Abstract
Understanding associations between circulating biomarkers and physical performance across the adult life span could aid in better describing mechanistic pathways leading to disability. We hypothesized that high concentrations of circulating biomarkers would be associated with lower functioning across study populations representing the adult life span. The data were from four intervention and two observational studies with ages ranging 22–89 years. Biomarkers assayed included inflammatory, coagulation, and endothelial function markers. Physical performance was measured either by VO2peak (studies of young and middle-aged adults) or usual gait speed (studies of older adults). Partialled (by age, body mass index, race, and sex) and weighted common correlations were calculated between biomarkers and physical performance. Homogeneity of the associations was also assessed. Interleukin-6 (weighted r = −.22), tumor necrosis factor receptor 2 (weighted r = −.19), D-dimer (weighted r = −.16), tumor necrosis factor receptor 1 (weighted r = −.15), granulocyte colony-stimulating factor (weighted r = −.14), and tumor necrosis factor alpha (weighted r = −.10) were all significantly inversely correlated with physical performance (p < .05). All significant correlations were homogeneous across studies. In summary, we observed consistent inverse associations between six circulating biomarkers and objective measures of physical performance. These results suggest that these serum biomarkers may be broadly applicable for detection, trajectory, and treatment monitoring of physical function across the life span or possibly for midlife predictors of functionally deleterious conditions.
Key Words: Physical performance, Circulating biomarkers, Aging life span
Aging is associated with dysregulation across multiple systems that may lead to reduced physical capacities and disability (1,2). The dysregulation that becomes clinically evident later in life is often the result of decades of gradual, and often unnoticed, declines in systems. Circulating biomarkers of inflammation, coagulation, and endothelial function can provide important insights related to the functioning of their representative systems (3–5). Similarly, measures of physical performance can provide insight into physical capacity as it relates to functional decline (6,7). Understanding associations between circulating biomarkers and physical performance could aid in better describing mechanistic pathways leading to disability. For example, contrasting theories that remain unresolved to date are whether aging-related degradations occur within compartments in a stochastic nature or in a more synchronized or “cross-talk” manner across physiologic systems (8,9). Measurement of multiple markers that represent signaling in inflammation, coagulation, and endothelial function can inform these theories and aid in the development of therapies or interventions by targeting the biologic pathways of decline (9). Further, it is important to study these associations across the adult life span to better understand the aging process; however, few studies do so because of disparate physical performance measures which lead to analytic challenges (10).
Meta-analysis as a statistical technique, independent of a systematic review that is typically performed prior to applying the statistical methodology, can be used to address the challenges that are posed when combining studies that use differing measures. This statistical method allows rich extant data sources to be combined and appropriately weighted based on sample size and associated variance in measures (11). The purpose of this report is to present new data, using meta-analytic methodology, which describe common associations between physical performance and multiple circulating biomarkers in six study cohorts including young, middle-aged, and older adults. We hypothesized that high concentrations of biomarkers of inflammation, coagulation, and endothelial dysfunction would be associated with lower functioning across different study populations representing ages across the life span.
Methods
Data Source
The data used were from six studies conducted at Duke and the Durham VA Medical Centers and coordinated by the Duke Claude Pepper Older Americans Independence Center. The participants’ ages ranged across 7 decades of life (from 22 to 89 years). Of the studies, four were interventional: (i) CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy, conducted 2002–2004) studied of the effects of 6 months of caloric restriction or caloric restriction plus exercise in overweight, nonobese men and women on body composition and related outcomes (12); (ii) STRRIDE (Studies Targeting Risk Reduction Interventions through Defined Exercise, conducted 1998–2003) examined the effects of different amounts and intensities of aerobic exercise training on insulin sensitivity in middle-aged moderately overweight men and women (13); (iii) LIFE (Veteran’s LIFE study, conducted 2004–2007) that compared the impact of a home-based physical activity counseling program versus usual primary care on physical activity and physical function in older veterans (14); and (iv) EF (Enhanced Fitness, conducted 2008–2011) that compared the impact of a home-based physical activity counseling program versus usual primary care on selected glycemic indicators in older veterans with impaired fasting glucose (15). There were two cohort studies: (v) POP (Prediction of Osteoarthritis Progression, conducted 2003–2008) was a longitudinal cohort study to evaluate predictors of knee osteoarthritis progression in men and women (16) and (vi) CARRIAGE (CARolinas Region Interaction of Aging, Genes and Environment, conducted 2002, 2004, and 2006) study of a large extended family of primarily African and Native American ethnicity evaluated for intermediate traits of cardiovascular disease and arthritis (17). Study sizes and demographics are summarized in Table 1.
Table 1.
Subject Demographic and Physical Characteristics for Individual Studies and Entire Group
| CALERIE | STRRIDE | CARRIAGE | POP | LIFE | Enhanced Fitness |
Entire Group |
|
|---|---|---|---|---|---|---|---|
| N | 41 | 68 | 30 | 137 | 74 | 253 | 603 |
| % women | 56.5 | 48.0 | 71.4 | 73.2 | 0 | 0 | 25 |
| % minority | 39.1 | 20.6 | 100 | 13.0 | 22.1 | 28.3 | 28 |
| Age (years), mean (SD) | 38.5 (6.4) | 51.2 (7.7) | 71.6 (5.5) | 65.9 (11.6) | 79.2 (4.8) | 67.2 (6.3) | 64.7 (12.8) |
| BMI (kg/m2), mean (SD) | 27.7 (1.7) | 30.5 (2.9) | 32.4 (6.9) | 31.3 (6.9) | 28.4 (4.1) | 31.3 (3.7) | 30.6 (4.8) |
| Usual gait speed, m/s, mean (SD) | — | — | 0.85 (0.22) | 0.99 (0.25) | 1.10 (0.32) | 1.27 (0.23) | 1.13 (0.28) |
| VO2peak, mL/kg/min, mean (SD) | 30.6 (5.7) | 28.3 (6.0) | — | — | — | — | 29.1(6.0) |
Note: BMI = body mass index; CALERIE = Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy; CARRIAGE = CARolinas Region Interaction of Aging, Genes and Environment; POP = Prediction of Osteoarthritis Progression; STRRIDE = Studies Targeting Risk Reduction Interventions through Defined Exercise.
Circulating Biomarkers
Blood was collected by venipuncture, separated to yield plasma and serum, and immediately stored at −80°C. With the exception of LIFE, all samples were drawn on fasting participants. Seventeen circulating biomarkers of inflammation, coagulation, and endothelial function were assayed. These biomarkers broadly represent physiologic systems that are known to dysregulate with aging and were chosen a priori for analysis by each of the six study’s research teams. Ten markers were measured as a group using a Luminex multiplex bead panel (Invitrogen, Carlsbad, CA): GCSF (granulocyte colony-stimulating factor), IL-1RA (interleukin-1 receptor antagonist), IL-2, IL-8, MCP-1 (monocyte chemotactic protein 1), RANTES (regulated upon activation, normal T-cell expressed, and secreted), TNFα (tumor necrosis factor alpha), TNFR1 (TNF receptor 1), TNFR2, and VEGF (vascular endothelial growth factor). Five additional markers were measured by immunoassay. These included: IL-6, which was measured by the MSD Ultrasensitive Assay (Meso Scale Discovery, Gaithersburg, MD) (18); MMP-3 (matrix metalloproteinase-3; BioSource, Camarillo, CA); TRAIL (TNF-related apoptosis-inducing ligand; BioSource); leptin (Millipore, Billerica, MA); and VCAM (soluble vascular cell adhesion molecule; R&D Systems, Minneapolis, MN). The organophosphatase-specific activity of paraoxonase was assayed using a kit from Invitrogen (Eugene, OR). All of the preceding analytes were measured in serum according to the manufacturers’ specifications. The coagulation marker, D-dimer, a fibrinolytic component, was measured in plasma by immunoassay, per the manufacturer’s protocol (American Diagnostica, Stamford, CT). All samples were analyzed in duplicate and analyses were repeated for out of range high values and for any duplicates with coefficients of variation above 10%. In total, 17 circulating biomarkers were analyzed for this study, although not all 6 studies included all 17 biomarkers.
Physical Performance Measures
The studies used one of two measures of physical performance—VO2peak or usual gait speed. We believe it is appropriate to combine these two measures of physical performance based on the previously reported association between the two measures. Malatesta and colleagues reported a strong correlation (r = .70) between VO2peak and usual gait speed in healthy older adults (19). Cunningham and colleagues reported that in men spanning 6 decades, the association between self-selected gait speed and VO2peak was independent of age (20). Further, Fiser and colleagues reported a strong association of usual gait speed and VO2peak after controlling for gender (partial r = .69, p < .001), which remained significant after additional control for age and muscle strength in a sample of 60–88 year olds (21). Lastly, the meta-analytic techniques used in this study enable generally related types of outcomes to be combined into a common variable of interest (11). A particular strength of using meta-analytic techniques is the ability to find common associations by combining variables with related outcomes of differing metrics. In the STRRIDE and CALERIE studies, physical performance was determined from a graded treadmill exercise tolerance test with gas exchange analysis to measure VO2peak, a measure of overall cardiorespiratory fitness. The standard unit of measure for this test is mL/kg/min. The four studies involving older adults (CARRIAGE, POP, LIFE, and EF) measured usual gait speed over an eight-foot course using the Short Physical Performance Battery (SPPB) protocol (22).
Statistical Analysis
Characteristics of study participants were calculated using appropriate univariate continuous or frequency statistics. All biomarker values were transformed by the natural log (with a constant of one added to the raw values to deal with values of zero) because of their skewed distribution. Partial Spearman correlations between each biomarker and physical performance (partialling age, body mass index, gender, and race via linear regression methods) were then estimated separately by study. Because correlation coefficient SEs are not normally distributed, to derive test statistics and confidence intervals, we transformed the estimates using Fisher’s Z transformation which is defined as (Equation 1): Fisher’s Z = 0.5 [ln(1 + r) − ln(1 − r)] (23). We were then able to apply standard meta-analytic techniques to the data, including weighting estimates based on study variance which is generally written as (Equation 2) of the overall effect and confidence intervals. The estimate was then algebraically transformed back to produce a weighted correlation coefficient, which was the statistic of interest for this study. Tests of homogeneity were conducted using the Q statistic, which is calculated as (Equation 3): Q = ∑w i (Z i − Z)2, where w i is the weighting factor for the ith study assuming a fixed-effects model, and Z is the sum of effect sizes weighted by their inverse variance. The Q statistic has a chi-square distribution with k − 1 degrees of freedom (24). All analyses were conducted using SAS v9.4 software (SAS, Cary, NC).
Results
There was considerable variability between studies with respect to subject characteristics. The number of study subjects ranged from 33 (CARRIAGE in which only subjects 65 years and older were evaluated for physical performance) to 253 (EF), and mean ages ranged from late thirties (CALERIE) to late seventies (LIFE). Overall, men were well represented largely due to LIFE and EF, which were studies conducted at a VA Medical Center. The mean usual gait speed in the CARRIAGE study was the slowest (0.85 ± 0.22 m/s) and indicated decreased mobility as a group; whereas, the EF group represented the highest mean gait speed of 1.27 ± 0.23 m/s. The mean VO2peak in the CALERIE (30.6 ± 5.7 mL/kg/min) and STRRIDE (28.3 ± 6.0 mL/kg/min) studies indicated low cardiovascular fitness, which was consistent with the target populations and inclusion criteria.
Individual Study Biomarker Concentrations
Median and interquartile ranges of biomarker concentrations for each study can be observed in Table 2. CARRIAGE and POP had data for all biomarkers; TNFα data from the LIFE study was not included due to large numbers of samples with values below the lower limit of detection. The remaining studies (CALERIE, STRRIDE, and EF) show the available biomarkers based on the assays planned a priori. There was considerable variability of concentrations within each study with a consistent pattern of right skewness. Generally, the younger cohorts (CALERIE and STRRIDE) had lower biomarker concentrations compared to the oldest cohort (CARRIAGE).
Table 2.
Median (interquartile range) Biomarker Concentrations by Study
| Circulating Biomarker (group) | CALERIE | STRRIDE | CARRIAGE | POP | LIFE | Enhanced Fitness |
|---|---|---|---|---|---|---|
| D-Dimer, ng/mL (C) | * | 223.4 (168.6) | 669.9 (577.8) | 399.1 (389.8) | 659.3 (516.7) | 403.4 (380.9) |
| GCSF, pg/mL (I) | * | 590.7 (252.6) | 302.4 (144.2) | 362.6 (134.7) | 128.0 (53.8) | * |
| IL-1RA, pg/mL (I) | * | 1,333 (1,124) | 2,131 (3,053) | 2,441 (1,651) | 212.2 (455.0) | * |
| IL-2, pg/mL (I) | * | 4.2 (17.6) | 12.7 (57.9) | 12.7 (52.0) | 13.2 (14.9) | * |
| IL-6, pg/mL (I) | 0.59 (0.48) | 0.69 (0.63) | 0.75 (0.72) | 1.07 (1.08) | 1.10 (1.15) | 1.65 (1.70) |
| IL-8, pg/mL (I) | * | 5.8 (10.7) | 88.8 (54.2) | 74.1 (45.5) | 24.9 (18.0) | 7.3 (3.4) |
| Leptin, ng/mL (I) | 16.3 (19.9) | 22.1 (24.0) | 23.1 (33.6) | 32.1 (32.1) | 12.8 (8.4) | * |
| MCP-1, pg/mL (I) | * | 1,033 (438) | 2,499 (1,076) | 2,418 (1,348) | 572.1 (307.0) | * |
| MMP-3, ng/mL (I) | * | * | 7.1 (4.7) | 4.7 (3.3) | 11.1 (6.4) | * |
| PXN, nmol/min/mL (E) | * | 3.1 (1.1) | 9.4 (4.8) | 6.3 (2.5) | 4.4 (1.5) | * |
| RANTES, pg/mL (I) | * | 21,273 (15,745) | 7,726 (7,831) | 6,827 (6,663) | 25,278 (35,852) | * |
| TNFα, pg/mL (I) | 5.9 (7.3) | 20.3 (9.2) | 19.5 (9.6) | 16.5 (4.7) | † | 6.7 (3.1) |
| TNFR1, pg/mL (I) | * | 2,981 (1,159) | 4,654 (2,920) | 3,755 (2,878) | 1,700 (788) | 2,064 (1,000) |
| TNFR2, pg/mL (I) | * | 4,249 (2,214) | 2,689 (2,031) | 1,624 (1,851) | 2,388 (1,468) | 5,063 (2,288) |
| TRAIL, pg/mL (I) | * | * | 438.0 (177.2) | 501.4 (240.0) | 596.8 (266.8) | * |
| VCAM, pg/mL (E) | * | * | 992.6 (134.6) | 838.7 (218.2 | 890.1 (173.7) | 881.4 (339.8) |
| VEGF, pg/mL (E) | * | † | 200.4 (234.4) | 254.2 (288.7) | 27.0 (13.3) | * |
Notes: Group refers to type of biomarker: I = inflammation related; C = coagulation related; E = endothelial function related. CALERIE = Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy; CARRIAGE = CARolinas Region Interaction of Aging, Genes and Environment; GCSF = granulocyte colony-stimulating factor; IL-1RA = interleukin-1 receptor antagonist; MCP = monocyte chemotactic protein; MMP = matrix metalloproteinase; POP = Prediction of Osteoarthritis Progression; PXN = paraoxonase; RANTES = regulated upon activation, normal T-cell expressed, and secreted; STRRIDE = Studies Targeting Risk Reduction Interventions through Defined Exercise; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor; TRAIL = tumor necrosis factor-related apoptosis-inducing ligand; VCAM = vascular cell adhesion molecule; VEGF = vascular endothelial growth factor.
*Biomarker not assayed.
†Biomarker not included due to high proportion of samples below the lower level of quantification.
Stratified Associations Between Biomarkers and Physical Performance
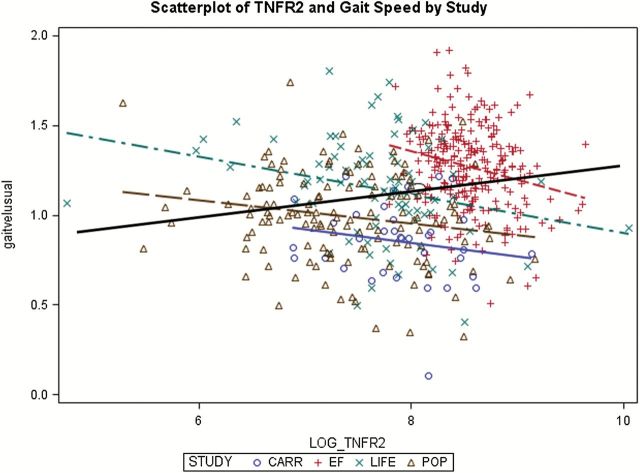
Bivariate Spearman correlations, done after combining VO2 (CALERIE and STRRIDE studies) and gait speed (CARRIAGE, POP, LIFE, and EF studies) data from the studies, generally indicated inverse associations between biomarkers and both physical performance measures (Table 3). Leptin (r = −.78) and D-dimer (r = −.51) had the strongest inverse associations with VO2, while TNFα (r = −.51) and IL-8 (r = −.44) were most strongly inversely associated with gait speed. Four biomarkers had correlations of differing directions with VO2 and gait speed, including: MCP-1 (r = .11 and −.25), RANTES (r = −.25 and .16), TNFR1 (r = .01 and −.41), and TNFR2 (r = −.17 and .20). However, combining data from multiple studies can lead to misinterpretation of associations of variables within individual studies. Figure 1 provides an example, as the correlation of r = .20 of TNFR2 and gait speed (solid black regression line) in the combined datasets misrepresents the inverse correlations found in the individual studies, which are appropriately maintained over all studies when meta-analytic techniques are used.
Table 3.
Associations (Spearman correlation coefficients) of VO2 peak and Gait Speed With Circulating Biomarkers
| Circulating Biomarker | VO2peak | Gait Speed |
|---|---|---|
| D-Dimer | −0.51 | −0.23 |
| GCSF | −0.03 | −0.26 |
| IL-1RA | −0.08 | −0.21 |
| IL-2 | −0.03 | −0.10 |
| IL-6 | −0.07 | −0.05 |
| IL-8 | −0.31 | −0.44 |
| Leptin | −0.78 | −0.25 |
| MCP-1 | 0.11 | −0.25 |
| MMP-3 | NA | 0.22 |
| PXN | −0.04 | −0.18 |
| RANTES | −0.25 | 0.16 |
| TNFα | −0.19 | −0.51 |
| TNFR1 | 0.01 | −0.41 |
| TNFR2 | −0.17 | 0.20 |
| TRAIL | NA | 0.12 |
| VCAM | NA | −0.15 |
| VEGF | NA | −0.25 |
Note: GCSF = granulocyte colony-stimulating factor; IL-1RA = interleukin-1 receptor antagonist; MCP = monocyte chemotactic protein; MMP = matrix metalloproteinase; PXN = paraoxonase; RANTES = regulated upon activation, normal T-cell expressed, and secreted; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor; TRAIL = tumor necrosis factor-related apoptosis-inducing ligand; VCAM = vascular cell adhesion molecule; VEGF = vascular endothelial growth factor; NA = not available.
Figure 1.
Example of potential misinterpretation when combining datasets for analysis.
Weighted Associations Between Biomarkers and Physical Performance
The weighted correlations between circulating biomarkers and physical performance can be observed in Table 4. Of the 17 biomarkers measured, 4 inflammatory markers (IL-6, TNFR2, TNFR1, and TNFα), 1 coagulation marker (D-dimer), and 1 inflammatory mediator (GCSF) were found to be inversely associated with physical performance after controlling for age, body mass index, gender, and race and adjusting for multiple comparisons. In order of strength of the association, IL-6 (weighted r = .22), TNFR2 (weighted r = −.19), D-dimer (weighted r = −.16), TNFR1 (weighted r = −.15), GCSF (weighted r = −.14), and TNFα (weighted r = −.10) were all significantly inversely correlated with physical performance (all p < .05). This indicates that higher circulating concentrations of these particular biomarkers were associated with poorer physical performance across the study cohorts. VCAM, a marker of endothelial activation, also tended to be associated with physical performance but was not significant (r = −.09; Fisher’s Z = −1.87 which equates to p = .06). With the exception of Leptin, the Q statistics were nonsignificant, indicating homogeneity in the associations between biomarkers and physical performance across the studies.
Table 4.
Weighted Correlations Between Circulating Biomarkers and Physical Performance
| Circulating Biomarker | Number of Studies | N Subjects | Weighted Correlation | Fisher’s Z | Q Statistic |
|---|---|---|---|---|---|
| IL-6 | 6 | 603 | −0.22 | −5.24* | 9.27 |
| TNFR2 | 5 | 562 | −0.19 | −4.40* | 0.31 |
| D-Dimer | 5 | 562 | −0.16 | −3.68* | 6.76 |
| TNFR1 | 5 | 562 | −0.15 | −3.54* | 2.85 |
| GCSF | 4 | 309 | −0.14 | −2.37* | 3.61 |
| TNFα | 5 | 525 | −0.10 | −2.31* | 5.78 |
| VCAM | 4 | 469 | −0.09 | −1.87 | 3.25 |
| IL-8 | 5 | 562 | −0.07 | −1.63 | 1.52 |
| RANTES | 4 | 309 | −0.09 | −1.49 | 1.59 |
| PXN | 3 | 241 | 0.09 | 1.39 | 0.43 |
| Leptin | 5 | 350 | −0.07 | −1.26 | 19.68† |
| TRAIL | 3 | 241 | 0.07 | 1.12 | 1.62 |
| VEGF | 3 | 241 | −0.05 | −0.74 | 3.38 |
| IL-1RA | 4 | 309 | −0.03 | −0.54 | 0.99 |
| IL-2 | 4 | 309 | −0.03 | −0.45 | 1.73 |
| MMP-3 | 3 | 241 | 0.03 | 0.46 | 2.21 |
| MCP-1 | 3 | 241 | 0.00 | −0.04 | 0.55 |
Notes: GCSF = granulocyte colony-stimulating factor; IL-1RA = interleukin-1 receptor antagonist; MCP = monocyte chemotactic protein; MMP = matrix metalloproteinase; PXN = paraoxonase; RANTES = regulated upon activation, normal T-cell expressed, and secreted; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor; TRAIL = tumor necrosis factor-related apoptosis-inducing ligand; VCAM = vascular cell adhesion molecule; VEGF = vascular endothelial growth factor.
*Adjusted p < .05 (adjusted for age, BMI, gender, and race).
†Indicates heterogeneity of correlation between studies.
Discussion
We present data that demonstrate significant inverse associations between six circulating biomarkers (IL-6, TNFR2, D-dimer, TNFR1, GCSF, and TNFα) and physical performance, across a diverse group of study cohorts. The mean ages in these study cohorts ranged from late thirties to late seventies; additional demographic measures, such as gender and race, varied considerably between studies. The studies available to us provided an opportunity to test, using meta-analytic methods, if associations exist among markers of disability in dissimilar cohorts. We view this as a unique strength to this work. Despite the demographic heterogeneity, the associations of biomarkers with physical performance were remarkably homogeneous across studies, demonstrating a consistent inverse correlation of six biomarker concentrations with physical performance. The biomarkers that demonstrated no association with physical performance in our study may not represent causal pathways in the genesis of functional decline, and/or their contribution may be too small to be detected by our cross-sectional, correlative analysis. Worth noting is that the pathways that these biomarkers represent, inflammation and coagulation, have considerable “cross-talk” with respect to upregulation of systems and disease sequelae (9). Thus, their unidirectional and consistent strength of association with physical performance as a group may represent similar activation of multiple inflammatory and coagulation pathways that would precede clinical detection in most instances.
Of the six biomarkers with associations with physical performance in our study, D-dimer and, particularly IL-6, have been most commonly discussed in previous work in this area (25). This has been work done primarily in aging cohorts (1,2,26–29) and in all the studies, higher concentrations of IL-6/D-dimer were associated with poor outcomes. Several published articles have used the Established Populations for Epidemiologic Studies of the Elderly cohorts to investigate these associations. These studies reported cross-sectional associations between IL-6 and functional disability (1), longitudinal associations between baseline IL-6/D-dimer and subsequent declines in physical functioning (2), and longitudinal associations between baseline IL-6 and incident disability (30). More recently, Adriaensen and coworkers (31) examined data from the BELFRAIL study (80+ year old cohort from Belgium) and reported cross-sectional associations between IL-6 and global functioning (physical and mental components) in men and women, and highly elevated serum IL-6 levels with poor physical performance. Additionally, while not reaching statistical significance, we observed a similar pattern of association with VCAM and physical performance, as previously described by our group (32).
We observed associations between both IL-6 and D-dimer and physical performance across all our cohorts. This both complements and extends the previous work described above. It is complementary in that it provides further confirmatory evidence of associations between IL-6/D-dimer and physical functioning when simultaneously testing associations with 15 other biomarkers. It extends previous work in that our functional assessments (gait speed and VO2peak) are objective measures of performance that are amenable to change and are a primary focus of interventions aimed at improving function in older adults (14,33,34).
Our finding of an association between TNFR1/TNFR2 and physical performance is a novel finding in populations whose primary diagnosis is not an inflammatory disease. Nicklas and coworkers (35) reported that after adjusting for age, race, sex, and body mass index, older individuals with osteoarthritis of the knee and homozygous for the major T allele of the TNFR2 +676 polymorphism had better physical function. In the current study, TNFR2 had the strongest association with physical performance (weighted r = −.20). Interestingly, the elevation of TNFR2 and its association with lower physical function may represent a counter measure as it has been shown that the TNFR2 signaling pathways appear to offer protective roles in several disorders by enhancing regeneration and repair processes. This has led to the proposal to consider using TNFR2 agonists in the treatment of such inflammatory diseases (3,36). Whether or not similar targeted therapies could improve physical performance across the life span certainly warrants consideration and future examination.
TNFα has been shown to relate to physical performance in several aged patient populations with inflammatory and cardiovascular diseases (37–39) and in well-functioning older adult cohorts such as the Health, Aging and Body Composition study (40). In our study, the CALERIE cohort (younger, overweight, and unfit) demonstrated the strongest inverse association between TNFα and physical performance (r = −.20). In a group of obese middle-aged women, Poelkens and coworkers found that, compared to those with metabolic syndrome, those who were metabolically healthy obese had significantly lower TNFα concentrations (41). Further study is needed to determine whether TNFα could be utilized as a preclinical marker of increased risk of developing metabolic syndrome in otherwise healthy, overweight, sedentary middle-aged adults.
The significant association of GCSF with physical performance is particularly novel. GCSF is a cytokine produced by endothelial cells, fibroblasts, and other immune cells. It is induced by, among others, TNF and ILs (42). It is a mediator of pathways found in this study to be associated with physical performance. To our knowledge, GCSF has not been comprehensively examined as a biomarker of low-level inflammation as part of the aging process. Based on its representation of multiple immune and inflammatory pathways, it may be of benefit to further examine GCSF as a global biomarker of systems dysregulation.
Limitations to this study include the cross-sectional design, potentially uncontrolled confounders, such as medication usage and exercise history, and the incomplete measurement of all biomarkers in all the studies. Another limitation, which is a significant issue with life span research, is lack of a uniform measure of physical performance between younger and older cohorts. Finally, while we used conservative methods for significance testing, Type I error due to multiple tests of association is a potential issue that should be considered when interpreting these findings.
This study provides impetus to continue to understand if and how physiologic dysregulation occurs in a more coordinated, “cross-talk” manner, as opposed to a stochastic manner (8), in which case simultaneously measuring multiple biomarkers representing the spectrum of physiologic systems may not be necessary to quantify preclinical aging-related functional impairment. Conversely, early and midlife concentrations of particular biomarkers (eg, TNFα) may have unique predictive characteristics for increased risk of specific diseases or syndromes that impact function (41). This could be addressed in future longitudinal studies with a standardized physical performance measures and a broad spectrum of biomarkers across multiple times points throughout the adult life span.
In summary, using six cohorts that were diverse in many ways, we observed consistent inverse associations between six biomarkers and objective measures of physical performance. The generalizability of these results in cohorts spanning adulthood suggests that these serum biomarker measures may be broadly applicable for detection, trajectory, and treatment monitoring of physical function across the life span or possibly for midlife predictors of functionally deleterious conditions.
Funding
This was work was supported by the Duke Claude D. Pepper Older Americans Independence Center from the National Institute on Aging at the National Institutes of Health (grant number 1P30 AG028716 to H.J.C.) and the National Cancer Institute at the National Institutes of Health (grant number KM1 CA156687 training fellowship to M.J.P.).
References
- 1. Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–M208. [DOI] [PubMed] [Google Scholar]
- 2. Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. [DOI] [PubMed] [Google Scholar]
- 3. Faustman DL, Davis M. TNF receptor 2 and disease: autoimmunity and regenerative medicine. Front Immunol. 2013;4:478. :10.3389/fimmu.2013.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94:3171–3182. :10.1210/jc.2008-2534 [DOI] [PubMed] [Google Scholar]
- 5. Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54(suppl):S29–S37. :10.1016/j.ypmed.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guralnik JM, Branch LG, Cummings SR, Curb JD. Physical performance measures in aging research. J Gerontol A Biol Sci Med Sci. 1989;44:M141–M146. [DOI] [PubMed] [Google Scholar]
- 7. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. [DOI] [PubMed] [Google Scholar]
- 8. Sanders JL, Ding V, Arnold AM, et al. Do changes in circulating biomarkers track with each other and with functional changes in older adults? J Gerontol A Biol Sci Med Sci. 2014;69:174–181. doi:10.1093/gerona/glt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Poll T, Levi M. Crosstalk between inflammation and coagulation: the lessons of sepsis. Curr Vasc Pharmacol. 2012;10:632–638. [DOI] [PubMed] [Google Scholar]
- 10. Malina RM. Tracking of physical activity and physical fitness across the lifespan. Res Q Exerc Sport. 1996;67:S48–S57. [DOI] [PubMed] [Google Scholar]
- 11. Van Cleave JH, Egleston BL, Bourbonniere M, McCorkle R. Combining extant datasets with differing outcome measures across studies of older adults after cancer surgery. Res Gerontol Nurs. 2011;4:36–46. :10.3928/19404921-20101201-02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. :10.1038/oby.2008.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kraus WE, Torgan CE, Duscha BD, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc. 2001;33:1774–1784. [DOI] [PubMed] [Google Scholar]
- 14. Morey M, Peterson M, Pieper C, et al. The Veterans Learning to Improve Fitness and Function in Elders Study: a randomized trial of primary care-based physical activity counseling for older men. J Am Geriatr Soc. 2009;57:1166–1174. :10.1111/j.1532-5415.2009.02301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morey MC, Pieper CF, Edelman DE, et al. Enhanced Fitness: a randomized controlled trial of the effects of home-based physical activity counseling on glycemic control in older adults with prediabetes mellitus. J Am Geriatr Soc. 2012;60:1655–1662. doi:10.1111/j.1532-5415.2012.04119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kraus VB, McDaniel G, Worrell TW, et al. Association of bone scintigraphic abnormalities with knee malalignment and pain. Ann Rheum Dis. 2009;68:1673–1679. :10.1136/ard.2008.094722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen HC, Shah S, Stabler TV, Li YJ, Kraus VB. Biomarkers associated with clinical phenotypes of hand osteoarthritis in a large multigenerational family: the CARRIAGE family study. Osteoarthritis Cartilage. 2008;16:1054–1059. :10.1016/j.joca.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson DK, Huffman KM, Kraus WE, Kraus VB. Critical appraisal of four IL-6 immunoassays. PLoS One. 2012;7:e30659. :10.1371/journal.pone.0030659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malatesta D, Simar D, Dauvilliers Y, et al. Aerobic determinants of the decline in preferred walking speed in healthy, active 65- and 80-year-olds. Pflugers Arch. 2004;447:915–921. [DOI] [PubMed] [Google Scholar]
- 20. Cunningham DA, Rechnitzer PA, Pearce ME, Donner AP. Determinants of self-selected walking pace across ages 19 to 66. J Gerontol. 1982;37:560–564. [DOI] [PubMed] [Google Scholar]
- 21. Fiser WM, Hays NP, Rogers SC, et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci. 2010;65:1332–1337. :10.1093/gerona/glq137 [DOI] [PubMed] [Google Scholar]
- 22. Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Biol Sci Med Sci. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 23. Fleiss J, Levin B, Paik M.Statistical Methods for Rates and Proportions. 3rd ed. New York City: Wiley; 2003. [Google Scholar]
- 24. Hedges L, Olkin I.Statistical Methods for Meta Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 25. Roubenoff R. The “cytokine for gerontologists” has some company. J Gerontol A Biol Sci Med Sci. 2014;69:163–164. :10.1093/gerona/glt184 [DOI] [PubMed] [Google Scholar]
- 26. Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. [DOI] [PubMed] [Google Scholar]
- 27. Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004;36:960–964. [DOI] [PubMed] [Google Scholar]
- 28. Beavers KM, Hsu FC, Houston DK, et al. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2013;68:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014;26:2–12. :10.1016/j.smim.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 30. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. [DOI] [PubMed] [Google Scholar]
- 31. Adriaensen W, Matheï C, van Pottelbergh G, et al. Significance of serum immune markers in identification of global functional impairment in the oldest old: cross-sectional results from the BELFRAIL study. Age (Dordr). 2014;36:457–467. :10.1007/s11357-013-9558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huffman KM, Pieper CF, Kraus VB, Kraus WE, Fillenbaum GG, Cohen HJ. Relations of a marker of endothelial activation (s-VCAM) to function and mortality in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1369–1375. :10.1093/gerona/glr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. :10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nicklas BJ, Mychaleckyj J, Kritchevsky S, et al. Physical function and its response to exercise: associations with cytokine gene variation in older adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2005;60:1292–1298. [DOI] [PubMed] [Google Scholar]
- 36. Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:165–173. 10.1093/gerona/glt023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683–693. [DOI] [PubMed] [Google Scholar]
- 38. Roubenoff R, Roubenoff RA, Cannon JG, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. [DOI] [PubMed] [Google Scholar]
- 41. Poelkens F, Eijsvogels TM, Brussee P, Verheggen RJ, Tack CJ, Hopman MT. Physical fitness can partly explain the metabolically healthy obese phenotype in women. Exp Clin Endocrinol Diabetes. 2014;122:87–91. :10.1055/s-0033-1363686 [DOI] [PubMed] [Google Scholar]
- 42. Fleiner HF, Radtke M, Ryan L, Moen T, Grill V. Circulating immune mediators are closely linked in adult-onset type 1 diabetes as well as in non-diabetic subjects. Autoimmunity. 2014;47:530–537. :10.3109/08916934.2014.938321 [DOI] [PubMed] [Google Scholar]