Abstract
Neuroblastoma (NB), which arises from embryonic neural crest cells, is the most common extra-cranial solid tumor of childhood. Approximately half of NB patients manifest bone metastasis accompanied with bone pain, fractures and bone marrow failure, leading to disturbed quality of life and poor survival. To study the mechanism of bone metastasis of NB, we established an animal model in which intracardiac inoculation of the SK-N-AS human NB cells in nude mice developed osteolytic bone metastases with increased osteoclastogenesis. SK-N-AS cells induced the expression of receptor activator of NF-κB ligand and osteoclastogenesis in mouse bone marrow cells in the co-culture. SK-N-AS cells expressed COX-2 mRNA and produced substantial amounts of prostaglandin E2 (PGE2). In contrast, the SK-N-DZ and SK-N-FI human NB cells failed to develop bone metastases, induce osteoclastogenesis, express COX-2 mRNA and produce PGE2. Immunohistochemical examination of SK-N-AS bone metastasis and subcutaneous tumor showed strong expression of COX-2. The selective COX-2 inhibitor NS-398 inhibited PGE2 production and suppressed bone metastases with reduced osteoclastogenesis. NS-398 also inhibited subcutaneous SK-N-AS tumor development with decreased angiogenesis and vascular endothelial growth factor-A expression. Of interest, metastasis to the adrenal gland, a preferential site for NB development, was also diminished by NS-398. Our results suggest that COX2/PGE2 axis plays a critical role in the pathophysiology of osteolytic bone metastases and tumor development of the SK-NS-AS human NB. Inhibition of angiogenesis by suppressing COX-2/PGE2 may be an effective therapeutic approach for children with NB.
Keywords: Osteoclasts, Receptor activator of NF-κB ligand, Vascular endothelial growth factor-A, Microvessel density, Adrenal gland, COX-2 inhibitor
1. Introduction
Neuroblastoma (NB), which arises from embryonal neural crest cells, is the most common extra-cranial solid tumor of childhood and accounts for more than 15% of all child cancer death [1], [2]. Patients with NB frequently manifest disseminated disease at first visit and prognosis of these patients is poor with 3-year survival rate less than 40% [1], [2]. It has been well-known that bone is one of the most common target sites of NB spread. More than 60% of advanced NB patients manifest bone metastases that are radiologically osteolytic or a mixture of osteolytic and osteoblastic type [3], [4]. Children with bone metastases suffer from complications including intolerable bone pain, pathological fractures and bone marrow failure. Currently-available anti-NB therapies are not satisfactorily effective at treating bone metastases and these complications. Accordingly, these children show miserable clinical courses and poor survival rate [5], [6]. Therefore, control of bone metastases is an important goal in the management of infants with NB [7] and effective therapeutic interventions designed based on the understanding of the pathophysiology of bone metastasis of NB have been awaited. However, the mechanism underlying the preferential metastasis of NB to bone remains poorly understood.
Recent studies suggest that the interactions between cancer cells and bone microenvironment are critical to the pathophysiology of bone metastasis [8], [9]. Bone provides a fertile soil for metastatic cancer cells by releasing growth factors such as insulin-like growth factors (IGFs) [10] and transforming growth factor β (TGFβ) [11] as a consequence of osteoclastic bone resorption during bone remodeling. Cancer cells stimulated by these bone-derived growth factors consequently produce increased levels of osteoclast-stimulating factors such as parathyroid hormone-related protein (PTH-rP) [11] and prostaglandin E2 (PGE2) [12], which in turn further promotes osteoclastic bone resorption, establishing a vicious cycle between bone-resorbing osteoclasts and metastatic cancer cells [8], [9]. Contribution of similar vicious cycle between NB and bone microenvironment [13] mediated by receptor activator of NF-κB ligand (RANKL) [14], [15] and IGF-1 [16] to the development and progression of NB bone metastasis has been also suggested.
In the present study, we developed an animal model of NB bone metastasis to advance our understanding of the mechanism of bone metastasis. We showed that an inoculation of the SK-N-AS human NB cells into the left heart ventricle of nude mice caused osteolytic bone metastases with increased osteoclastogenesis. SK-N-AS cells expressed COX-2 mRNA and produced substantial amounts of PGE2. The selective COX-2 inhibitor NS-398 inhibited PGE2 production and osteolytic bone metastases with reduced osteoclastic bone resorption and subcutaneous SK-N-AS tumor enlargement with reduced angiogenesis. These results suggest that COX-2/PGE2 system plays a critical role in the pathophysiology of osteolytic bone metastases and tumor progression of NB. Inhibition of COX-2/PGE2 is a potential therapeutic intervention for bone metastases in children with NB.
2. Materials and methods
2.1. Reagents and antibodies
Recombinant human osteoprotegerin (OPG) was purchased from BioSource International (Camarillo, CA). 1,25-dihydroxyvitamin D3 (1,25D3) was from BIOMOL International (Plymouth Meeting, PA). Dimethylsulfoxide (DMSO), PGE2, and indomethacin were obtained from Sigma Chemical Co. (St. Louis, MO). NS-398, a selective COX-2 inhibitor, was purchased from Cayman Chemical Co. (Ann Arbor, MI). For in vitro use, indomethacin and NS-398 were reconstituted in DMSO and diluted with culture media. The final concentration of DMSO in the culture media was less than 0.1%.
Polyclonal rabbit anti-vascular endothelial growth factor-A (VEGF-A) and polyclonal rabbit anti-proliferation cell nuclear antigen (PCNA) were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit anti-COX-2 and polyclonal rabbit anti-factor VIII were from Zymed Laboratories, Inc. (South San Francisco, CA).
2.2. Human neuroblastoma cells
Human NB cell lines, SK-N-DZ, SK-N-AS, SK-N-FI, were obtained from American Type Culture Collection (Rockville, MD). All cells were maintained in Dulbecco׳s modified Eagle minimal essential medium (DMEM, Life Technologies, Inc., Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Life Technologies, Inc.) at 37 °C under 5% CO2 in air.
2.3. Tumorigenicity of human NB cell lines
Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science center at San Antonio and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Animals were kept in our specific pathogen-free animal facilities. SK-N-DZ, SK-N-AS or SK-N-FI human NB cells (2.5×106) were suspended in 0.1 ml of phosphate buffered saline (PBS) and inoculated subcutaneously into the right flank of 4-week-old BALB/c-nu/nu (nude) mice (National Cancer Institute, Frederick, MD) (Day 0). Tumor volume was determined weekly based on the calculation suggested by the National Cancer Institute formula: Volume (cm3)=(L cm×I2 cm)/2, in which L is the largest diameter, and I is the smallest diameter of the tumor. Body weight (BW) of mice bearing with or without NB tumor was measured weekly until sacrifice. Subcutaneous (sc) tumors were excised and their wet weight was determined. A portion of the tumor tissue was fixed in 10% formalin for subsequent histological examinations and remaining tissues were snap-frozen in liquid nitrogen and stored at −80 °C for RNA extraction.
2.4. Bone metastases of human NB cell lines
Bone metastasis of SK-N-DZ, SK-N-AS or SK-N-FI human NB cells was studied using a well-characterized animal model we described previously [17]. Briefly, NB cells (5×105) were suspended in 0.1 ml of PBS and injected into the left heart ventricle of 4-week-old nude mice with 27-gauge needle under anesthesia with pentobarbital (0.05 mg/g body weight) (Day 0). Development of bone metastases was monitored weekly by X-ray. At 5 weeks, mice were euthanized and blood was collected for Ca2+ measurement and all bones and soft tissues were harvested and fixed in formalin for subsequent histological analyses.
2.5. Radiological monitoring of bone metastases
Animals were anesthetized deeply, then x-rayed in a prone position against the film (22×27 cm2 X-Omat AR, Eastman Kodak Co., Rochester, NY) and exposed with X-rays at 35 KVP for 6 s using a Faxitron radiographic inspection unit (43855A; Faxitron X-ray Corporation, Buffalo Grove, IL) as described [17]. The radiographs were scanned and the area of osteolytic lesions was measured using NIH Image 1.62 and the results were expressed in square millimeters. The number of bone metastases was also counted in bilateral femurs and tibiae.
2.6. Ca2+ measurement
Ca2+ concentrations were measured in whole blood using a Ciba Corning 634 ISE Ca2+/pH analyzer (Corning Medical and Scientific, Medfield, MA) and adjusted using the internal algorithm of the instrument to pH 7.4 as described previously [18].
2.7. Administration of a selective COX-2 inhibitor NS-398
Three days after the tumor cell inoculation, mice were divided into two groups, one group of mice received daily intraperitoneal (ip) injections of 100 µl vehicle (50% v/v DMSO) and another group of mice received NS-398 (15 mg/kg body weight) [12] until the end of the experiments.
2.8. Histology
Soft tissues and bones were removed from mice at the time of sacrifice, fixed in 10% buffered formalin for 2 days. Bones were further decalcified in 14% EDTA solution for 2 weeks at room temperature with gentle stirring. Subsequently, they were embedded in paraffin and stained with hematoxylin and eosin (H&E). Tartrate-resistant acid phosphatase (TRAP) staining was also performed according to the procedure described [12].
2.9. Immunohistochemistry
Paraffin sections (5 µm) were deparaffinized in xylene and rehydrated through graded alcohol [100%, 95%, 80% ethanol/double distilled H2O (v/v)], and then rehydrated in PBS (pH 7.4). Sections were microwaved in 10 mM trisodium citrate (pH 6.0) for 4 min for antigen retrieval and then endogenous peroxidase activity was blocked by incubation in 3% H2O2/methanol for 30 min at room temperature. After a 30-min treatment with 10% normal goat serum diluted in PBS, sections were incubated overnight at 4 °C with a 1:100–200 dilution of anti-VEGF-A, anti-PCNA, anti-COX-2 or anti-factor VIII antibody. Antibodies were diluted with PBS containing 1% normal goat serum. Normal rabbit IgG was used as negative control. The specimens were then rinsed and incubated with biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for 30 min at room temperature. Next, they were stained according to the avidin–biotin-peroxide complex (ABC) method using a commercial kit (Vectastain Elite ABC; Vector Laboratories). Subsequently, the specimens were counterstained with hematoxylin (VEGF-A, COX-2, and factor-VIII).
2.10. Co-cultures of human NB Cells and mouse bone marrow cells
Mouse bone marrow cells (BMCs) and spleen cells were isolated from 4- to 8-week-old male C57BL mice (Harlan, Indianapolis, IN) as described [19], [20]. Briefly, femurs and tibiae were excised aseptically, cut off both ends. The marrow cavity of femurs and tibiae was flushed with α-minimum essential medium (αMEM, Life Technologies, Inc.) and then BMCs were collected. The collected BMCs (5×105 cells/48-well for TRAP stain, 4×106 cells/6-well for RNA extraction) were co-cultured with NB cells (0.5×104 cells/48-well for TRAP stain, 4×104 cells/6-well for RNA extraction) in αMEM containing 10% FCS in 48-well plates (0.2 ml/well) or 6-well plates (1.2 ml/well) for 6 days. As a positive control, BMCs were cultured in the presence of 10−8 M 1,25D3.
Spleen cells were obtained from splenic tissues aseptically collected from mice. NB cells (0.5×104 cells/well in a 48-well plate) were co-cultured with spleen cells (5×105 cells/well in a 48-well plate) in the presence or absence of bone marrow stromal cells (5×105 cells/well) for 8 days. The cultures were fed every 3 days by replacing a half of the spent medium with fresh medium. At the end of cultures, conditioned media were harvested and the cells adhering to the well surface were stained for TRAP using commercially available kits (Leukocyte acid phosphatase A kit, Sigma) or extracted RNA. TRAP-positive multinucleated cells (TRAP (+) MNCs) containing three or more nuclei per cell were counted as osteoclast-like cells (OC).
2.11. Prostaglandin E2 (PGE2) assay
Culture media were harvested at the end of experiments and frozen at −80 °C. Concentrations of PGE2 in the culture media were determined using an EIA kit (Cayman Chemical Co.) according the protocol provided by the manufacture. Measurements were made in triplicate wells.
2.12. Reverse transcribed polymerase chain reaction (RT-PCR)
Total RNA was isolated using the TRI reagent (Sigma Chemical Co.) according to the manufacture׳s protocol. First-strand complementary DNAs (cDNAs) were synthesized from total RNA (1 µg) in 20 µl of reaction mixture containing oligo (dT) primer and superscript reverse transcriptase (Invitrogen, Carlsbad, CA). One microliter of this reaction was amplified using PCR primer sets for mouse RANKL [21], mouse OPG [21], mouse glyceraldehydes 3-phosphate dehydrogenase (GAPDH) [21], human COX-1 [22], human COX-2 [23], human VEGF-A [24], and human GAPDH [21] according to the manufacture׳s instruction (Invitrogen). The PCR products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining under UV light.
2.13. Western analysis
After overnight serum starvation, cells were washed three times with ice-cold PBS, and lyzed in the lysis buffer [20 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM EGTA, 1.5 mM MgCI2, 10% glycerol, 1% Triton X-100, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF, 0.1 mM sodium orthovanadate]. The cell lysates were boiled in SDS sample buffer containing 0.5 M beta-mercaptoethanol, separated by SDS-PAGE, transferred to nitrocellulose membranes and immunoblotted with anti-COX-2 antibody as described [12]. Separated proteins were visualized with horseradish peroxidase coupled with protein A (Kirkegaard & Perry laboratories, Inc., Gaithersburg, MD) with enhancement by chemiluminescence using ECL Western Blotting Detection Reagent (Amersham Bioscience Corp., Piscataway, NJ).
2.14. Statistical analysis
Results are expressed as the mean±SE. Data were analyzed by either one-factor ANOVA followed by Turkey-Kramer post-hoc test, Mann-Whitney U test, or unpaired Student׳s t test. P values of<0.05 were considered significant. All statistical analyses were carried out using Statview ver 5 (SAS Institute Inc., Berkeley, CA).
3. Results
3.1. Establishment of an animal model of bone metastasis of Human NB
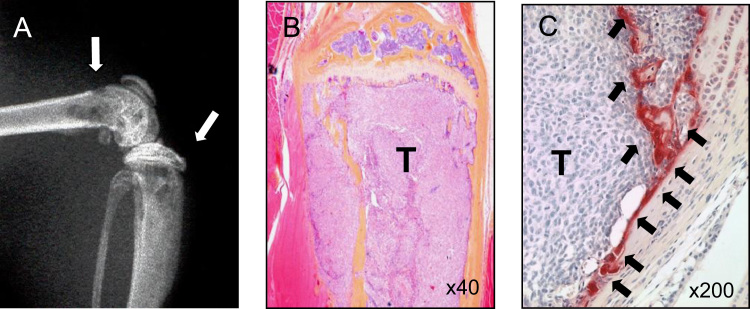
Three human NB cell lines (SK-N-DZ, SK-N-AS, and SK-N-FI) developed tumors after sc inoculation. However, these sc tumors failed to develop radiologically-detectable bone metastases. Histological examination revealed no metastases to soft tissue organs and bones even at 12 weeks. Accordingly, we inoculated these NB cells into the left ventricle of the heart, which is a well-established technique to develop bone metastases [17]. Among three NB cell lines tested, only SK-N-AS cells reproducibly developed discernible osteolytic lesions in the limbs on X-ray over a period of 5–6 weeks (Fig. 1A). Some mice (~40%) showed hind limb paralysis with metastases to spine and spinal cord. Mice inoculated with SK-N-AS cells also demonstrated metastases to adrenal gland, kidney and lung but not liver, with a decrease in body weight, an average of 15.5% loss compared to control mice. Histological examination demonstrated extensive tumor colonization in the bone marrow cavity and destruction of trabecular bone (Fig. 1B). Increased numbers of TRAP (+) osteoclasts were observed along the endosteal bone surface (Fig. 1C). Blood Ca2+ concentration remained normal in mice received sc or heart injection of SK-N-AS cells (sc injection: 1.28±0.06 mM, heart-injection: 1.26±0.05 mM, control; 1.29±0.03 mM, n=5). SK-N-A-DZ and SK-N-FI cells rarely developed bone metastases following heart inoculation.
Fig. 1.
Bone metastasis of the SK-N-AS human NB cell line. (A) Radiograph of hindlimbs of mouse bearing SK-N-AS tumors 40 days after intracardiac inoculation of 5×105 SK-N-AS tumor cells. Osteolytic bone metastases are indicated by arrows. (B) Histological pictures of bone metastases stained with H&E (40×). The bone marrow cavity was occupied by SK-N-AS tumor and trabecular bones were completely destroyed. (C) Histological pictures of bone metastases stained with TRAP (200×). Histological sections were made using proximal tibial midsection of mice bearing SK-N-AS cells. SK-N-AS tumor cells (T) extensively colonized bone marrow cavity with increased numbers of OCs (arrows) along the endosteal surface of bone.
3.2. TRAP (+) MNC formation in the co-cultures of NB cells with mouse BMCs
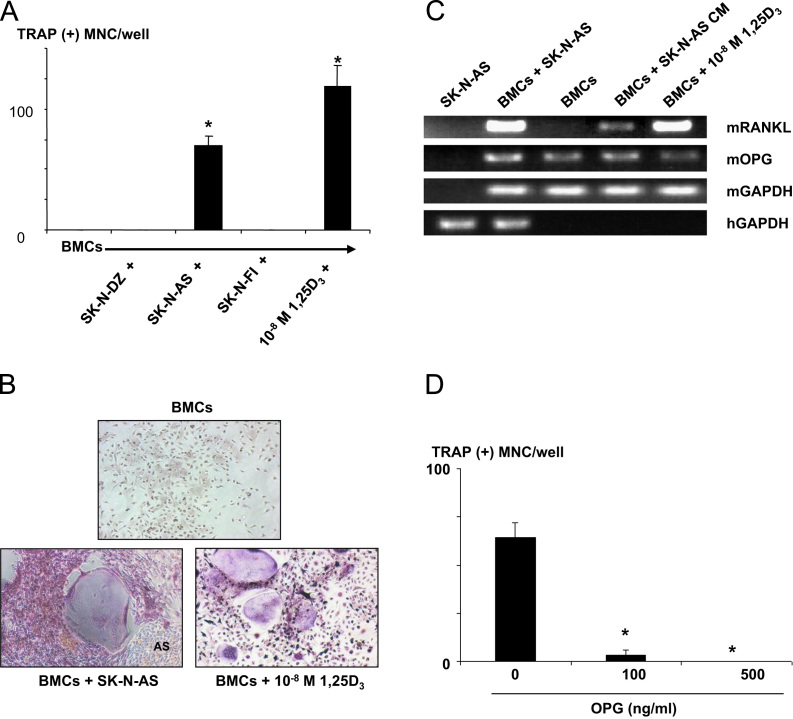
To determine the mechanism of increased osteoclasts in the bone metastases of SK-N-AS cells, we examined whether SK-N-AS cells supported osteoclast formation in the co-cultures with mouse BMCs. We found that TRAP (+) MNCs formed in the co-cultures of SK-N-AS cells and mouse BMCs (Fig. 2A and B). These TRAP (+) MNCs cells formed resorption pits on dentine slices (data not shown). In contrast, co-cultures of mouse BMCs with SK-N-DZ or SK-N-FI cells that failed to develop bone metastases showed no induction of osteoclastogenesis (Fig. 2A). Since receptor activator of NF-κB ligand (RANKL) expressed in marrow stromal cells plays a central role in osteoclastogenesis [25], we next examined whether SK-N-AS cells induced RANKL expression in mouse BMCs. RT-PCR analysis showed an induction of RANKL mRNA expression in mouse BMCs co-cultured with SK-N-AS cells (Fig. 2C). The conditioned medium (CM) harvested from SK-N-AS cells also induced mRANKL mRNA expression in mouse BMCs (Fig. 2C). Consistent with up-regulation of mRANKL in the co-cultures of mouse BMCs and SK-N-AS cells, osteoprotegerin (OPG), a natural antagonist of RANKL [25], reduced TRAP (+) MNC formation in the co-cultures in a dose-dependent manner (Fig. 2D). SK-N-AS cells did not change mRNA expression of OPG in mouse BMCs. These results collectively suggest that a soluble factor produced by SK-N-MS cells induces RANKL expression in mouse BMCs, leading to an induction of osteoclastogenesis.
Fig. 2.
TRAP (+) MNCs formation in the co-cultures of human NB cells and mouse BMCs. Mouse BMCs (5×105 cells/well) were co-cultured with or without (−) SK-N-DZ, SK-N-AS or SK-N-FI human NB cells (0.5×104 cells/well) without addition of osteoclastogenic cytokine for 6 days. 1,25D3 (10−8 M) served as positive control. (A) Formation of TRAP (+) MNCs. Data are expressed as mean±SE (n=3). There was no TRAP (+) MNC formation in the cultures of BMCs alone and co-cultures of BMCs and SK-N-DZ or SK-N-FI cells in the absence of osteoclastogenic cytokine. In contrast, the co-cultures of BMCs and SK-N-AS cells produced TRAP (+) MNC in the absence of osteoclastogenic cytokine to an equivalent level to that of 1,25D3 (10−8 M). *Significantly different from SK-N-DZ and SK-N-FI (p<0.01). (B) Representative pictures of TRAP (+) MNCs formed in the co-cultures of BMCs and SK-N-AS cells. (C) RT-PCR analysis of RANKL expression in mouse BMCs. Mouse BMCs (4×106 cells/well) were co-cultured with or without SK-N-AS cells (4×104 cells/well) or cultured in the presence of the conditioned medium from SK-N-AS cells (SK-N-AS CM, 20% v/v) for 6 days. Mouse BMCs co-cultured with SK-N-AS cells or treated with SK-N-AS CM showed increased mRANKL mRNA expression. mRANKL was undetectable in untreated BMCs. 1,25D3 served as positive control. (D) Effects of OPG on TRAP (+) MNC formation in the co-cultures of SK-N-AS cells and mouse BMCs. Mouse BMCs (5×105 cells/well) were co-cultured with SK-N-AS cells (0.5×104 cells/well) in the presence of OPG for 6 days and TRAP (+) MNCs were counted. OPG inhibited TRAP formation in mouse BM cells induced by SK-N-AS cells in a dose-dependent manner. Data are expressed as mean±SE (n=3). *Significantly different from untreated group (p<0.01).
3.3. Expression of COX-2 and PGE2 production in SK-N-AS cells
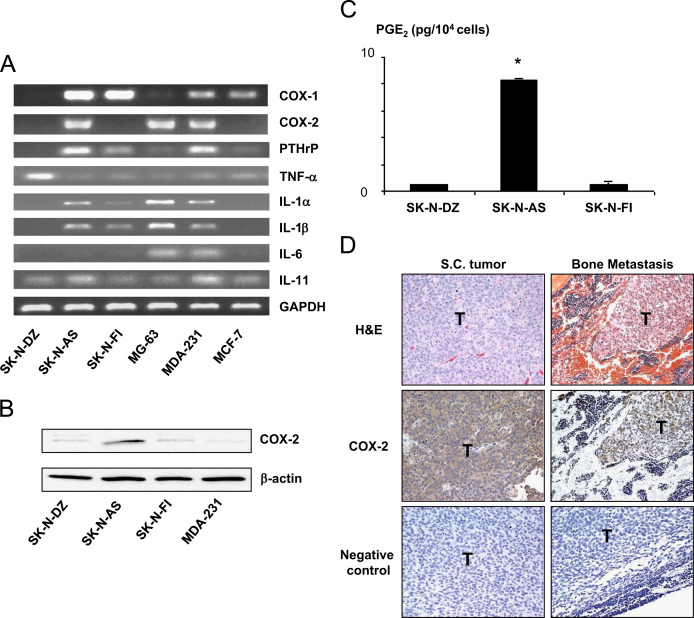
To search for a soluble factor responsible for the induction of osteoclastogenesis, we examined mRNA expression of several known osteoclastogenic cytokines in SK-N-AS cells by RT-PCR. As shown in Fig. 3A, SK-N-AS cells expressed mRNA of parathyroid hormone-related protein (PTH-rP), interleukin-1 α (IL-1α), IL-1β and IL-11 but not tumor necrosis factor α (TNFα) and IL-6. Of note, COX-2 mRNA expression was observed only in SK-N-AS cells in three human NB cell lines studied here. COX-2 is a rate-limiting enzyme in PG synthesis [26]. PGs, especially PGE2, are known as potent osteoclastogenic factors [12]. Western analysis also showed that only SK-N-AS cells expressed COX-2 protein (Fig. 3B). Furthermore, EIA measurement demonstrated that SK-N-AS cells produced larger amounts of PGE2 than SK-N-DZ and SK-N-FI cells (Fig. 3C). Immunohistochemical examination showed that sc tumor and bone metastases of SK-N-AS were positive for COX-2 (Fig. 3D). Taken together, these results suggest that PGE2 produced by SK-N-AS cells plays a role in increased osteoclastogenesis in the co-cultures.
Fig. 3.
(A) RT-PCR analysis of the expression of osteoclastogenic cytokine mRNA in SK-N-AS, SK-N-DZ, SK-N-FI human NB cells and a variety of human tumors. MG-63: human osteosarcoma cell line; MDA-231: estrogen-independent human breast cancer cell line; MCF-7: estrogen-dependent human breast cancer cell line. Note that COX-2 was expressed in SK-N-AS cells but not SK-N-DZ and SK-N-FI cells. (B) Western analysis of COX-2 expression in SK-N-AS, SK-N-DZ, SK-N-FI human NB cells. SK-N-AS cells showed strong COX-2 expression. (C): PGE2 production in SK-N-AS, SK-N-DZ, SK-N-FI human NB cells in culture. Confluent cells were cultured in serum-free medium for 48 h and CMs were harvested and determined PGE2 concentrations using an EIA kit as described in the text. Data were normalized for cell number. Data are expressed as mean±SE (n=3). *Significantly greater than SK-N-DZ and SK-N-FI cultures (p<0.01). (D): COX-2 expression in SK-N-AS tumor in vivo. Paraffin-embedded specimens from sc tumors and bone metastases of SK-N-AS were stained with H&E and anti-COX-2 antibody as described in the text. COX-2 expression was detected in both sc tumor and bone metastasis. T: tumor; Negative control: no primary antibody. Original magnification, 200×.
3.4. Effect of indomethacin and NS-398 on TRAP (+) MNC formation in co-cultures
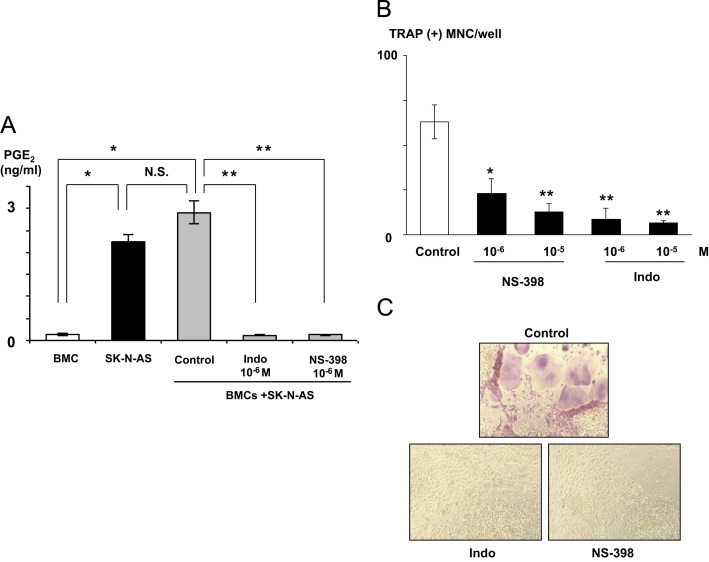
To verify the role of COX-2/PGE2 in the TRAP (+) MNC formation in the co-cultures, the effects of a non-selective COX inhibitor indomethacin and a selective COX-2 inhibitor NS-398 were examined. Both agents inhibited PGE2 production (Fig. 4A) and TRAP (+) MNC formation (Fig. 4B and C) in the co-cultures. Addition of 10−6 M PGE2 to the NS-398-treated co-cultures restored the TRAP (+) MNC formation, suggesting that the effects of NS-398 were not due to toxicity (data not shown).
Fig. 4.
(A) Effects of COX inhibitors on PGE2 production in the co-cultures of mouse BMCs and SK-N-AS cells. Indomethacin (Indo) and NS-398 significantly suppressed PGE2 production in the co-cultures. Mouse BMCs (5×105 cells/well) were co-cultured with SK-N-AS cells (0.5×104 cells/well) in the absence or presence of indomethacin (10−6 M) or NS-398 (10−6 M) for 6 days. At the end of the experiments, CM were harvested and measured for PGE2 concentrations (A). Remaining adherent cells were then stained for TRAP (B). (B) Effects of COX inhibitors on TRAP (+) MNC formation in the co-cultures of mouse BMCs and SK-N-AS cells. Indomethacin and NS-398 inhibited PGE2 production and TRAP (+) MNC formation in the co-cultures. Data are expressed as mean±SE (n=3). *Significantly different from BMC (p<0.01). **Significantly different from control (p<0.01). (C) Representative pictures of TRAP (+) MNC formation in the co-cultures of mouse BMCs and SK-N-AS cells in the absence (top) or presence of indomethacin (bottom left) or NS-398 (bottom right).
3.5. Effects of NS-398 on SK-N-AS bone metastasis
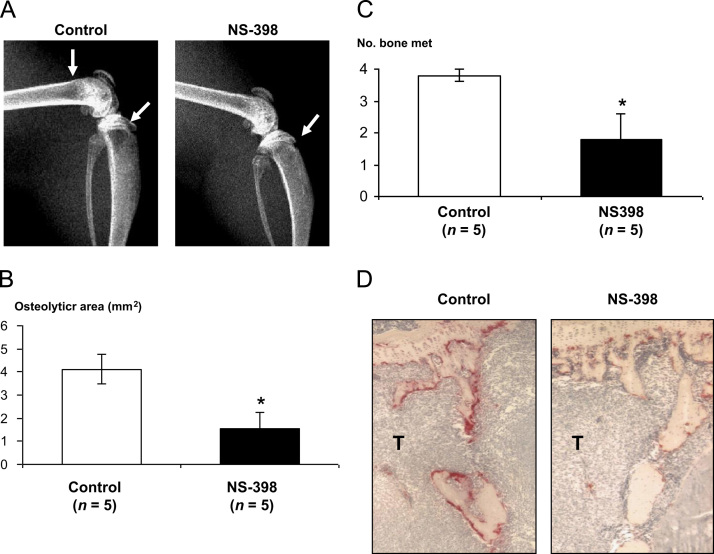
Next, we examined whether COX-2 played a role in bone metastasis. As shown in Fig. 7A, control mice developed extensive osteolytic bone metastases 5 weeks after intracardiac inoculation of SK-N-AS cell inoculation. Treatment with NS-398 (15 mg/kg body weight) markedly reduced osteolytic lesions on radiographs (Figs. 5A and B; 4.12±0.65 mm2 (untreated) vs 1.53±0.71 mm2 (NS-398); p<0.05). The number of bone metastases also significantly reduced in NS-398-treated mice compared to untreated mice (Fig. 5C; 3.8±0.2 (untreated) vs 1.8±0.8 (NS-398); p<0.05). Histological examination revealed that NS-398 markedly reduced the number of OCs compared with untreated group (Fig. 5D). NS-398 also significantly prevented body weight loss in tumor-bearing mice (17.72±0.82 g (untreated) vs 21.04 ±0.82 g (NS-398); p<0.05).
Fig. 7.
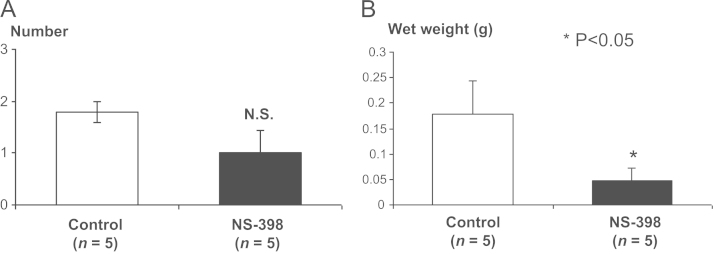
Effects of NS-398 on SK-N-AS adrenal gland metastasis. Mice inoculated with SK-N-AS cells (5×105 cells) via the left cardiac ventricle were treated as described in Fig. 7. Five weeks after tumor inoculation, adrenal glands with metastases were excised, wet weight was measured and examined for the number of metastatic foci under dissecting microscope. SN-N-AS metastases were verified by histological examination. (A) Number of adrenal gland metastases. Data are expressed as mean±SE (n=5). (B) Wet weight of adrenal gland with metastases. Data are expressed as mean±SE (n=5). *Significantly different from control (p<0.05).
Fig. 5.
Effects of NS-398 on SK-N-AS bone metastasis. Mice inoculated with SK-N-AS cells (5×105 cells) via the left cardiac ventricle were treated with either vehicle (control) or NS-398 (15 mg/kg. ip). Five weeks after tumor inoculation, radiographs were taken and bones were harvested for histological analyses. (A) Representative radiographs of hindlimbs of mice. Osteolytic lesions are indicated by arrows. (B) Quantitative measurement of the area of the osteolytic lesions on radiographs as assessed by computerized image analysis. MNS-398 significantly decreased osteolytic area on radiographs. Data are expressed as mean±SE (n=5). *Significantly different from control (p<0.05). (C): Number of osteolytic lesions in hindlimbs of mice on radiographs. Data are expressed as mean±SE (n=5). *Significantly different from control (p<0.05). (D): TRAP staining of the midtibial metaphysis of representative mice treated with either vehicle (control) or NS-398. In mice treated with NS-398, there were less TRAP (+) OCs along trabecular bone compared to control mice. T: tumor.
3.6. Effect of NS-398 on subcutaneous SK-N-AS tumor formation and angiogenesis
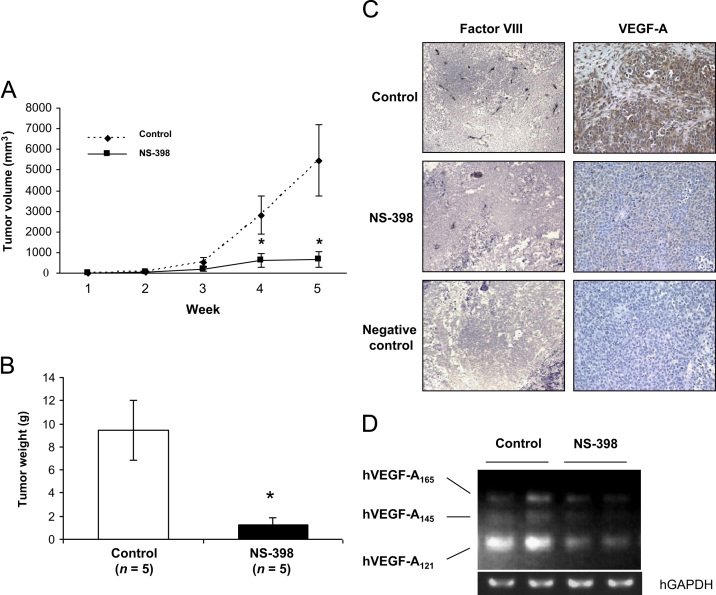
We next investigated the effects of NS-398 on subcutaneous SK-N-AS tumor formation. Daily treatment with NS-398 (15 mg/kg body weight) significantly inhibited tumor enlargement as determined by volume (Fig. 6A). Average tumor wet weight at sacrifice was 9.44±2.60 g in untreated mice and 1.22±0.69 g in NS-398-treated mice (Fig. 6B). SK-N-AS cell proliferation in the subcutaneous tumor determined by immunohistochemical staining for PCNA was not apparently different between untreated and NS-398-treated tumors (data not shown). In addition, NS-398 had no effects on the cell proliferation of SK-N-AS cells in culture at concentrations as high as 200 μM assessed by MTT assay (data not shown). These results suggest that the inhibition of subcutaneous SK-N-AS tumor formation by NS-398 is not due to direct inhibition of SK-N-AS cell proliferation.
Fig. 6.
Effects of NS-398 on subcutaneous SK-N-AS tumor development. Three days after sc inoculation of SK-N-AS cells (2.5×106 cells), mice received daily injections of NS-398 (15 mg/kg, ip) or vehicle (control) for the following 5 weeks (n=5 for each group). (A): Development of sc SK-N-AS tumors. Data are expressed as mean±SE (n=5). *Significantly different from control (p<0.05). (B) Tumor wet weight at 5 weeks after sc inoculation. Treatment with NS-398 significantly inhibited tumor wet weight. Data are expressed as mean±SE (n=5). *Significantly different from control (p<0.05). (C) Immunohistochemical examination of subcutaneous SK-N-AS tumors. Tumor sections were stained with anti-factor VIII antibody and anti-VEGF-A antibody as described in Materials and Methods. Tumors from NS-398-treated mice showed a decrease in microvessel density (factor VIII staining) and reduced intensity of VEGF-A staining compared to control. Negative control: no primary antibody. Original magnification, x50 (factor VIII); x200 (VEGF-A). (D) Effects of NS-398 on VEGF-A mRNA expression in SK-N-AS tumor in vivo. One µg of total RNA from sc tumors was subjected to RT-PCR analysis in duplicate. NS-398 down-regulated hVEGF-A121 mRNA.
To investigate the mechanism by which NS-398 reduced tumor development independent of cell proliferation, we examined the effects of NS-398 on tumor-associated angiogenesis. Vascularization was identified by staining with an anti-factor VIII antibody. Representative sections from untreated and NS-398-treated tumors are shown in Fig. 6C. Blood vessels in the tumors of untreated mice showed intensive staining of factor VIII, indicating well-developed vascular networks. In contrast, there were few blood vessels in the tumors of NS-398-treated mice. To determine whether the inhibitory effects of NS-398 on the angiogenesis was due to the suppression of VEGF, a powerful stimulator of angiogenesis, the expression of VEGF-A was studied by immunohistochemistry. VEGF-A expression was reduced in NS-398-treated mice compared with untreated mice (Fig. 6C). RT-PCR displayed that NS-398 inhibited the expression of VEGF-A mRNA, in particular VEGF-A121 isoform in SK-N-AS tumor (Fig. 6D). We also found that SK-N-AS cells expressed PGE2 receptors, EP1, EP2, EP3 and EP4 and PGE2 increased VEGF mRNA expression by RT-PCR in SK-N-AS cells (data not shown). These results suggest that PGE2 is an autocrine stimulator of angiogenesis via up-regulation of VEGF-A expression in SK-N-AS cells.
3.7. Effects of NS-398 on SK-N-AS adrenal gland metastasis
Since NB tumor arises in adrenal gland [1], [2] and adrenal gland is one of the preferential sites of metastasis in the heart injection model [27], we next examined the effects of NS-398 on adrenal gland metastases of SK-N-AS cells following intracardiac inoculation. Although not statistically significant, NS-398 decreased the number of adrenal gland metastases (1.8±0.2 (untreated) vs 1.0±0.4 (NS-398)) on histological sections (Fig. 7A), while organ weight of adrenal gland including metastatic SK-N-AS tumors in NS-398-treated mice was significantly lesser than untreated mice (0.178±0.064 g (untreated) vs 0.0482±0.023 g (NS-398); p<0.05) (Fig. 7B). These results suggest that NS-398 inhibited metastases not only to bone but also adrenal glands. Histological examination of gastric mucosa and liver showed no toxic effects of NS-398 in mice during the experiments.
3.8. Contribution of PTH-rP to SK-N-AS bone metastasis
PTH-rP is a potent osteoclastogenic factor and has long been implicated in breast cancer metastasis to bone [8], [9]. SK-N-AS cells express PTH-rP mRNA. However, the levels of PTH-rP protein produced by SK-N-AS cells in culture were low compared with the MDA-MB-231 human breast cancer cells that cause extensive bone metastases (basal level: 0.51±0.05 pmol/105 SK-N-AS cells vs 1.46±0.14 pmol/105 MDA-MB-231 cells). TGFβ, which is a potent stimulator of PTH-rP production [8], [9], did not increase PTH-rP production in SK-N-AS cells in culture (PTH-rP production in the presence of 10 ng/ml TGFβ: 0.75±0.04 pmol/105 SK-N-AS cells vs 3.84±0.29 pmol/105 MDA-MB-231 cells). Furthermore, SK-N-FI cells that had no capacity to develop bone metastases following intracardiac inoculation also expressed PTH-rP mRNA by RT-PCR. Therefore, it is unlikely that PTH-rP contributes to the osteolytic bone metastases of SK-N-AS NB.
4. Discussion
In the present study, we have shown that, of the three human NB cell lines examined, only the SK-N-AS human NB cell line reproducibly developed radiologically and histologically discernible osteolytic bone metastases following intracardiac inoculation in nude mice. Histological examination demonstrated that there were numerous TRAP-positive osteoclasts present along the endosteal surface of eroded bone in the SK-N-AS bone metastases. In the co-cultures with mouse BMCs, SK-N-AS cells induced TRAP (+) MNC formation in the absence of osteoclastogenic factor. The induction was RANKL-dependent, since mRANKL mRNA expression was induced in mouse BMCs and TRAP (+) MNC formation was decreased by OPG in the co-culture with SK-NAS cells. On the other hand, SK-N-DZ and SK-N-FI cells that failed to develop bone metastases did not induce TRAP (+) MNC formation in the co-cultures with mouse BMCs. These results suggest that SK-N-AS cells possess the capacity to cause osteolytic bone metastases accompanied with RANKL-dependent osteoclastogenesis. Subsequently, we attempted to understand this capacity at molecular levels. The result that SK-N-AS CM increased TRAP (+) MNC formation in mouse BMCs suggests that SK-N-AS cells produce a soluble osteoclastogenic factor. We found that SK-N-AS cells strongly expressed COX-2 and produced substantial levels of PGE2, while non-bone metastatic SK-N-DZ and SK-N-FI cells did not express COX-2 and produced little PGE2. Furthermore, the selective COX-2 inhibitor NS-398 significantly reduced bone metastases and osteoclastogenesis in vivo and in vitro. Taken together, these experimental data suggest that COX-2 expression and consequent PGE2 production are responsible for causing bone metastases with increased osteoclastogenesis in SK-N-AS human NB cells. Consistent with our results, it was reported that elevated COX-2 expression and PGE2 production and induction of apoptosis and inhibition of tumor growth in vivo by the nonsteroidal anti-inflammatory drugs in NB [28], [29]. Thus, we show here an establishment of a reproducible animal model of osteolytic bone metastases of human NB in which COX-2/PGE2 plays a critical role. We believe that this model leads us to deepen our understanding of the mechanism of bone metastasis of NB.
The animal model described here is also expected to lead us to design mechanism-based therapeutic interventions for bone metastases of NB. In this context, it is notable that the selective COX-2 inhibitor NS-398, which is known to selectively inhibit COX-2 without affecting COX-1 activity [30], inhibited bone metastases of SK-N-AS cells. Consistent with the results described here, we reported that NS-398 significantly reduced osteolytic bone metastases of MDA-MB-231 human breast cancer cells [12]. Moreover, Sabino et al. [31] described that NS-398 not only decreased 2472 sarcoma colonization in bone and bone destruction but also bone pain associated with tumor growth, suggesting an additional potential beneficial effect of COX-2 inhibitors for NB treatment. Bone pain is one of the most common and devastating complications in bone metastases in children with NB [32]. Another notable finding in our study is that NS-398 inhibited not only bone metastases but also adrenal metastases. Adrenal gland is a preferential site for NB to initiate and expand, which leads to death [1], [2]. Thus, selective COX-2 inhibitors may have beneficial effects on not only quality of life but survival in infants with NB. Clinical studies also reported that the selective COX-2 inhibitor celecoxib prevented the occurrence of colorectal adenomas [33] and showed some therapeutic effects in patients with breast [34], head and neck [35], cervical [36], non-small cell lung [37], colorectal [38] and ovarian cancer [39]. Of note, a recent clinical study described that oral celecoxib prevented the progression of neuroblastoma [40]. Further experimental and clinical studies are needed to verify the beneficial effects of selective COX-2 inhibitors for the treatment of infants with NB. The NB model described here would provide with a suitable preclinical model to conduct these studies.
Since the selective COX-2 inhibitor NS-398 did not inhibit the proliferation of SK-N-AS cells in vivo and in vitro, we reasoned that the agent suppressed tumor-associated angiogenesis. Angiogenesis is essential for tumor cells to initiate, grow, survive, invade and spread [41]. VEGF, a specific mitogen for neovascularization, is a major factor in the promotion of tumor angiogenesis and growth of human cancers [42]. The importance of VEGF expression associated with angiogenesis in tumor behavior is also suggested in NB [43]. Several recent experimental studies have reported that COX-2 inhibitors inhibit tumor angiogenesis through down-regulation of VEGF expression, thereby suppressing tumor growth in breast [44], colorectal [45] and pancreatic cancer [46]. Furthermore, it has been shown that celecoxib combined with irinotecan inhibits the growth of neuroblastoma xenografts through induction of apoptosis and suppression of angiogenesis with decreased VEGF expression [47]. Consistent with these previous studies, we found that NS-398 decreased vascularization and VEGF-A expression in subcutaneous SK-N-AS tumors. COX-2/PGE2 is a well-known stimulator of angiogenesis [44], [45], [46]. Collectively, these results suggest that the inhibition by NS-398 of subcutaneous tumor growth and bone metastases of SK-N-AS NB is due to the suppression of VEGF and angiogenesis.
Based on the pathophysiology bone metastasis [8], [9], inhibition of osteoclastic bone resorption is a mechanism-based treatment for bone metastases regardless of cancer type. Bisphosphonates (BPs), which are potent and specific inhibitors of osteoclastic bone resorption, have been widely used for the treatment of bone metastases [48]. An experimental animal study reported that the BP ibandronate inhibited osteolytic bone lesions developed after intra-femoral injection of NB cells [49]. However, caution is required for the use of BPs for the treatment of bone metastases of NB children, since administration of BP may have adverse effects on skeletal growth in growing infants [50]. In this context, COX-2 inhibitors could be an alternative agent in the management of bone metastases in infants with NB with less adverse effects on skeletal growth.
In summary, we have established an animal model of human NB bone metastasis in which COX-2/PGE2 plays a critical role. We provide pre-clinical data supporting the use of COX-2 inhibitors in the treatment of bone metastases in NB.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgment
This study was supported by NIH Grant RO1 CA103034-01 (TY) and grants-in-aid 11557136 (TY), 17014058 (TY) and 21st Century Center of Excellence Program (12137205, TY) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Brodeur G.M. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Maris J.M. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuBois S.G., Kalika Y., Lukens J.N., Brodeur G.M., Seeger R.C., Atkinson J.B., Haase G.M., Black C.T., Perez C., Shimada H., Gerbing R., Stram D.O., Matthay K.K. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Granchi D., Corrias M.V., Garaventa A., Baglìo S.R., Cangemi G., Carlini B., Paolucci P., Giunti A., Baldini N. Neuroblastoma and bone metastases: clinical significance and prognostic value of Dickkopf 1 plasma levels. Bone. 2011;48:152–159. doi: 10.1016/j.bone.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Matthay K.K., Villablanca J.G., Seeger R.C., Stram D.O., Harris R.E., Ramsay N.K., Swift P., Shimada H., Black C.T., Brodeur G.M., Gerbing R.B., Reynolds C.P. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children׳s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 6.Hero B., Simon T., Horz S., Berthold F. Metastatic neuroblastoma in infancy: what does the pattern of metastases contribute to prognosis? Med Pediatr Oncol. 2000;35:683–687. doi: 10.1002/1096-911x(20001201)35:6<683::aid-mpo43>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Ladenstein R., Philip T., Lasset C., Hartmann O., Garaventa A., Pinkerton R., Michon J., Pritchard J., Klingebiel T., Kremens B., Pearson A., Coze C., Paolucci P., Frappaz D., Gadner H., Chauvin F. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–965. doi: 10.1200/JCO.1998.16.3.953. [DOI] [PubMed] [Google Scholar]
- 8.Yoneda T., Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 9.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraga T., Myoui A., Hashimoto N., Sasaki A., Hata K., Morita Y., Yoshikawa H., Rosen C.J., Mundy G.R., Yoneda T. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Res. 2012;72:4238–4249. doi: 10.1158/0008-5472.CAN-11-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin J.J., Selander K., Chirgwin J.M., Dallas M., Grubbs B.G., Wieser R., Massagué J., Mundy G.R., Guise T.A. TGFβ signaling blockade inhibits PTH-rP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiraga T., Myoui A., Choi M.E., Yoshikawa H., Yoneda T. Stimulation of cyclooxygenase-2 expression by bone-derived transforming growth factor β enhances bone metastases in breast cancer. Cancer Res. 2006;66:2067–2073. doi: 10.1158/0008-5472.CAN-05-2012. [DOI] [PubMed] [Google Scholar]
- 13.Sohara Y., Shimada H., DeClerck Y.A. Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer Lett. 2005;228:203–209. doi: 10.1016/j.canlet.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 14.Michigami T., Ihara-Watanabe M., Yamazaki M., Ozono K. Receptor activator of nuclear factor kappaB ligand (RANKL) is a key molecule of osteoclast formation for bone metastasis in a newly developed model of human neuroblastoma. Cancer Res. 2001;61:1637–1644. [PubMed] [Google Scholar]
- 15.Granchi D., Garaventa A., Amato I., Paolucci P., Baldini N. Plasma levels of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in patients with neuroblastoma. Int J Cancer. 2006;119:146–151. doi: 10.1002/ijc.21783. [DOI] [PubMed] [Google Scholar]
- 16.van Golen C.M., Schwab T.S., Kim B., Soules M.E., Su Oh. S., Fung K., van Golen K.L., Feldman E.L. Insulin-like growth factor-I receptor expression regulates neuroblastoma metastasis to bone. Cancer Res. 2006;66:6570–6578. doi: 10.1158/0008-5472.CAN-05-1448. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A., Boyce B.F., Story B., Wright K.R., Chapman M., Boyce R., Mundy G.R., Yoneda T. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995;55:3551–3557. [PubMed] [Google Scholar]
- 18.Yoneda T., Lowe C., Lee C.H., Gutierrez G., Niewolna M., Williams P.J., Izbicka E., Uehara Y., Mundy G.R. Herbimycin A a pp60c-src tyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J Clin Invest. 1993;91:2791–2795. doi: 10.1172/JCI116521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi N., Yamana H., Yoshiki S., Roodman G.D., Mundy G.R., Jones S.J., Boyde A., Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988;122:1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- 20.Udagawa N., Takahashi N., Akatsu T., Sasaki T., Yamaguchi A., Kodama H., Martin T.J., Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 21.Thomas R.J., Guise T.A., Yin J.J., Elliott J., Horwood N.J., Martin T.J., Gillespie M.T. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 22.Souza R.F., Shewmake K., Beer D.G., Cryer B., Spechler S.J. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]
- 23.Ohshiba T., Miyaura C., Ito A. Role of prostaglandin E produced by osteoblasts in osteolysis due to bone metastasis. Biochem Biophys Res Commun. 2003;300:957–964. doi: 10.1016/s0006-291x(02)02937-6. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Majid R.M., Marshall J.S. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 25.Lacey D.L., Boyle W.J., Simonet W.S., Kostenuik P.J., Dougall W.C., Sullivan J.K., San Martin J., Dansey R. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 26.Menter D.G., Schilsky R.L., DuBois R.N. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin Cancer Res. 2010;16:1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michigami T., Hiraga T., Williams P.J., Niewolna M., Nishimura R., Mundy G.R., Yoneda T. The effect of the bisphosphonate ibandronate on breast cancer metastasis to visceral organs. Breast Cancer Res Treat. 2002;75:249–258. doi: 10.1023/a:1019905111666. [DOI] [PubMed] [Google Scholar]
- 28.Johnsen J.I., Lindskog M., Ponthan F., Pettersen I., Elfman L., Orrego A., Sveinbjörnsson B., Kogner P. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210–7215. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- 29.Rasmuson A., Kock A., Fuskevåg O.M., Kruspig B., Simón-Santamaría J., Gogvadze V., Johnsen J.I., Kogner P., Sveinbjörnsson B. Autocrine prostaglandin E2 signaling promotes tumor cell survival and proliferation in childhood neuroblastoma. PLoS One. 2012;7:e29331. doi: 10.1371/journal.pone.0029331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano M., Denda N., Matsumoto M., Kawamura M., Kawakubo Y., Hatanaka K., Hiramoto Y., Sato Y., Noshiro M., Harada Y. Interaction between cyclooxygenase (COX)-1- and COX-2-products modulates COX-2 expression in the late phase of acute inflammation. Eur J Pharmacol. 2007;559:210–218. doi: 10.1016/j.ejphar.2006.11.080. [DOI] [PubMed] [Google Scholar]
- 31.Sabino M.A., Ghilardi J.R., Jongen J.L., Keyser C.P., Luger N.M., Mach D.B., Peters C.M., Rogers S.D., Schwei M.J., de Felipe C., Mantyh P.W. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002;62:7343–7349. [PubMed] [Google Scholar]
- 32.Paulino A.C. Palliative radiotherapy in children with neuroblastoma. Pediatr Hematol Oncol. 2000;20:111–117. doi: 10.1080/0880010390158702. [DOI] [PubMed] [Google Scholar]
- 33.Arber N., Eagle C.J., Spicak J., Rácz I., Dite P., Hajer J., Zavoral M., Lechuga M.J., Gerletti P., Tang J., Rosenstein R.B., Macdonald K., Bhadra P., Fowler R., Wittes J., Zauber A.G., Solomon S.D., Levin B., PreSAP Trial Investigators Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 34.Perroud H.A., Rico M.J., Alasino C.M., Queralt F., Mainetti L.E., Pezzotto S.M., Rozados V.R., Scharovsky O.G. Safety and therapeutic effect of metronomic chemotherapy with cyclophosphamide and celecoxib in advanced breast cancer patients. Future Oncol. 2013;9:451–462. doi: 10.2217/fon.12.196. [DOI] [PubMed] [Google Scholar]
- 35.Shin D.M., Zhang H., Saba N.F., Chen A.Y., Nannapaneni S., Amin A.R., Müller S., Lewis M., Sica G., Kono S., Brandes J.C., Grist W.J., Moreno-Williams R., Beitler J.J., Thomas S.M., Chen Z., Shin H.J., Grandis J.R., Khuri F.R., Chen Z.G. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: preclinical and clinical studies. Clin Cancer Res. 2013;19:1244–1256. doi: 10.1158/1078-0432.CCR-12-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doll C.M., Winter K., Gaffney D.K., Ryu J.K., Jhingran A., Dicker A.P., Weidhaas J.B., Miller B.E., Magliocco A.M. COX-2 expression and survival in patients with locally advanced cervical cancer treated with chemoradiotherapy and celecoxib: a quantitative immunohistochemical analysis of RTOG C0128. Int J Gynecol Cancer. 2013;23:176–183. doi: 10.1097/IGC.0b013e3182791efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sörenson S., Fohlin H., Lindgren A., Lindskog M., Bergman B., Sederholm C., Ek L., Lamberg K., Clinchy B. Predictive role of plasma vascular endothelial growth factor for the effect of celecoxib in advanced non-small cell lung cancer treated with chemotherapy. Eur J Cancer. 2013;49:115–120. doi: 10.1016/j.ejca.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 38.Almhanna K., El-Rayes B., Sethi S., Dyson G., Heilbrun L., Philip P.A., Sarkar F. Association between COX-2 expression and effectiveness of COX-2 inhibitors in a phase II trial in patients with metastatic colorectal adenocarcinoma. Anticancer Res. 2012;32:3559–3563. [PMC free article] [PubMed] [Google Scholar]
- 39.Reyners A.K., de Munck L., Erdkamp F.L., Smit W.M., Hoekman K., Lalisang R.I., de Graaf H., Wymenga A.N., Polee M., Hollema H., van Vugt M.A., Schaapveld M, Willemse P.H., DoCaCel Study Group A randomized phase II study investigating the addition of the specific COX-2 inhibitor celecoxib to docetaxel plus carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer, Fallopian tube or primary peritoneal carcinomas: the DoCaCel study. Ann Oncol. 2012;23:2896–2902. doi: 10.1093/annonc/mds107. [DOI] [PubMed] [Google Scholar]
- 40.Robison N.J., Campigotto F., Chi S.N., Manley P.E., Turner C.D., Zimmerman M.A., Chordas C.A., Werger A.M., Allen J.C., Goldman S., Rubin J.B., Isakoff M.S., Pan W.J., Khatib Z.A., Comito M.A., Bendel A.E., Pietrantonio J.B., Kondrat L., Hubbs S.M., Neuberg D.S., Kieran M.W. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer. 2014;61:636–642. doi: 10.1002/pbc.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Mittal K., Ebos J., Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41:235–251. doi: 10.1053/j.seminoncol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Shusterman S., Maris J.M. Prospects for therapeutic inhibition of neuroblastoma angiogenesis. Cancer Lett. 2005;228:171–179. doi: 10.1016/j.canlet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 44.Xin X., Majumder M., Girish G.V., Mohindra V., Maruyama T., Lala P.K. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest. 2012;92:1115–1128. doi: 10.1038/labinvest.2012.90. [DOI] [PubMed] [Google Scholar]
- 45.Ninomiya I., Nagai N., Oyama K., Hayashi H., Tajima H., Kitagawa H., Fushida S., Fujimura T., Ohta T.. Antitumor and anti-metastatic effects of cyclooxygenase-2 inhibition by celecoxib on human colorectal carcinoma xenografts in nude mouse rectum. Oncol Rep. 2012;28:777–784. doi: 10.3892/or.2012.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayorek N., Naftali-Shani N., Grunewald M. Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS One. 2010;5:e12715. doi: 10.1371/journal.pone.0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaneko M., Kaneko S., Suzuki K. Prolonged low-dose administration of the cyclooxygenase-2 inhibitor celecoxib enhances the antitumor activity of irinotecan against neuroblastoma xenografts. Cancer Sci. 2009;100:2193–2201. doi: 10.1111/j.1349-7006.2009.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coleman R., Gnant M., Morgan G., Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104:1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 49.Sohara Y., Shimada H., Scadeng M., Pollack H., Yamada S., Ye W., Reynolds C.P., DeClerck Y.A. Lytic bone lesions in human neuroblastoma xenograft involve osteoclast recruitment and are inhibited by bisphosphonate. Cancer Res. 2003;63:3026–3031. [PubMed] [Google Scholar]
- 50.Whyte M.P., Wenkert D., Clements K.L., McAlister W.H., Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]