Healthcare worker (HCW) exposure to measles results in high-intensity, high-cost exposure investigations. Our measles outbreak experience highlights the importance of N95 use and education and furlough of exposed HCWs at the first sign of illness, regardless of immunity.
Keywords: measles, exposure investigation, healthcare worker immunization, transmission of communicable disease, measles immunity
Abstract
Background. When caring for measles patients, N95 respirator use by healthcare workers (HCWs) with documented immunity is not uniformly required or practiced. In the setting of increasingly common measles outbreaks and provider inexperience with measles, HCWs face increased risk for occupational exposures. Meanwhile, optimal infection prevention responses to healthcare-associated exposures are loosely defined. We describe measles acquisition among HCWs despite prior immunity and lessons from healthcare-associated exposure investigations during a countywide outbreak.
Methods. Primary and secondary cases, associated exposures, and risk factors were identified during a measles outbreak in Orange County, California from, 30 January 2014 to 21 April 2014. We reviewed the effect of different strategies in response to hospital exposures and resultant case capture.
Results. Among 22 confirmed measles cases, 5 secondary cases occurred in HCWs. Of these, 4 had direct contact with measles patients; none wore N95 respirators. Four HCWs had prior evidence of immunity and continued working after developing symptoms, resulting in 1014 exposures, but no transmissions. Overall, 13 of 15 secondary cases had face-to-face contact with measles patients, 8 with prior evidence of immunity.
Conclusions. HCWs with unmasked, direct contact with measles patients are at risk for developing disease despite evidence of prior immunity, resulting in potentially large numbers of exposures and necessitating time-intensive investigations. Vaccination may lower infectivity. Regardless of immunity status, HCWs should wear N-95 respirators (or equivalent) when evaluating suspected measles patients. Those with direct unprotected exposure should be monitored for symptoms and be furloughed at the earliest sign of illness.
A decade after measles was declared nonendemic, the United States saw a rise in outbreaks, most associated with importation by unvaccinated individuals traveling internationally [1, 2]. More measles cases were reported nationally in 2014 than in any year since elimination was declared in 2000 [1]. Measles outbreaks significantly impact public health systems and healthcare facilities, costing an estimated $2.7 to $5.3 million in 2011 alone [3, 4].
Healthcare worker (HCW) inexperience with measles contributes to delayed recognition and diagnosis, increasing the potential for healthcare-setting exposures. HCWs caring for measles patients frequently have face-to-face contact, placing them at high acquisition risk. If they subsequently develop illness, HCWs can potentially expose large numbers of patients and staff [5]. Evidence of measles immunity by history or laboratory confirmation significantly lowers, but does not eliminate, the risk of secondary infection [6, 7]. Despite this, guidance has not always been uniform in requiring N95 respirator use among HCWs with documented immunity to measles; when recommended, the necessity of N95 respirator use has been questioned by practitioners [8, 9]. Furthermore, although most HCWs are required to have evidence of measles immunity as a condition of employment, enforcement of such policies is variable. While occupational health standards for immunization may be stringent upon hire, documentation of immunity in HCWs after hire is less consistent [10]. Infection control and occupational health strategies often treat historical documentation of measles immunity as absolute, despite the low but present risk for measles infection in persons with evidence of immunity.
In this study, we report an outbreak of measles in Orange County, California, in which 4 secondarily exposed HCWs developed disease despite history of vaccination and immunity, resulting in multiple labor-intensive exposure investigations with no further cases identified. We found that N95 respirator use by HCWs regardless of immunity and prompt follow-up for assessment of exposed staff could dramatically reduce healthcare-associated exposures. We also identify key primary prevention strategies to limit healthcare-associated exposures adopted at our facility.
METHODS
The Orange County Health Care Agency (OCHCA) was notified of suspect primary measles cases by local medical providers; secondary suspect cases were reported by clinical providers or identified directly by OCHCA. Measles testing was arranged for suspect cases presenting with rash with some combination of fever (temperature, > 101°F), cough, coryza, or conjunctivitis. Oropharyngeal polymerase chain reaction (PCR) testing was performed for patients presenting within 3 days of rash onset, with urine PCR and measles serum immunoglobulin M (IgM) testing whenever possible. Patients presenting days 4–10 after rash onset had oropharyngeal and urine PCR testing, frequently with measles IgM testing as well. Patients with rash beginning more than 10 days post-exposure had measles IgM testing, with oropharyngeal and/or urine PCR testing on a case-by-case basis. Exposures were defined, per California Department of Public Health (CDPH) guidance, as persons sharing the same airspace with a measles patient within 4 days before or after rash onset or who were in these areas within 1 hour of an infectious measles case [11].
Exposed county residents were assessed by OCHCA. Healthcare facility patient exposures were co-managed with OCHCA. Healthcare facility staff exposures were managed primarily by the facility with OCHCA support. Clinical information for suspected and confirmed cases was obtained from medical chart reviews and phone/in-person interviews; the latter was attempted for all close contacts (household, social, workplace, or other contact with risk for prolonged exposure). Acceptable evidence of immunity for those working in high-risk occupations (healthcare/daycare settings) was defined as written documentation of 2 measles, mumps, and rubella (MMR) vaccine doses or positive measles serum IgG. Non-high–risk exposures were defined as those exposed (sharing the same room for any length of time) to an infectious measles patient without close contact, underlying medical conditions including pregnancy and immunocompromised status, or high-risk occupations such as HCW or daycare worker.
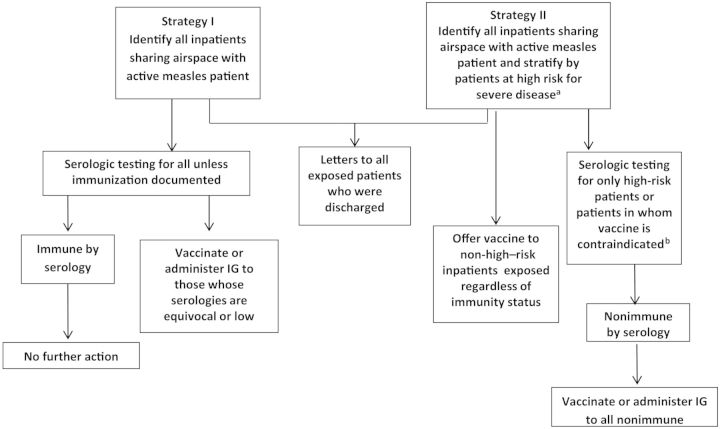
OCHCA or hospital infection prevention program staff attempted to reach all identified contacts. Contacts were informed of their exposure, educated on measles symptoms, and asked to report any illness to OCHCA. High-risk contacts were monitored for symptoms by periodic follow-up phone calls. Strategies for inpatient exposure investigations used by an academic medical center during this outbreak are shown in Figure 1.
Figure 1.
Strategies for exposure investigation used by academic health center during 2 measles exposure investigations. aPregnant, Age <12 months, or Immunocompromised; bPatients with vaccine allergy, severe immunocompromise (malignancy, neutropenia, on short or long term immunosuppressives, AIDS), or pregnancy. Abbreviation: IG, immunoglobulin.
Serology was performed for measles-specific IgM/IgG at OCHCA or at the facilities where patients presented. All positive samples from community laboratories were confirmed by OCHCA, which used the measles virus IgM/IgG antibody test system (MBL Bion, Des Plaines, Illinois; www.mblintl.com). All measles viral RNA reverse transcription polymerase chain reaction (RT-PCR) virologic testing was performed at OCHCA using RT-PCR assay with the following 3 gene targets: nucleoprotein (N3), hemagglutinin (H1), and fusion (F1). The CDPH Viral and Rickettsial Disease Laboratory performed measles genotype sequencing [12].
RESULTS
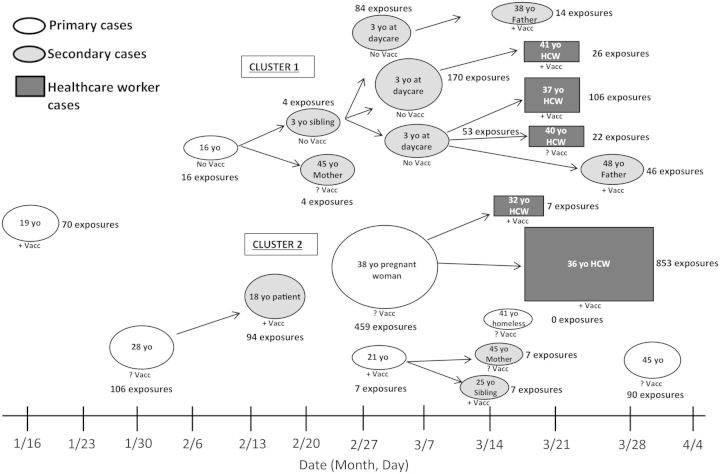
The outbreak involved 22 confirmed cases diagnosed in Orange County from 16 January 2014 to 21 April 2014; 7 were primary (with no known epidemiologic link to a source case) and 15 were secondary cases (Figure 2). Among 2245 exposures, 1994 (88%) were healthcare associated; 6 secondary cases resulted from exposure in a healthcare setting, 5 of these were HCWs. Among secondary cases, 13 (86.6%) had direct face-to-face contact with measles patients; among these, 8 (61.5%) had evidence of immunity against measles. PCR confirmed measles in 16 cases. Genotyping of PCR-positive samples revealed B3 measles virus (associated with strains in Philippines outbreaks) in all 16 cases. Four cases identified retrospectively were not PCR tested but were measles IgM positive. One patient refused testing but met clinical criteria for measles based on classic presentation and known close contact with a confirmed case. One HCW with 2 previously documented MMR vaccine doses was PCR negative (oropharyngeal and urine samples) and serum IgM negative but met criteria for measles based on history of descending rash with fever and cough developing after an appropriate incubation period following exposure to a known case. Serologic testing was performed on 19 of 22 cases; 11 were IgM positive.
Figure 2.
Measles outbreak and identified exposures across outbreak period. Abbreviations: HCW, healthcare worker; +Vacc, vaccinated; ?Vacc, unknown vaccination status; No Vacc, not vaccinated; yo, years old.
The first identified measles case was a 19-year-old female with known exposure to measles during travel to the Philippines who developed disease despite 3 documented MMR vaccine doses. Subsequently, 6 additional primary cases were identified, none with measles exposure history or recent travel to measles-endemic countries. The outbreak consisted of 2 clusters. Cluster 1 occurred in an affluent community and began with illness in a vaccine-refusing family with subsequent spread in a daycare center. Cluster 2 centered on a less affluent Latino adult community in a small geographic area over a short period of time, with likely unrecognized common exposures. Among the affected Latinos, vaccination history was mostly unknown or vaccinated; none were identified to have refused vaccination.
Demographic and clinical characteristics stratified by primary vs secondary measles cases and vaccination status is shown in Table 1. Among cases with known immunization status, all 5 unvaccinated individuals were aged <16 years and white. Two secondary cases acquired measles following household exposure to a single primary case who had 2 documented MMR vaccine doses; 12 occurred after exposure to 5 unvaccinated measles cases; and 1 acquired disease after exposure to a primary case with unknown immunity.
Table 1.
Patient Characteristics Among Confirmed Measles Cases for the 2014 Measles Outbreak in Orange County, California
| Patient Characteristic | Primary vs SecondaryCases |
Vaccinated vs Unvaccinated Casesa |
||
|---|---|---|---|---|
| Primaryb (n = 7) | Secondaryc (n = 15) | Vaccinatedd (n = 10) | Unvaccinatede (n = 5) | |
| Demographics | ||||
| Age, y (median, range) | 30 (16–45) | 36 (3–48) | 34 (18–48) | 3 (3–16) |
| Male, % | 57.1 | 53.3 | 50 | 80 |
| Race/Ethnicity (n, %) | ||||
| White, Non-Hispanic | 0 (0) | 8 (53) | 4 (40) | 3 (60) |
| Hispanic | 5 (71) | 4 (27) | 4 (40) | 0 (0) |
| Asian/Pacific Islander | 2 (29) | 3 (20) | 2 (20) | 2 (40) |
| Clinical | ||||
| Day of illness on presentation (median, range) | 5 (2–6) | 3 (1–5) | 4 (1–5) | 3 (1–4) |
| Days from first presentation to diagnosis (median, range) | 4 (1–20) | 2 (1–8) | 4 (1–5) | 3 (1–4) |
| Overall clinical presentation with typical measles featuresf (%) | 71.4 | 53.3 | 20 | 100 |
| Complications associated with measlesg | 0 | 0 | 0 | 0 |
| Presence of comorbidityh (%) | 42.9 | 0 | 0 | 0 |
| Primary clinical diagnosis other than measles on first presentation (%) | 57.1 | 60 | 40 | 80 |
| Documented history of vaccination or positive titer (%) | 28.6 | 53.3 | … | … |
| Travel history | 1 | 0 | 1 | 0 |
| Admitted to hospital (%) | 57.1 | 20 | 10 | 40 |
| Percent immunoglobulin M+ during active illness (%) | 71.4 | 53.3 | 0 | 100 |
| Public health and infection control factors | ||||
| Primary locations of measles diagnosis | ||||
| Emergency department (%) | 71.4 | 33.3 | 20 | 40 |
| Inpatient (%) | … | 6.7 | … | 20 |
| Public health center (%) | 28.6 | 60 | 80 | 40 |
| Number of medical visits prior to diagnosis (median, range) | 2.0 (1–3) | 1.0 (1–3) | 1 (1–2) | 3 (2–3) |
| Time between arrival and placement in airborne isolation, median hours (range) | 4.42 (0–8.0) | 2.0 (0.08–48) | … | … |
| Number exposed | 748 | 1497 | 1230 | 327 |
| Healthcare exposures | 706 | 1288 | 1053 | 281 |
| Household exposures | 30 | 75 | 49 | 28 |
| Social events | 0 | 23 | 23 | 0 |
| Daycare setting | 12 | 14 | 8 | 18 |
| School exposures | 0 | 69 | 69 | 0 |
| Work exposures | 0 | 28 | 28 | 0 |
| Number of transmissions | 7 | 8 | 2 | 10 |
a Vaccination or immunity status unknown for 7 cases.
b Primary cases = index patient and/or patient in whom exposure is not clearly associated with known measles case.
c Secondary cases = patient with exposure to known measles case.
d Vaccinated cases defined as patients presenting with measles with documented history of childhood measles, mumps, and rubella (MMR) vaccination series completion and/or known previous serologic testing confirming immunization titers.
e Unvaccinated cases defined as patients presenting with measles with history of not receiving any or receiving incomplete childhood MMR vaccination.
f Typical clinical presentation defined as presence of fever (subjective or objective), rash, and either cough, coryza, or conjunctivitis.
g Complications defined as otitis media, pneumonia, seizures, encephalitis.
h One patient was pregnant, 1 with schizophrenia, 1 with controlled diabetes mellitus and hypertension. No patients were immunocompromised.
Clinical presentation with typical signs and symptoms of measles (prodromal temperature ≥101°F [38.3°C] and some combination of cough, coryza, and/or conjunctivitis followed by descending maculopapular rash) were found in 10 of 11 cases who were not known to have had measles exposure when initially seen by an HCW. Nevertheless, only 2 of 11 (18%) had measles on initial differential diagnosis. The majority of secondary cases and those occurring in vaccinated individuals had known measles exposure and diagnosed by public health personnel, resulting in earlier diagnoses.
Seven patients, including all HCWs, were known to be exposed to measles and evaluated by public health personnel immediately upon rash onset, causing no healthcare exposures during their medical assessment. Three cases were identified retrospectively, and healthcare exposure information was unavailable. One patient was identified retrospectively and reported not seeking medical care. No patients developed pneumonia or encephalitis, and all recovered without sequelae.
Median time to airborne isolation was twice as long among cases without exposure, the longest being for patients admitted with a primary diagnosis other than measles. For example, of two 3-year-olds exposed to measles at the same daycare center, 1 was diagnosed with Kawasaki disease and the other with an unknown febrile illness. Both presented with typical measles symptoms and neither was placed in airborne precautions until 48 and 24 hours, respectively. Similarly, a pregnant woman diagnosed with a “viral exanthem” was placed in airborne precautions 8 hours after arrival, resulting in 450 exposures. Among those placed in airborne precautions ≥2 hours after arrival to a healthcare facility, all had typical measles presentation.
The majority of identified exposures in this outbreak were healthcare associated and fueled by secondary measles among HCWs. All HCWs had been informed of their exposure and assessed for evidence of immunity prior to illness. HCW-associated facility exposures occurred while working during prodromal symptoms. Table 2 displays clinical and epidemiologic details of the 5 HCWs who acquired measles, resulting in 1014 exposures. None wore N95 respirators on initial examination of measles patients. All but 1 had evidence of immunity. Of note, 4 of 5 HCWs had direct, close contact with measles patients; 1 HCW with documented serologic immunity was working in the same emergency room but did not recall direct interaction with the measles case. Only 2 HCWs who acquired measles presented with typical symptoms, one of whom was the HCW with uncertain vaccination history. The 3 HCWs who continued working while symptomatic (prior to recognition of measles) generated the majority of exposures, had prior acceptable evidence of immunity, and had mild prodromal symptoms. No secondary cases occurred among the large number of patients and staff who these HCWs exposed.
Table 2.
Secondary Measles Cases Among Healthcare Workers
| Age, y | Gender | Measles Immunity Prior to Exposure | Date of Exposure | Date of Illness Onseta | Fever | Cough | Coryza | Date of Rash Onset | Days Infectious While Asymptomatic | Days Working During Active Symptoms | Number of Patients Exposed |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 32 | F | IgG titer positive | 3/3/2014 | 3/17/2014 | Y | Y | N | 3/18/14 | 3 | 0 | 0b |
| 36 | F | IgG titer positive | 3/3/2014 | 3/14/2014 | Y | N | N | 3/18/14 | 0 | 4 | 850 |
| 41 | M | 2 MMR vaccine doses | 3/7/2014 | 3/18/2014 | Y | N | N | 3/20/14 | 2 | 2 | 26 |
| 37 | M | 4 MMR vaccine doses, IgG titer positive | 3/7/2014 | 3/16/2014 | N | Y | N | 3/20/14 | 0 | 4 | 72 |
| 40 | F | Uncertain vaccine history, IgG titer equivocal | 3/7/2014 | 3/19/2014 | Y | Y | Y | 3/21/14 | 2 | 0 | 22c |
Abbreviations: IgG, immunoglobulin G; MMR, measles, mumps, and rubella.
a No healthcare workers presented with conjunctivitis.
b Not scheduled for work for 4 days prior to rash developing.
c Furloughed from work, but went to emergency department to be seen.
Two strategies used at an academic healthcare center for 2 patients presenting with measles are shown in Figure 2. The first case generated 140 exposures among inpatients sharing the same airspace with the measles patient, including all hospital floors sharing air circulation with the emergency room and the medical floor the patient occupied before placement in airborne precautions. Using strategy I, all inpatients underwent serologic testing. Among 64 inpatients, all except 4 were measles IgG positive; among these, 2 were equivocal and 2 were negative. All 4 received measles post-exposure prophylaxis vaccination within 72 hours of exposure. Similarly high levels of immunity were found on public health serologic evaluation of identified exposed individuals not meeting CDPH criteria for likely immunity. Even among those in this group, more than 95% had positive measles titers [11]. In another case, strategy II was adopted and revealed 62 inpatients exposed to measles, 27 of whom received post-exposure vaccination. The remainder declined vaccination, many of whom recalled prior MMR vaccination. Among 11 requiring serology, all had positive titers. None of the exposed inpatients in either case developed disease.
We calculated the number of known persons exposed for each measles case and identified strategies that could have prevented these exposures if implemented. Immediate triage into airborne isolation of patients presenting with 1–3 measles symptoms could have averted 980 (43%) exposures. N95 respirator use by HCWs regardless of immunization status could have prevented up to 1014 (100%) of all HCW-associated exposures. Once exposed, daily monitoring of HCWs for symptoms and furlough at the first sign of illness could have potentially prevented 922 (91%) identified HCW-related exposures and 41% of all identified exposures.
DISCUSSION
This measles outbreak highlights HCW risk of becoming infected while caring for measles patients, regardless of presumptive evidence of immunity. History of immunity provided false reassurance to HCWs with unprotected face-to-face exposures during the outbreak, leading them to continue working even when prodromal symptoms appeared. Our findings emphasize the importance of adherence to the recent Centers for Disease Control and Prevention (CDC) recommendation for use of N95 or equivalent respirator for suspect measles cases regardless of immunity status [13, 14]. This presents a challenge for primary care facilities that frequently do not stock N95 respirators or have their staff fit-tested. However, even in facilities where N95 respirators are available and fit-testing is standard, compliance with this recommendation is variable [8].
Timely N95 respirator use relies on provider suspicion of measles, which may not be appreciated until after direct contact with infected patients, particularly in post-elimination era settings where clinical experience with measles is low. In our study, 80% of patients required multiple visits before diagnosis. This finding underscores the need for continued, periodic education on previously eliminated diseases of front-line HCWs. The cost of missed diagnosis of this highly communicable disease obligates consideration of conservative primary prevention strategies in triage. After this outbreak, our facility began immediate triage of patients presenting with any rash, using signage to guide patients to enter the facility away from the emergency room waiting area and directly into airborne isolation until further evaluation by nursing staff. Subsequently, 2 patients with suspected measles immediately triaged in this manner (ultimately testing negative) would have resulted in zero healthcare-associated exposures. Triaging on rash alone may not be feasible in the primary care setting. Importantly, we estimate that the addition of other symptoms to this triaging strategy could still avoid a substantial number of exposures. Strategies for primary prevention of exposures used at this academic hospital were subsequently refined and are detailed in Supplementary Table 4.
The mild nature of prodromal symptoms in previously vaccinated HCWs likely further delayed diagnosis. Identification and furlough at the earliest signs of illness could have prevented 91% of healthcare exposures. HCWs with evidence of measles immunity who have face-to-face contact with a measles patient can continue to work; however, they should be made aware of their risk (albeit low) for developing illness despite prior immunity. These HCWs should be monitored for ongoing symptoms and be furloughed at the earliest sign of illness.
Measles immunization was highly protective in our county's outbreak, with only 6 secondary cases in healthcare settings despite more than 1000 exposures. Moreover, vaccination appeared to minimize infectivity among the few who developed breakthrough disease despite vaccination. None of the previously vaccinated individuals (including HCWs) who acquired measles resulted in further transmission despite 1053 identified exposures. Meanwhile, 5 unvaccinated individuals who exposed far fewer individuals (281) resulted in 6 secondary cases. Efforts by hospital infection prevention and occupational health program staff charged with maintaining concurrent and complete employee vaccination rates prove critical in the setting of exposures, as in this outbreak. State immunization requirements, investments in automated systems to augment these efforts, and mandatory vaccination strategies can substantially mitigate risks and optimize resource use in response to exposures [15–18].
Measles exposure investigations represent high-cost, high-stress efforts for local public health departments and hospital infection prevention programs in the post-elimination era, with unclear net returns [4]. While extensive guidance exists on case definition and high-risk exposure investigation, the recommended extent and scale of investigation for non-high–risk exposures is less well outlined [19]. The CDC includes both persons sharing the same room and those sharing the same airspace as “high priority groups for contact investigation” [19]. It is noteworthy that 86.6% of secondary cases seen in our outbreak had documented face-to-face contact with measles patients. These direct encounters carried a risk of infection despite history of prior vaccination or immunity. Persons with only airspace exposure (most often persons sharing the same waiting room in a clinic or emergency department setting) to cases were unlikely to develop disease. While shared airspace transmissions in healthcare settings have occurred, the majority of these have been in settings where measles is endemic, where vaccination rates have not achieved herd immunity, or where structural engineering of facilities is less well developed [5]. Moreover, surveying low-risk, nondirect contact exposures was labor intensive and identified few nonimmune persons. Based on our experience during this outbreak, OCHCA no longer surveys all exposed patients for evidence of immunity but rather works with healthcare facilities to notify low-risk patients of their exposure, with follow-up recommendations if symptoms develop. Serologic testing is reserved for high-risk exposures, infants, pregnant women, the immunocompromised, and those working in locations such as healthcare and daycare settings.
Almost 15 years have passed since measles was declared eliminated from the United States [20]. Though we still face challenges in maintaining high vaccination rates in some communities, overall, the majority of the population has been vaccinated or has natural immunity. While it is understood that previously vaccinated individuals who acquire measles may present atypically, clinical presentation or laboratory diagnostic criteria in the post-elimination era has not been systematically characterized [21–23]. Documented transmission of measles from a previously vaccinated individual has been reported once previously and was seen in our outbreak, but seems rare. Information from sporadic outbreaks suggests that vaccinated individuals who acquire measles are less infectious [4, 6, 24, 25]. How much less infectious such cases are is unknown. Further definition could substantially streamline exposure investigations. Our findings also raise additional questions about the prevalence and significance of waning immunity in previously vaccinated persons, particularly HCWs. Waning immunity has not been well studied in adults in the post-elimination era.
Our investigation has several limitations. We were unable to identify or reach all community contacts of measles cases. In the healthcare setting, not all contacts could be reached. Not all family members accompanying case patients were identified or reachable. HCWs or patients who may have developed unrecognized measles are not reflected in our findings. While reporting measles cases to public health is mandated, such cases are not always identified or reported. Finally, seroimmunity rates in our county are consistently higher than 98%; communities with lower immunization rates may need to consider response and contact investigation strategies that are different from those suggested here.
Current recommendations for measles exposure investigations in healthcare settings emphasize the high risk of transmission and the protective value of immunization; our county's outbreak experience affirmed these principles. However, we also found that face-to-face exposure to someone with measles conferred substantial risk of infection, independent of immunity status. In our study, nondirect airborne exposures within healthcare facilities did not result in additional cases. For HCWs, breakthrough cases of disease can occur despite meeting CDC criteria for acceptable evidence of immunity. Investigation of measles exposures in healthcare facilities should ensure that exposed HCWs are educated on the possibility of breakthrough disease. Regardless of immunity status, HCWs should wear N-95 respirators on room entry when evaluating suspect measles patients, and those with face-to-face unprotected exposure should be monitored closely for symptoms, with consideration of furlough at the earliest sign of illness.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Michael Brown from the Orange County Health Care Agency and Abiy Tadesse at the California Department of Public Health for their work in polymerase chain reaction genotype sequencing and analysis of the measles samples.
Potential conflicts of interest. S. S. H. conducted a clinical trial for which participating hospitals are receiving contributed product from Sage Products and Molnycke. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Measles cases in the United States reach 20-year high, 2014. Available at: http://www.cdc.gov/media/releases/2014/p0529-measles.html Accessed August 2014.
- 2.Chen TH, Kutty P, Lowe LE, et al. Measles outbreak associated with an international youth sporting event in the United States, 2007. Pediatr Infect Dis J 2010; 29:794–800. [DOI] [PubMed] [Google Scholar]
- 3.Ortega-Sanchez IR, Vijayaraghavan M, Barskey AE, Wallace GS. The economic burden of sixteen measles outbreaks on United States public health departments in 2011. Vaccine 2014; 32:1311–7. [DOI] [PubMed] [Google Scholar]
- 4.Chen SY, Anderson S, Kutty PK, et al. Health care-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis 2011; 203:1517–25. [DOI] [PubMed] [Google Scholar]
- 5.Komitova R, Kunchev A, Mihneva Z, Marinova L. Nosocomial transmission of measles among healthcare workers, Bulgaria, 2010. Euro Surveill 2011; 16:pii:19842. [PubMed] [Google Scholar]
- 6.Rosen JB, Rota JS, Hickman CJ, et al. Outbreak of measles among persons with prior evidence of immunity, New York City, 2011. Clin Infect Dis 2014; 58:1205–10. [DOI] [PubMed] [Google Scholar]
- 7.Willy ME, Koziol DE, Fleisher T, et al. Measles immunity in a population of healthcare workers. Infect Control Hosp Epidemiol 1994; 15:12–7. [DOI] [PubMed] [Google Scholar]
- 8.Alberta Health Services. HCW Personal Protective Equipment: Measles Guidelines. Available at: http://www.albertahealthservices.ca/9914.asp Accessed 2 May 2014.
- 9.Oregon State Public Health. Measles Investigative Guidelines, 2015. Available at: https://public.health.oregon.gov/DiseasesConditions/CommunicableDisease/ReportingCommunicableDisease/ReportingGuidelines/Documents/measles.pdf Accessed 13 July 2015.
- 10.Baxter D. Specific immunization issues in the occupational health setting. Occup Med (Lond) 2007; 57:557–63. [DOI] [PubMed] [Google Scholar]
- 11.California Department of Public Health. Measles Investigation Quicksheet, 2013. Available at: http://www.cdph.ca.gov/programs/immunize/Documents/CDPHMeaslesInvestigationQuicksheet.pdf Accessed 15 April 2015.
- 12.Rota PA, Brown KE, Hubschen JM, et al. Improving global virologic surveillance for measles and rubella. J Infect Dis 2011; 204(suppl 1):S506–13. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Measles: For Healthcare Professionals, 2015. Available at: http://www.cdc.gov/measles/hcp/ Accessed 30 April 2015.
- 14.Shefer A, Atkinson W, Friedman C, et al. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep 2011; 60(RR07):1–45. [PubMed] [Google Scholar]
- 15.Atkinson WL, Markowitz LE, Adams NC, Seastrom GR. Transmission of measles in medical settings—United States, 1985–1989. Am J Med 1991; 91:320S–4. [DOI] [PubMed] [Google Scholar]
- 16.Lindley MC, Horlick GA, Shefer AM, Shaw FE, Gorji M. Assessing state immunization requirements for healthcare workers and patients. Am J Prev Med 2007; 32:459–65. [DOI] [PubMed] [Google Scholar]
- 17.Backer H. Counterpoint: in favor of mandatory influenza vaccine for all health care workers. Clin Infect Dis 2006; 42:1144–7. [DOI] [PubMed] [Google Scholar]
- 18.Russi M, Buchta WG, Swift M, et al. Guidance for occupational health services in medical centers. J Occup Environ Med 2009; 51:1e–18. [DOI] [PubMed] [Google Scholar]
- 19.CDC. Manual for the Surveillance of Vaccine-Preventable Diseases, 2014. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html. Accessed 15 April 2014.
- 20.Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. J Infect Dis 2004; 189(suppl 1):S1–3. [DOI] [PubMed] [Google Scholar]
- 21.Edmonson MB, Addiss DG, McPherson JT, Berg JL, Circo SR, Davis JP. Mild measles and secondary vaccine failure during a sustained outbreak in a highly vaccinated population. JAMA 1990; 263:2467–71. [PubMed] [Google Scholar]
- 22.Pedersen IR, Mordhorst CH, Glikmann G, von Magnus H. Subclinical measles infection in vaccinated seropositive individuals in arctic Greenland. Vaccine 1989; 7:345–8. [DOI] [PubMed] [Google Scholar]
- 23.Huiss S, Damien B, Schneider F, Muller CP. Characteristics of asymptomatic secondary immune responses to measles virus in late convalescent donors. Clin Exp Immunol 1997; 109:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugerman DE, Barskey AE, Delea MG, et al. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics 2010; 125:747–55. [DOI] [PubMed] [Google Scholar]
- 25.Marin M, Nguyen HQ, Langidrik JR, et al. Measles transmission and vaccine effectiveness during a large outbreak on a densely populated island: implications for vaccination policy. Clin Infect Dis 2006; 42:315–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.