, integrated National Cancer Institute-Designated Comprehensive Cancer Center Network. Int J Radiat Oncol Biol Phys. 2012;83(4):1246–1251. doi: 10.1016/j.ijrobp.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 35.Hess G., Barlev A., Chung K., Hill J.W., Fonseca E. Cost of palliative radiation to the bone for patients with bone metastases secondary to breast or prostate cancer. Radiat Oncol. 2012;7:168–174. doi: 10.1186/1748-717X-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bekelman J.E., Epstein A.J., Emanuel E.J. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. J Am Med Assoc. 2013;310(14):1501–1502. doi: 10.1001/jama.2013.277081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellsworth S.G., Alcorn S.R., Hales R.K., McNutt T.R., DeWeese T.L., Smith T.J. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89(5):1100–1105. doi: 10.1016/j.ijrobp.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekelman J.E., Sylwestrzak G., Agiro A., Barron J., Andrew E., Malin J. Use of single fraction radiation therapy for bone metastases, 2006–2012. Int J Radiat Oncol. 2014;90(1S):S62–S63. [Google Scholar]
- 39.Holt T.R., Yau V.K. Innovative program for palliative radiotherapy in Australia. J Med Imaging Radiat Oncol. 2010;54(1):76–81. doi: 10.1111/j.1754-9485.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 40.Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24(2):112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Sze W.M., Shelley M., Held I., Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy – a systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;2(2):CD004721. doi: 10.1002/14651858.CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J.S., Wong R., Johnston M., Bezjak A., Whelan T., Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55(3):594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 43.Townsend P.W., Smalley S.R., Cozad S.C., Rosenthal H.G., Hassanein R.E. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31(1):43–49. doi: 10.1016/0360-3016(94)E0310-G. [DOI] [PubMed] [Google Scholar]
- 44.Konski A., James J., Hartsell W., Leibenhaut M.H., Janjan N., Curran W. Economic analysis of radiation therapy oncology group 97-14: multiple versus single fraction radiation treatment of patients with bone metastases. Am J Clin Oncol. 2009;32(4):423–428. doi: 10.1097/COC.0b013e31818da9f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van den Hout W.B., van der Linden Y.M., Steenland E., Wiggenraad R.G., Kievit J., de Haes H. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222–229. doi: 10.1093/jnci/95.3.222. [DOI] [PubMed] [Google Scholar]
- 46.Wu JS, Wong R, Johnston M, Bezjak A, Whelan T, Laetsch N, et al. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases. Toronto (ON): Cancer Care Ontario; 2003 Mar 14. Program in Evidence-based Care Practice Guideline: 13-2 [Archived 2013].
- 47.Lievens Y., Van den Bogaert W., Rijnders A., Kutcher G., Kesteloot K. Palliative radiotherapy practice within Western European countries: impact of the radiotherapy financing system? Radiother Oncol. 2000;56(3):289–295. doi: 10.1016/s0167-8140(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 48.Nieder C., Pawinski A., Dalhaug A. Continuous controversy about radiation oncologists’ choice of treatment regimens for bone metastases: should we blame doctors, cancer-related features, or design of previous clinical studies? Radiat Oncol. 2013;8:85–92. doi: 10.1186/1748-717X-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo S.S., Fakiris A.J., Chang E.L., Mayr N.A., Wang J.Z., Papiez L. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7(1):44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 50.Owen D., Laack N.N., Mayo C.S., Garces Y.I., Park S.S., Bauer H.J. Outcomes and toxicities of stereotactic body radiation therapy for non-spine bone oligometastases. Pract Radiat Oncol. 2014;4(2):e143–e149. doi: 10.1016/j.prro.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stinauer M.A., Kavanagh B.D., Schefter T.E., Gonzalez R., Flaig T., Lewis K. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34–41. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masucci G.L., Yu E., Ma L., Chang E.L., Letourneau D., Lo S. Stereotactic body radiotherapy is an effective treatment in reirradiating spinal metastases: current status and practical considerations for safe practice. Expert Rev Anticancer Ther. 2011;11(12):1923–1933. doi: 10.1586/era.11.169. [DOI] [PubMed] [Google Scholar]