Abstract
Primary bone tumors of the jaw are rare. The neoplastic cells in these tumors are the osteoblasts and osteoclasts. The gnathic bone tumors have also been referred to as borderline. The clinicopathologic approach towards these bony lesions have been reviewed.
Keywords: Borderline, Gnathic, Histopathology, Osteoid
1. Introduction
Primary tumors of jaw bone are uncommon [1]. Osteoid-producing primary bone tumors are encountered in gnathic apparatus, albeit far less in incidence as compared to their skeletal counterparts. The neoplasms covered in this review are those in which the osteoid or bone formation and its progenitor cells are responsible for the primary pathology [2].
Osteoid is the homogenously eosinophilic organic nonmineralised matrix of bone, produced by osteoblasts. The main constituent, type 1 collagen determines by its alignment whether the bone is lamellar or woven. The fiber arrangement is parallel to one another in lamellar bone and randomly distributed in woven. Association of reactive elements like giant cells, hemorrhage and edematous non-atypical spindle cell stroma is indicative of secondary repair or fracture callus. In reactive conditions, the bone formation is focal; progressively maturing and the osteoid islands are parallel to one another [3].
Bone producing lesions have overlapping histological features. The term “borderline” has been used throughout the literature for denoting these overlapping features seen in gnathic bone tumors [4]. Their distinct clinical and radiographic characterisitcs are used to provide an accurate diagnosis.
Osseous tumors are defined by the World Health Organization (WHO) as neoplasms that produce an osseous matrix. These lesions are divided into benign and malignant on the basis of their biological behavior [5]. Lesions that are included are the benign tumors—osteoma, exostosis, osteoid osteoma, osteoblastoma, giant cell tumor as well as the malignant neoplasm, osteosarcoma [1], [6]. Fibro-osseous lesions like juvenile ossifying fibroma, ossifying fibroma and fibrous dysplasia are excluded from the discussion as they are essentially fibrogenic in origin. Computed tomography imaging shows a benign bone tumor as a well circumscribed lesion with the matrix of the tumor; characteristics such as cortical breakthrough, bone destruction, a permeative pattern and associated soft-tissue masses suggest a malignant bone neoplasm [7].
2. Torus
Exostosis or tori are described simply as bony overgrowths. On the palate, the exostosis occurs posterior to midline and tends to be noticeable only by the third decade. In case of the torus mandibularis, the tumor presents itself in the lingual aspect of mandible opposite the mental foramen. Torus palatinus and torus mandibularis are essentially composed of compact bone with larger specimens associated with cancellous core. Tori are removed only if they are large enough to interfere with speech or denture stability [2].
3. Benign bone tumors
3.1. Gnathic osteoma
Presenting as a superficial mass or at an endosteal location, osteoma is the most common benign tumor of the paranasal sinus and posterior body of mandible or the condyle. Its incidence is between 0.014% and 0.43% [8].
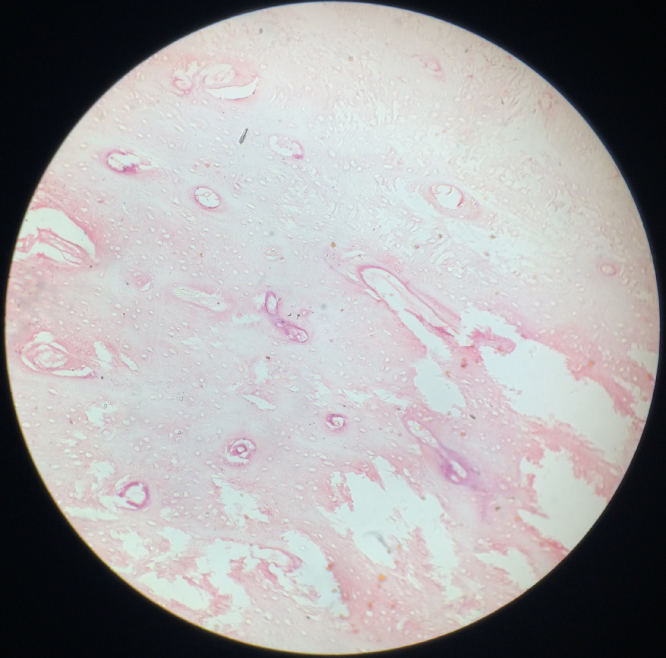
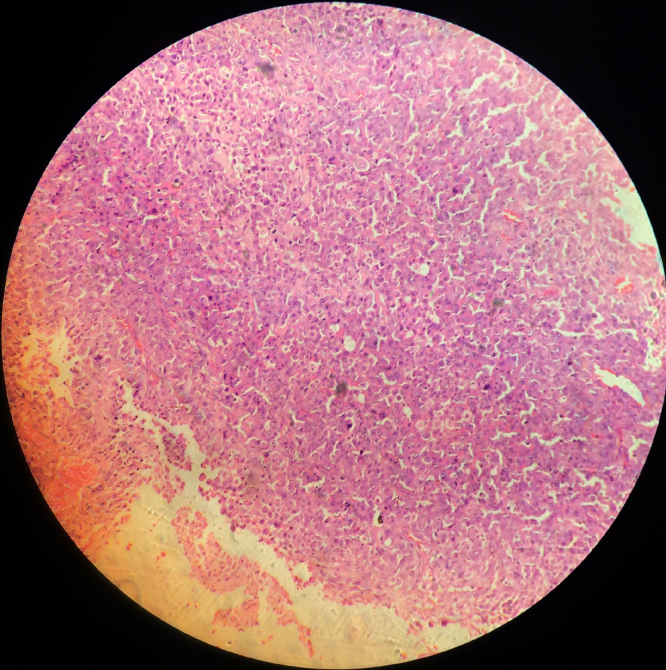
Osteomas present as unilateral, well defined mushroom like radio opaque bone like mass [9]. Compact osteoma is a slow growing lesion consisting of dense and parallel lamellae of bone interspersed with marrow spaces. No haversian systems can be discerned (Fig. 1, Fig. 2). Cancellous osteomas have trabeculae of bone with intervening marrow spaces and thin cortical bone.
Fig. 1.
Compact osteoma with minimal marrow tissue and osteons. Hematoxylin and eosin stain, 100×.
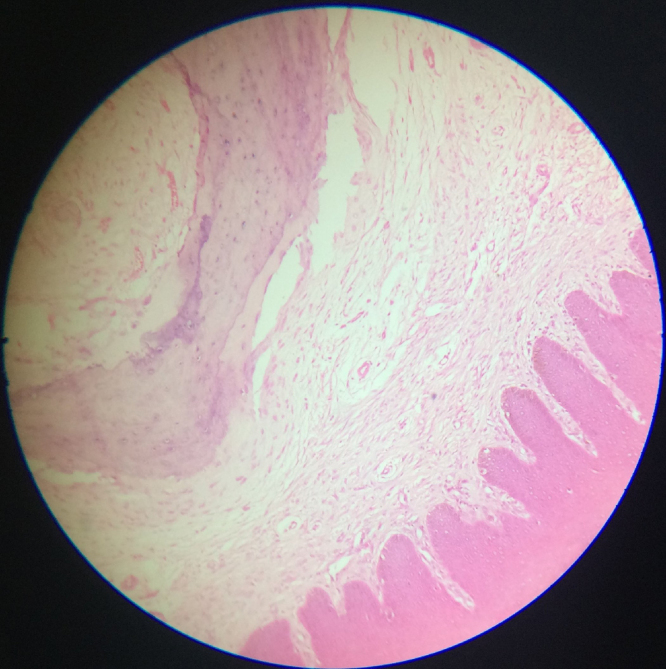
Fig. 2.
Peripheral osteoma in gingival region. Hematoxylin and eosin, 100×.
Gardner׳s syndrome is associated with multiple supernumerary teeth, multiple osteomas, premalignant polyposis coli and multiple epidermal cysts. These osteomas occur at the alveolar ridge to medullary spaces to periosteal surfaces of jaws [2].
3.2. Osteochondroma (cartilage capped osteoma)
Primarily occurring in the condylar and coronoid process of the mandible, osteochondroma presents as mushroom shaped ossification beneath a calcified cartilage layer. The microscopic appearance resembles the epiphysis with a cap of hyaline cartilage overlying bone. The bone is usually of the cancellous variety and poorly demarcated from the cortex and medulla of the rest of the jaw bone. Treatment involves complete excision with overlying periosteum. Sarcomatous transformation in the oro-facial region has not been documented.
3.3. Osteochondromatosis
Just as in the case of Gardner׳s syndrome, the face and jaws are rarely affected. Both osteochondroma and multiple osteochondromatosis cease to grow after pubertal growth spurts. Malignant transformation is rare. The tongue is the most common extra-skeletal tissue affected [2].
3.4. Gnathic osteoid osteoma and osteoblastoma [OB]
Although more common than osteoblastoma elsewhere in body, osteoid osteoma is still very rare in the mandible. Osteoid osteoma of the jaw typically occurs in the age range of 5–24 years. The association of pain with osteoid osteoma in gnathic sites is a worrying feature. Since clinicopathologic correlations are important in delineating osteoid osteoma from osteoblastoma, it is safest to characterize all osteoblastic benign tumors of the jaws under the umbrella term of gnathic osteoblastoma. Even if the lesion is small it may well represent an early stage of osteoblastoma [10], [11].
3.5. Gnathic osteoblastoma
Gnathic osteoblastoma is a rare, expansile, locally aggressive lesion with a higher frequency in males. Radiographically it usually presents as a medullary radiolucency with radio-opaque foci of more than 2 cm in diameter. Sunray appearance or Codman׳s triangle has also been reported in up to 25% cases [2]. Also referred to as giant osteoid osteoma, the osteoblasts are large and epithelioid in appearance.
Osteoblastomas that occur as bony outgrowths without evidence of central destruction are termed periosteal benign osteoblastoma. The reported frequency of osteoblastoma in the jaws may be artificially high because tumors are often located in vicinity of a tooth root. Cementoblastoma is the odontogenic counterpart of jaw osteoblastoma.
In more cellular variants of OB, osteoid is difficult to detect. There is a histological continuum between conventional osteoblastoma and osteosarcoma with osteoblastoma variants in the middle [11]. Imaging studies describe OB as an expansile mixed or sclerotic lesion possessing cortical shell with non-specific signal intensity [7].
Osteoblastoma affect the facial region inclusive of jaws in 10% of cases, more so second and third decades. A mandibular predilection is noted. If the lesion is adjacent to teeth it may lead to tooth loosening. Endodontic treatment and extractions may be attempted for alleviating pain attributed to osteoblastoma.
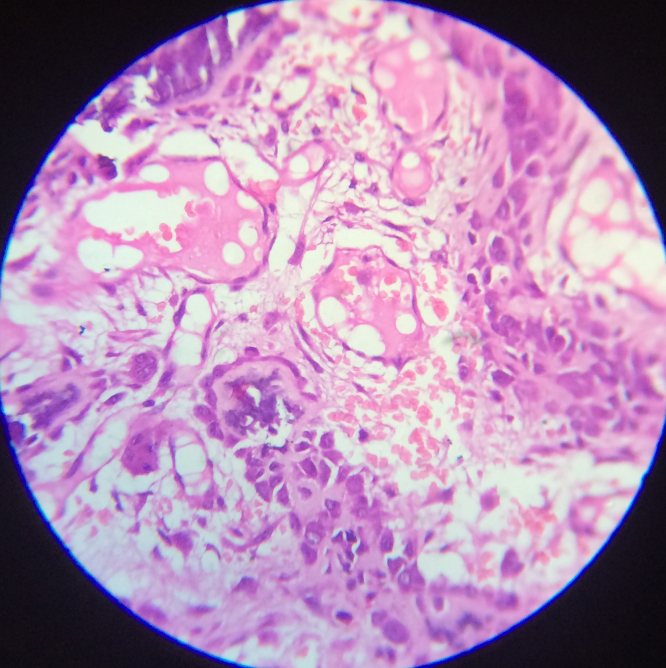
Benign osteoblastoma are well circumscribed with loosely arranged polymorphous small uniform cells, mitosis is rare, and sarcoma giant cells are absent. The tumor is nonpermeative at the borders [12]. Heavily calcified immature bone, also known as “blue bone” may be seen (Fig. 3, Fig. 4). Extensive intralesional hemorrhage in fibro-vascular spindle cell stroma is identified. Secondary aneurysmal bone cyst changes have been seen [13]. Osteoblasts do not fill the intertrabecular stromal spaces [14]. The word “toxic” osteoblastoma is used when severe systemic symptoms accompany osteoblastoma [5]. Storkel et al. reported a case of psammous desmo-osteoblastoma in desmal preformed craniofacial bones [15].
Fig. 3.
Osteoblastoma with large polygonal monomorphic osteoblasts lining osteoid island in a single line. Vascularity and giant cells noteworthy (450×).
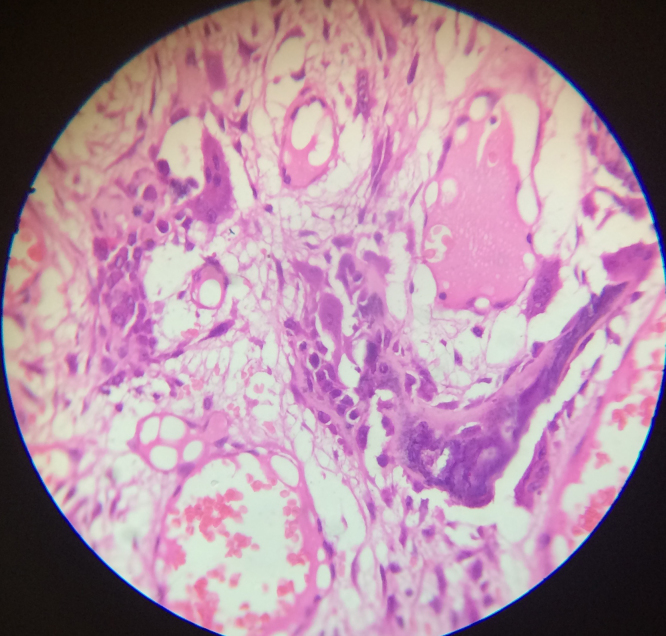
Fig. 4.
Presence of giant cells and blue bone in benign osteoblastoma (450×).
Pseudo-malignant osteoblastoma is used to describe degenerative cytological changes characterized by cells with large degenerated nuclei and smudged chromatin mimicking a malignant tumor.
Multinodular or multifocal osteoblastoma represents osteoblastoma with multiple nidi or growth centers within a single tumor, separated by reactive bone or spindle cell stroma [13].
The term “aggressive” or “epithelioid” or “malignant” osteoblastoma are rare borderline lesions, non-metastasing but locally aggressive behavior and with a higher frequency of local recurrence. Osteoblasts are large and epithelioid with abundant eosinophilic cytoplasm twice the size of conventional osteoblasts. They arrange themselves in sheets with little or no intervening osteoid [5], [13].
The incidence of aggressive osteoblastoma (AO) is unknown. The radiographic appearance of AO consists of a circumscribed lytic defect sometimes surrounded by a sclerotic rim, including occasional significant cortical expansion and destruction. AO tend to be clinically and radiographically larger (4 cm) than conventional osteoblastoma. Permeation into or entrapment of host bone is not seen with either variants [16]. In many cases, the aggressive form has more multinucleated giant cells of the osteoclast type and more abundant atypical osteoid. It has been reported that the recurrence rate of aggressive osteoblastoma is 50% and that of osteoblastoma is 13.6% [17]. They are often mistaken for osteosarcomas which are histologically differentiated by compact sheets of malignant osteoblasts blending with osteoid matrix, higher degree of hyperchromatism, mitotic activity and infiltration into surrounding normal tissue [15], [18]. A complete conservative excision generally suffices.
A differential diagnosis to benign osteoblastoma is that of a solid form of aneurysmal bone cyst that represents 5% of all aneurysmal bone cyst cases. It is a non-cystic variant with solid gray white tissue, hemorrhagic foci and abundant fibroblastic and histiocytic elements with osteoclast like giant cells. Differentiated areas with osteoid and calcified fibromyxoid tissue complete the picture [19].
3.6. Gnathic osteosarcoma
Osteosarcoma [OS] is the most common malignant tumor of bone with the presence of tumor osteoid being directly formed by tumor cells. Gnathic osteosarcoma [GOS] is uncommon and different from skeletal osteosarcoma because it exhibits tremendous incessant growth and rare distant metastases [20].
A variable incidence rate of 4–13.3% is reported. Data show GOS is more prevalent between the ages of 20 and 30 years. The mean age for OS of the long bones has been reported to be 10.15 years lesser than GOS [21], [22]. 60% of GOS occur in males with studies indicating the mandible and maxilla to be almost equally affected [22]. Mandibular tumors arise more frequently in the horizontal ramus, while the maxillary lesions are commonly discovered in the alveolar ridge, sinus floor, and palate [23]. Mandibular neoplasms have a better prognosis than maxillary counterparts. A symphyseal lesion has better prognosis than body, angle or ramus. 5 yr survival rate for gnathic osteosarcoma is 21.5–35% [21].
A typical clinical picture is progressive loosening of teeth, paresthesia, symmetric widening of periodontal spaces, widening of inferior dental canal and sunray and Codman׳s effect at the periosteal surface. Early lung metastasis may result in respiratory symptoms. Osteosarcoma of long bones presents as pain during activity compared to osteosarcoma of jaw bones where swelling is the commonest finding. Most patients related the occurrence of tumor to previous dental treatment, most commonly, dental extractions [2]. Radiographic presentation is of a sclerotic lesion with calcified matrix and sunburst periosteal reaction or it may be mixed or lytic lesion [7]. Osteosarcoma should always be considered in differentials of exophytic and expansile lesions of the jaws such as peripheral pyogenic granulomas, ossifying fibroma and epulis fissuratum [24]. Other unusual clinical presentations may be increased diastema, buccal or distal repositioning of teeth, and radio opaque expansion of alveolar crest. Cases of extra skeletal soft tissue high grade osteosarcoma of tongue have been reported with prognostic factors being size [>5 cm] and regional metastasis [2], [26].
Abundant biopsy specimens should be sampled and multiple expert consultations should be sought [25]. Broders grading is done to assess extent of anaplasia. A high grade conventional central osteosarcoma represents high grade of 3 or 4; parosteal or low grade (grade 1) central osteosarcoma also exist. If a focus of anaplasia exists in otherwise grade 1 tumor it is termed as “dedifferentiated” [20].
Osteosarcoma can be categorized into four major groups:
-
1)
Conventional, high grade osteosarcoma and its histologic subtypes (75–85%),
-
2)
High grade osteosarcoma that arises in a diseased bone (10%),
-
3)
Intramedullary, well-differentiated (1%),
-
4)
Surface osteosarcoma (5–10%) [27].
GOS has a high microscopic grade in most cases [22]. The estimated proportion of high grade mandibular OS is 58%. However, low-grade mandibular osteosarcomas may be underestimated as they are mostly reported as case reports. Estimated weighted-mean proportions of chondroblastic and osteoblastic mandibular OS are 37% and 46%, respectively [28].
Central osteosarcoma can be further classified as high grade conventional, telengiectatic, small cell, epitheliod, osteoblastoma like, chondroblastoma like, fibrohistiocytic, giant cell rich, sclerosing, clear cell osteosarcoma [1], [20]. Central osteosarcoma can be a low grade lesion. Low grade osteosarcoma has been described as fibrous dysplasia type, desmoplastic fibroma type, nonossifying fibroma type, osteoblastoma type, chondromyxoid fibroma type [2], [29].
Surface osteosarcomas are low grade, intermediate type and high grade. Low grade is referred to as parosteal. Intermediate variety is referred to as periosteal and high grade is surface or dedifferentiated parosteal. Parosteal osteosarcomas have a relatively better prognosis than the more centrally located endosteal medullary lesions. All the same this classification is more apt for the extremities than the gnathic region [2].
The tumor osteoid is dense pink often described as “hard”. It may show a lace like or sheet like appearance. The osteoid is curvilinear with small nubs and arborization that appears to be abortive, lacunae formation. The thickness of the osteoid is highly variable with the “thinnest” variant referred to as “filigree”. Osseous matrix has also the predisposition for appositional deposition upon previously existing normal bone trabeculae (scaffolding). Tumor cells may be condensed around the osteoid in palisaded pattern [27].
The tumor cells have a tendency to grow in around vascular elements giving a “basket-weave” or “cording” pattern [1]. Matrix formation may not necessarily be the predominant feature with tumor cells described as pleomorphic, hyper chromatic and showing mitotic figures [2]. The term “osteogenic sarcoma with no predominant growth pattern” is used when histologic sub-typing is not possible despite generous sampling. Tumor cells are round to polyhedral, recognized as osteoblasts, fibroblasts and cartilage cells. Fibroblastic variants have better prognosis than the osteoblastic type.
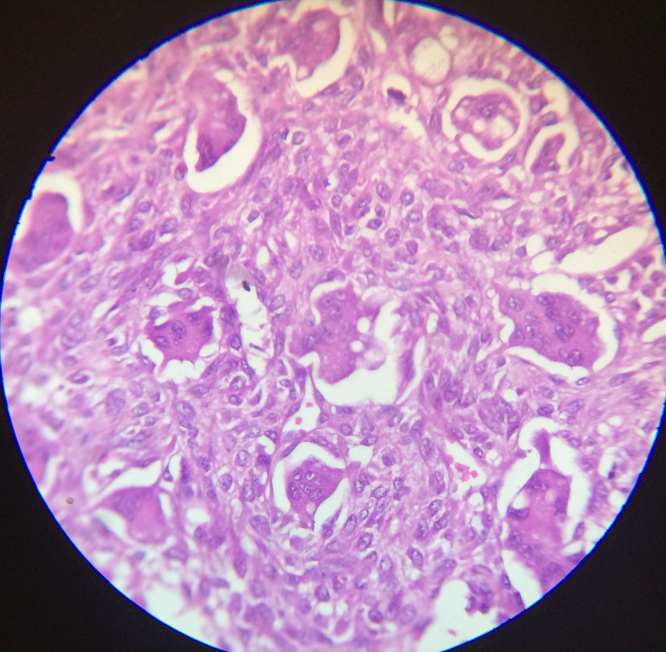
In osteoblastic osteosarcoma, the tumor cells are apposed over the surface of the neoplastic woven bone, exhibiting thin, lace like osteoid interweaving between neoplastic cells, or broad, large sheets of coalescing trabeculae [27] (Fig. 5, Fig. 6).
Fig. 5.
Osteosarcoma showing polygonal neoplastic osteoblasts. Hematoxylin and eosin, 100×.
Fig. 6.
Pleomorphic osteoblasts with minimal osteoid; bizarre mitosis and giant nuclei of osteosarcoma [arrow], hematoxylin and eosin, 450×.
In the jaws, the chondroblastic variant is most common. The neoplastic chondrocytes are mostly characterized by severe cytologic atypia and reside in lacunar spaces, hyaline matrix or float singly or in cords in myxoid matrix. Myxoid and other forms of cartilage are also common. The central areas of lesion are richer in osteoid than the more cellular peripheral zones and have a “normalizing” influence on the appearance of the neoplastic cells [21]. Chemotherapy if given after a biopsy but before definitive surgery often causes normalization along with necrosis. This is referred to as “maturation”.
In sclerosing osteosarcoma, the osteoid is deposited along septa between adipocytes in the marrow cavity. The surface tension between marrow adipocytes forces the tumor cells and their matrix production along the interseptal tissue making it look thicker than normal.
Telengiectatic osteosarcoma is characterized by bone destruction, asymmetric expansion and interrupted periosteal reaction. At higher power the nuclear atypia and high mitotic rate is present whereas at lower power the blood filled sinusoids are seen. Permeation into adjacent marrow tissue or cortical spaces exists. Osteoid production is sparse. Giant cell rich osteosarcomas like areas are commonly seen. Cells lining the blood lakes are pleomorphic. Telengiectatic osteosarcomas are reminiscent of aneurysmal bone cysts both radiologically and histologically [20]. Aneurysmal bone cysts can easily be interpreted as a giant cell tumor or an osteoblastoma, and, on occasion, can be mistaken for osteogenic malignancies [30].
Small cell osteosarcoma combines features of osteosarcoma and Ewing׳s sarcoma. Reciprocal translocation between 11 and 22 chromosomes is seen in small cell osteosarcomas. Some authors believe that small cell osteosarcomas are actually Ewing sarcoma family tumors with divergent differentiation. The difference arises when spindle cell foci and osteoid are seen in a round cell rich picture. This differentiation is crucial as Ewing sarcoma is radiosensitive whereas osteosarcomas are radio resistant.
Epithelioid osteosarcoma is an osteosarcoma in which tumor cells are so poorly differentiated that it is difficult to determine histologically whether it is sarcoma or carcinoma. Tumor cells are round or polygonal and they appear cohesive. Post-radiation osteosarcomas are more often seen demonstrating this pattern. Crossed polarizers can be employed after picrosirius red staining to detect these lesions.
Giant cell rich osteosarcomas are difficult to diagnose as they are confused with those present in giant cell tumor or telengiectatic osteosarcoma [20]. The giant cells need to be distributed uniformly in solid parts of the tumor to differentiate them from telengiectatic osteosarcoma. Numerous pleomorphic cells are associated with telengiectatic osteosarcoma. The giant cell rich osteosarcomas are seen in skeletally immature persons and associated with periosteal reaction as compared to giant cell tumor which occurs in skeletally mature and not associated with periosteal reaction.
True giant cell tumors may also develop malignancy. A recurring giant cell tumor is termed secondary malignant giant cell tumor. Sarcomatous tissue identified in otherwise typical giant cell tumor is primary malignant giant cell tumor.
Low grade lesions of mandible are rare and represent 2% of osteosarcomas reported. Low grade osteosarcomas have a predilection for the mandible with longstanding painless swelling as presentation and minimal mitotic activity but the radiological findings usually suggest the diagnosis [29]. Low grade tumor consists of spindle cells and limited osteoid formation [10].
The differential diagnosis for chondromyxoid fibroma like variant of osteosarcoma (a low grade variant) is chondroblastic osteosarcoma. Fibrous dysplasia and desmoplastic fibroma also are confused with low grade osteosarcomas [5]. Mutational analysis of GNAS1 reveals positive findings in fibrous dysplasia but not in osteosarcoma [10]. Multiple serial sectioning of representative lesion and obtaining large biopsy tissues in the first place prevent misdiagnosis as fibrous dysplasia, ossifying fibroma.
3.7. Osteoblastoma like and chondroblastomas like osteosarcoma
Osteobalstoma-like osteosarcoma is a low-grade lesion with radiographic features that can vary from lytic to sclerotic to mixed. Lesion borders can be well defined, suggesting a benign process, or can be ill defined and have cortical destruction. Borders of the tumor show infiltration and entrapment of bone. A recent publication has reported a novel three-way translocation involving chromosomes 1, 2 and 14 [16]. The dysplastic changes in stromal cells and osteoblasts are the elements that enable the differentiation of osteoblastoma from low-grade osteosarcoma [17].
Multicentric or multifocal osteosarcomas first described by Silverman in 1936 represent 1–2% of osteosarcomas. They are synchronous if multiple tumor foci occur within 5 months of initial presentation or metachronous if multiple tumor foci occur 5 months after initial presentation. They respond poorly to multimodal therapy. Synchronous multicentric osteosarcoma affecting the jaw is even rarer with only four cases having been reported [31].
Another entity found in up to 10% of osteosarcomas is “skip” metastases. Here a second focus of osteosarcoma is found in the same bone or in an adjacent bone across a joint.
Well-differentiated osteosarcomas may represent an entirely different entity, one that is related to an actual osteosarcoma. This would explain the dilemma evidenced in literature on histological and radiological diagnosis, differences in response to treatment and rarity of metastatic disease [32], [33].
Biopsy tissues may be obtained via intraoperative procedures such as aspiration biopsy, imaging assisted core needle biopsies or open surgical biopsies. In addition to clinicopathologic correlations teamed with routine staining, there exists the need to conduct immunohistochemistry, cytogenetic techniques [5]. In the event of new bone formation, alkaline phosphatase levels are increased. Hematoxylin and eosin, picrosirius red, ponceau trichrome, and Masson trichrome staining methods are most frequently used [22].
Osteogenic differentiation of mesenchymal stem cells can be monitored by using bone morphogenetic proteins (BMPs) and their downstream mediators, such as the Id proteins. Connective tissue growth factor (CTGF), as early markers, alkaline phosphatase and osterix as early/middle markers and osteocalcin and osteopontin as late markers of bone formation may be valuable [27].
Cell Adhesion Molecule1 [CADM1] is a novel osteoblastic adhesion molecule that is expressed transiently during osteoblastic maturation, and a useful diagnostic marker for osteosarcoma cells [34]. Recently, it was reported that a combination of MDM2 and CDK4 by immunohistochemical analysis shows 100% sensitivity and 97.5% specificity for the diagnosis of low-grade osteosarcoma [35].
Dissemination of osteosarcoma to other organs, especially early dissemination to the lung, is common, but metastasis to the jaw has only rarely been reported [36]. Studies comparing jaw osteosarcoma to nonjaw locations conclude that in an osteosarcoma in old age, treatment is more often confined to surgery or radiotherapy, and from the age of 60 years onwards chemotherapy is less often given in malignant diseases. Outcome is less favorable with increasing age. No significant differences in relation to age were found based on histology of the neoplasm or based on site of the osteosarcoma (maxilla, mandible, extragnathic sites) [37]. Whereas peripheral OS is regarded as systemic disease at the time of diagnosis in which >90% of patients develop lung metastases without multimodality treatment, the vast majority of GOS patients seem to be curable by complete resection only [38].
Jaw osteosarcomas are characterized by late metastasis but local invasiveness. Rate of recurrence is 50% with most recurrent cases being local. Therefore radical excision with clear surgical margins is advocated. [2] Local failure is the main cause of death in GOS compared to extragnathic sites [28].
4. Giant cell tumor of jaws [GCT]
GCTs arising in the head and neck region constitute approximately 2% of all GCTs. They are locally aggressive, recurrent and non-metastasing. Neoplastic giant cell are very rare in jaws. GCT usually occurs in children and young adults, predominantly females. GCTs are usually mono-ostotic, although they may occasionally present in a polyostotic form, when they are usually of a high grade [39]. Radiographic features are of an expansile radiolucent mass crossing the midline that tends to destroy and remodel the adjacent bone. A blurred border of the radiolucent area goes in favor of giant cell tumor.
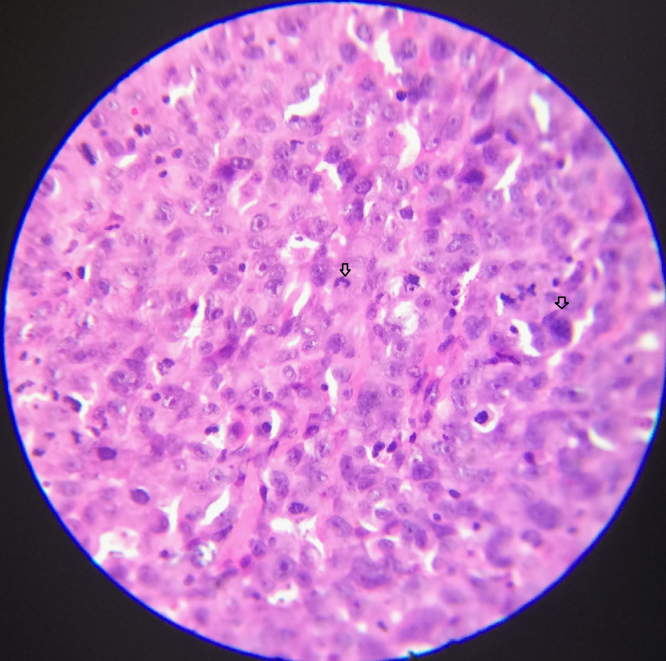
The giant cells represent osteoclast precursors. Numerous uniformly dispersed large giant cells with nuclei are seen (Fig. 7). Hemorrhagic elements are rare. Osteoid is not produced [21], [40].
Fig. 7.
Giant cell tumor. Photomicrograph, 450×.
Due to the similarity of the biological behavior of central giant cell granulomas [CGCG] of the jaw with that of GCT of long bones, CGCG of the jaw may be considered as a low grade tumor and the differences between both may be due to the variations in the anatomical sites, since the presence of the teeth in addition to the histological structure of the jaw bone and bone marrow activity, could influence the biological behavior of CGCG comparatively. p53 activity can be used to chart its aggressiveness. The recurrence rate of CGCG and GCT was 40–45% [41]. Sarcomatous areas may also develop in giant cell tumor [5].
The primary bone cell derived neoplasms of the gnathic apparatus show a wide variation from their extragnathic counterparts in both diagnosis and treatment approach. The jaw tumors especially are insidious and often under diagnosed. Repeated follow ups of all expansile jaw lesions should be the protocol to counter deceptive clinical presentations.
Conflict of interest statement
None.
References
- 1.Fletcher C.D.M., Unni K.K., Mertens F., editors. IARC Press; Lyon: 2002. World health organization classification of tumors. Pathology and genetics of tumors of soft tissue and bone. [Google Scholar]
- 2.Cawson R.A., Binnie W.H., Speight P., Barrett A.W., Wright J.M. 5th ed. Churchill Livingstone; London, Edinburgh, New York, Philadelphia, Sydney and Toronto: 1998. Lucas׳s pathology of tumors of the oral tissues. [Google Scholar]
- 3.Hameed O., Wei S., Siegel Gene P. Springer-Verlag New York Inc.; United States: 2011. Bone/osteoid producing lesions in Book Frozen section library: bone; pp. 5–24. (chapter 2) [Google Scholar]
- 4.Dorfman H.D., Weiss S.W. Borderline osteoblastic tumours: problems in the differential diagnosis of aggressive osteoblastoma and low-grade osteosarcoma. Semin Diagn Pathol. 1984;1:215–234. [PubMed] [Google Scholar]
- 5.Davies M., Sundaram M., James S.L.J. Imaging of bone tumors and tumor-like lesions. In: Baert A.L., Brady L.W., Heilmann H.P., Knauth M., Molls M.C., editors. Techniques and applications. Medical radiology: diagnostic imaging and radiation oncology, series. Nieder Springer-Verlag; Berlin, Heidelberg: 2009. [Google Scholar]
- 6.White L.M., Kandel R. Osteoid-producing tumours of bone. Semin Musculoskelet Radiol. 2000;4:25–43. doi: 10.1055/s-2000-6853. [DOI] [PubMed] [Google Scholar]
- 7.Abdel Razek A. Imaging appearance of bone tumours of the maxillofacial region. World J Radiol. 2011;28(3):125–134. doi: 10.4329/wjr.v3.i5.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyung-Soo Nah. Osteomas of the craniofacial region. Imaging Sci Dent. 2011;41:107–113. doi: 10.5624/isd.2011.41.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durãoa A.R., Chilvarquerb I., Hayekb J.E., Provenzanob M., Kendall A.R. Osteoma of the zygomatic arch and mandible: report of two cases. Rev Port Estomatol Med Dent Cir Maxilofac. 2012;53:103–107. [Google Scholar]
- 10.Alawi F. Benign fibro osseous diseases of the maxillofacial bones. A review and differential diagnosis. Am J Clin Pathol. 2002;118:S50–S70. doi: 10.1309/NUXA-JUT9-HA09-WKMV. [DOI] [PubMed] [Google Scholar]
- 11.Jones A.C., Prihoda T.J., Kacher J.E., Odingo N.A., Freedman P.D. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:639–650. doi: 10.1016/j.tripleo.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Ivkovic T., Vuèkovic N., Gajanin R., Karalic M., Stojiljkovic B., Panjkovic M. Benign osteoblastoma of the mandible. Arch Oncol. 2000;8:73–74. [Google Scholar]
- 13.ucas D.R. Osteoblastoma. Arch Pathol Lab Med. 2010;134:1460–1466. doi: 10.5858/2010-0201-CR.1. [DOI] [PubMed] [Google Scholar]
- 14.Rawal Y.B., Angiero F., Allen C.M., Kalmar J.R., Sedghizadeh P.P., Steinhilber A.M. Gnathic osteoblastoma: a clinicopathologic review of seven cases with long term follow up. Oral Oncol. 2006;42:123–130. doi: 10.1016/j.oraloncology.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Storkel S., Wagner W., Makek M.S. Psammous desmo-osteoblastoma. Ultra structural and immunohistochemical evidence for an osteogenic histogenesis. Virchows Arch A Pathol Anat Histopathol. 1987;411:561–568. doi: 10.1007/BF00713287. [DOI] [PubMed] [Google Scholar]
- 16.Harrington C., Accurso B.T., Kalmar J.R., Iwenofu O.H., Agrawal A., Allen C.M. Aggressive osteoblastoma of the maxilla: a case report and review of the literature. Head Neck Pathol. 2011;5:165–170. doi: 10.1007/s12105-010-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angiero F., Mellone P., Baldi A., Stefani M. Osteoblastoma of the jaw: report of two cases and review of the literature. In Vivo. 2006;20:665–670. [PubMed] [Google Scholar]
- 18.Chrysomali E., Schoinohoriti O., Theologie–Lygidakis N., Goutzanis L., Iatrou I. Osteoblastoma of the mandible: a case report with immunohistochemical evaluation. Open J Stomatol. 2011;1:207–211. [Google Scholar]
- 19.Capote-Moreno A., Acero J., Garcia-Recuero I., Ruiz J., Serrano R., de Paz V. Giant aneurysmal bone cyst of the mandible with unusual presentation. Med Oral Patol Oral Cir Bucal. 2009;14:E 137–E 140. [PubMed] [Google Scholar]
- 20.Klein M.J., Seigal G.P. Osteosarcoma. Am J Clin Pathol. 2006;125:555–581. doi: 10.1309/UC6K-QHLD-9LV2-KENN. [DOI] [PubMed] [Google Scholar]
- 21.Batsakis J.G. Tumors of the head and neck, clinical and pathological considerations. 2nd ed. Williams and Wilkins; Baltimore, London: 1981. Non odontogenic tumors of jaws; pp. 381–419. (chapter 20) [Google Scholar]
- 22.Azizi T., Motamedi M.H., Jafari S.M. Gnathic osteosarcomas: a 10-year multicenter demographic study. Indian J Cancer. 2009;46:231–233. doi: 10.4103/0019-509X.52958. [DOI] [PubMed] [Google Scholar]
- 23.George A., Mani V. Gnathic osteosarcomas: review of literature and report of two cases in maxilla. J Oral Maxillofac Pathol. 2011;15:138–143. doi: 10.4103/0973-029X.84476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shakib P.A., Aminio Foroughi R., Seyed Majid M. Osteosarcoma of the maxilla: a rare case with unusual clinical presentation. J Dent Res Dent Clin Dent Prospect. 2013;7:177–181. doi: 10.5681/joddd.2013.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padilla R.J., Murrah V.A. The spectrum of gnathic osteosarcoma: caveats for the clinician and the pathologists. Head Neck Pathol. 2011;5:92–99. doi: 10.1007/s12105-010-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loyzaga J.M., Machin P.F., Sala J. Osteogenic sarcoma of the tongue. Case report and review of literature. Pathol Res Pract. 1996;191:75–78. [PubMed] [Google Scholar]
- 27.Helen trihia and christos valavanis. Histopathology and molecular pathology of bone and extraskeletal osteosarcomas, Osteosarcoma, Dr. Manish Agarwal (Ed.), 2012; ISBN: 978-953-51-0506-0, InTech, Available from: 〈http://www.intechopen.com/books/osteosarcoma/histopathology-and-molecular-pathology-ofbone-and-extraskeletal-osteosarcomas〉
- 28.Thariat J., Julieron M., Brouchet A., Italiano A., Schouman T., Marcy P.Y. Osteosarcomas of the mandible: Are they different from other tumor sites? Crit Rev Oncol Hematol. 2012;82:280–295. doi: 10.1016/j.critrevonc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Sinha R., Roychowdhury S.K., Chattopadhyay P.K., Rajkumar K. Low grade osteosarcoma of the mandible. J Maxillofacial Oral Surg. 2010;9:186–190. doi: 10.1007/s12663-010-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H.M., Cho K.S., Choi K.U., Roh H.J. Aggressive aneurysmal bone cyst of the maxilla confused with telengiectatic osteosarcoma. Auris Nasus Larynx. 2012;39:337–340. doi: 10.1016/j.anl.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q., Li Y., Gao N., Huang Y., Li L.J. Synchronous multicentric osteosarcoma involving mandible and maxillas. Int J Oral Maxillofac Surg. 2011;40:446–449. doi: 10.1016/j.ijom.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Nthumba P.M. Osteosarcoma of the jaws: a review of literature and a case report on synchronous multicentric osteosarcomas. World J Surg Oncol. 2012;10:240. doi: 10.1186/1477-7819-10-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babazade F., Mortazavi H., Jalalian H. Bilateral metachronous osteosarcoma of the mandibular body: a case report. Chang Gung Med J. 2011;34:66–69. [PubMed] [Google Scholar]
- 34.Inoue T., Hagiyama M., Enoki E., Sakurai M.A., Tan A., Wakayama T. Cell adhesion molecule 1 is a new osteoblastic cell adhesion molecule and a diagnostic marker for osteosarcoma. Life Sci. 2013;17(92):91–99. doi: 10.1016/j.lfs.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary M., Chaudhary S.D. Osteosarcoma of jaws. J Oral Maxillofac Pathol. 2012;16:233–238. doi: 10.4103/0973-029X.99075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angiero F., Moltrasio F., Cattoretti G., Valente M.G. Clinical and histopathological profile of primary or secondary osteosarcoma of the jaws. Anticancer Res. 2011;31:4485–4489. [PubMed] [Google Scholar]
- 37.van den Berg H, Schreuder WH, de Lange J, Osteosarcoma: A Comparison of Jaw versus Nonjaw Localizations and Review of the Literature. Sarcoma 2013; 2013 (316123): 9 p. 10.1155/2013/316123 [DOI] [PMC free article] [PubMed]
- 38.Baumhoer D., Brunner P., Eppenberger-Castori S., Smida J., Nathrath M., Jundt G. Osteosarcomas of the jaws differ from their peripheral counterparts and require a distinct treatment approach. Experiences from the DOESAK Registry. Oral Oncol. 2014;50:147–153. doi: 10.1016/j.oraloncology.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Park S.R., Chung S.M., Lim J.Y., Choi E.C. Giant cell tumor of the mandible. Clin Exp Otorhinolaryngol. 2012;5:49–52. doi: 10.3342/ceo.2012.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabhlok S., Shaikh M.I., Tripathy R., Mishra S. Giant cell tumor of the maxilla in an 8 year old boy. Int J Med Dent Sci. 2012;1:23–27. [Google Scholar]
- 41.Kader O., Bashar H., Abdullah B.H., Edward M.L. Histopathological and immunohistochemical study of giant cell granuloma of the jaw and giant cell tumor of long bones (comparative study) Iraqi Postgrad Med J. 2011;10:33–39. [Google Scholar]