Introduction
Leprosy or Hansen's disease is a non-fatal, slowly progressive, chronic granulomatous infection caused by Mycobacterium leprae and was first mentioned in ancient Indian texts from sixth century B.C.1 There is preferential involvement of the skin, peripheral nervous system, upper respiratory tract, eyes and testes due to their lower temperature than the core body temperature. The neurotropism of the bacteria and immune-mediated reactions cause much debilitation and mutilation and consequent psychosocial damage. Based on the individual's immune status, leprosy presents as a continuum extending from polar tuberculoid to polar lepromatous leprosy. Patients with all forms of leprosy, but particularly those with the borderline tuberculoid (BT) form may develop abscesses of nerves, most commonly the ulnar nerve (1). This article illustrates the ultrasonographic and magnetic resonance imaging (MRI) appearance of ulnar nerve abscess in a patient with leprosy.
Case report
A 34-year-old male patient of BT leprosy who had completed two years of multi-drug therapy two years ago, presented with occasional pain and tingling sensation along the inner aspect of his left lower arm of six month's duration. There was no history of fever. He also noticed a superficial thick cord-like swelling at the same place and impaired sensations along the inner aspect of his hand.
Physical examination revealed tender thickening of the left ulnar nerve for approximately 8.0 cm proximal to the medial epicondyle of the humerus. Multiple soft nodules were palpable along the course of the nerve. ‘Ulnar claw’ was noted with inability to extend the interphalangeal joints of the 4th and 5th digits. Thenar and hypothenar muscle wasting along with ‘guttering’ of the 1st web space were present. Sensations were impaired for all modalities along the medial aspect of the hand and the fifth digit. There were no local trophic changes. The rest of the physical examination including the eyes, testes and nose did not reveal any abnormality.
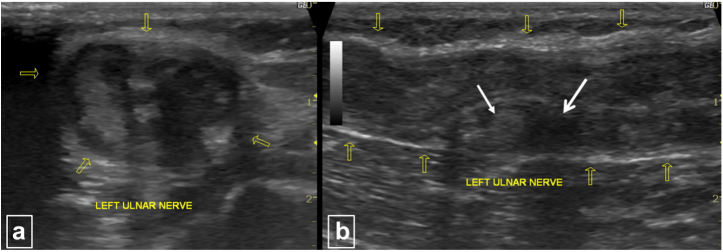
High-resolution ultrasonography using a linear probe at 12 MHz (Logiq Pro 5, GE Healthcare, Waukesha, Wisconsin, USA) revealed nodular thickening of the left ulnar nerve proximal to the medial epicondyle for a length of approximately 8.0 cm. The maximum diameter of the nerve was 12.4 mm. The nerve fascicles were swollen and appeared heterogeneously hypoechoic, surrounded by the echogenic perineurium and epineurium (Fig. 1). Small focal outpouchings were also noted arising from the nerve.
Fig. 1.
(a) & (b) Axial (a) and longitudinal (b) ultrasound scans show thickening of the left ulnar nerve with loss of the normal fascicular pattern, granuloma (thin white arrow) and abscess (thick white arrow) formation and thickening of the nerve epineurium (yellow arrows). The maximum thickness of the nerve was 12.0 mm.
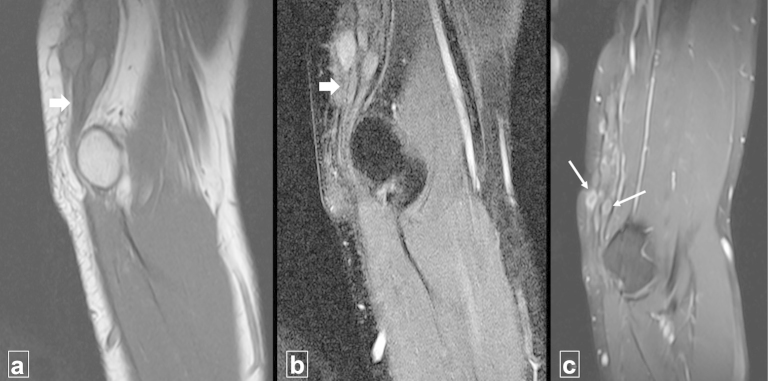
Non-contrast as well as contrast-enhanced MRI (Siemens Avanto 1.5 T MRI scanner, Erlangen, Germany) of the patient revealed nodular thickening of the left ulnar nerve for an approximate length of 8.0 cm proximal to the medial epicondyle. The maximum thickness of the nerve was 12.0 mm. The nerve fascicles appeared swollen and mildly hyperintense to muscle on T1-weighted images and markedly hyperintense on fat-saturated (FS) proton density (PD) and T2-weighted images. The perineurium and epineurium appeared hypointense on all sequences and appeared thick. There were multiple disruptions in the perineurium and epineurium with small contiguous variable sized outpouchings. A ‘horse-shoe’ shaped and epineural collection (mildly hyperintense to muscle on T1-weighted images and strongly hyperintense on FS PD and T2-weighted images) was also noted between the nerve and the triceps muscle. Minimal epineural hyperintense signal on PD-FS images was present. Post-contrast images revealed enhancement of the nerve and rim-enhancement of these neural outpouchings (Fig. 2, Fig. 3). Based on these imaging findings a diagnosis of ulnar neuritis with ulnar nerve abscesses was made.
Fig. 2.
(a)–(c) The panel shows (a) coronal T1-weighted, (b) coronal fat-suppressed proton density weighted and (c) coronal fat suppressed T1-weighted post-contrast MRI images. The ulnar nerve is thickened (thick white arrows) and multiple enhancing outpouchings are noted arising from the nerve (thin white arrows).
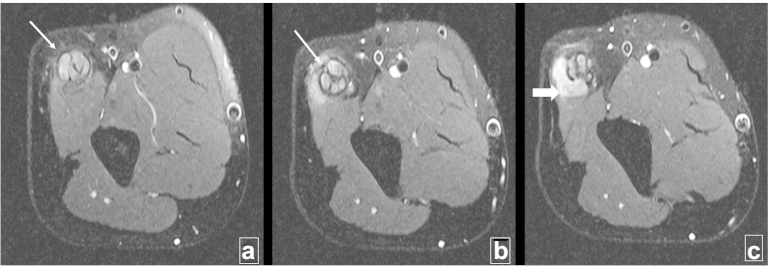
Fig. 3.
(a)–(c) The panel shows fat-suppressed proton density weighted axial sections through the distal left arm (proximal to distal). There is thickening of the ulnar nerve (arrows). The swollen nerve fascicles appear hyperintense and are surrounded by the thickened hypointense perineurium and epineurium. A ‘horse-shoe’ shaped perineural collection is noted between the nerve and the triceps muscle (thick arrow).
The patient was assumed to have relapsed. He was put on a course of steroids and multi-drug therapy was reinstituted. Slit skin smear examination for acid-fast bacilli was negative. He was also referred for decompressive surgery. On follow-up after a month, the patient was asymptomatic with significant reduction in the ulnar nerve thickening without any increase in the sensorimotor deficit.
Discussion
With an estimated 600,000 new cases detected annually, of which 60% are from India, leprosy can be considered the most common treatable neuropathy in the endemic zones.2M. leprae is an obligate intracellular bacterium due to the reductive evolutionary loss of genes encoding for metabolic and respiratory pathways.1 A unique trisaccharide in the wall of M. leprae binds to the basal lamina of Schwann cells and enables the invasion of Schwann cells and endoneural and perineural macrophages.1, 3 Nerve involvement in leprosy is noted across the disease spectrum and in lepra reactions. Usually the neural lesion is a granuloma, however uncommonly they may form an abscess, particularly in patients with BT leprosy.4 In tuberculoid leprosy, there is aggressive infiltration of epithelioid and lymphoid cells into the nerve causing thickening of the epineurium and perineurium and destruction of fascicles. At the lepromatous pole, the immune response is milder and active proliferation of bacilli occurs, with resultant better preservation of the neural architecture. It has been hypothesized that tissue anoxia secondary to pressure from inflamed swollen neuronal fascicles leads to avascular necrosis and abscess formation.5 The sites of nerve swelling in leprosy are similar to those of entrapment neuropathies; however in leprosy the nerve enlargement is more extensive.6
Ulnar nerve is the commonest nerve to develop abscesses.1 In India, a nerve abscess develops in approximately 1.3 per cent of leprosy patients and some of these may calcify.7 The incidence of nerve abscesses in leprosy has been rising and has been attributed to wider implementation of multi-drug therapy programmes without the necessary infrastructure to detect and treat early neuritis.8 Young children and teenagers account for the majority of cases.8
The role of imaging has been limited in leprosy so far. Recent improvements in high-frequency transducers permit detailed evaluation of nerves. Normal nerves have a fascicular pattern on longitudinal scans due to the neuronal fascicles (multiple hypoechoic parallel linear areas) separated by the perineurium/epineurium (hyperechoic bands). On transverse scans, nerves reveal a honeycomb-like appearance with rounded hypoechoic neuronal fascicles in a hyperechoic background.9 Involved nerves reveal focal thickening (more marked proximal to the medial epicondyle), hypoechoic focal areas (granulomata), peripheral hyperechogenicity (epineural fibrosis), abscesses and increased vascularity on colour-Doppler imaging (in lepra reactions).2, 9 Elias et al2 have proposed that altered neural anatomy on sonography with preserved electrophysiological tests is highly suggestive of leprosy and may be useful in evaluating asymptomatic household contacts.
References of MRI features of peripheral neuropathy in leprosy are scarce in literature. MRI may show diffuse oedema and swelling of the involved nerve. These findings are non-specific and may also be seen in other hypertrophic neuropathies like Refsum's disease, amyloid infiltration, chronic relapsing polyneuritis and Guillain–Barre syndrome. Presence of nodules or nerve sheath granulomas is suggestive of leprosy.10 Nerve abscesses appear hypointense on T1-weighted images and hyperintense on T2-wighted images and show peripheral enhancement on post-contrast study.4, 10 In the setting of leprosy, apart from nerve abscess reversal reaction should also be considered. This differentiation is important as reversal reaction can be managed conservatively with steroids whereas as a nerve abscess may need surgical decompression. The indications for surgery in the case of a nerve abscess are – symptoms not controlled by steroids, requirement of high doses of steroids and increasing sensorimotor deficit.5 MRI is also very sensitive in detecting early neuroarthropathic changes in asymptomatic patients, such as degradation and interruption of the subcutaneous fat and effusion and synovitis of the metatarsophalangeal joints.11
Conclusion
Leprosy is the most common preventable neuropathy in endemic areas. With the significant improvements in imaging technology, the hitherto limited role of ultrasonography and MRI in leprosy is set to rise, particularly in the evaluation nerves including nerve abscesses.
Conflicts of interest
All authors have none to declare.
References
- 1.Gelber R.H. Leprosy. In: Longo D.L., Fauci A.S., Kasper D.L., Hauser S.L., Jameson J.L., Loscalzo J., editors. Harrison's Principles of Internal Medicine. 18th ed. McGraw Hill; New York: 2012. pp. 1359–1366. [Google Scholar]
- 2.Elias J., Nogueira-Barbosa M.H., Feltrin L.T. Role of ulnar nerve sonography in leprosy neuropathy with electrophysiologic correlation. J Ultrasound Med. 2009;28:1201–1209. doi: 10.7863/jum.2009.28.9.1201. [DOI] [PubMed] [Google Scholar]
- 3.McAdam A.J., Sharpe A.H. Infectious diseases. In: Kumar V., Abbas A.K., Fausto N., Aster J.C., editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Saunders Elsevier; Philadelphia: 2010. pp. 372–374. [Google Scholar]
- 4.Kulkarni M., Chauhan V., Bharucha M., Deshmukh M., Chhabra A. MRI imaging of ulnar leprosy abscess. JAPI. 2009;57:175–176. [PubMed] [Google Scholar]
- 5.Thomas M., Emmanuel M. Facial nerve abscess in a case of leprosy – a case report. Neurol Asia. 2009;14:41–42. [Google Scholar]
- 6.Bianchi S., Martinoli C. Ultrasound of the musculoskeletal system. In: Baert A.L., Knauth M., Sartor K., editors. Medical Radiology: Diagnostic Imaging and Radiation Oncology. Springer–Verlag; Berlin Heidelberg: 2007. pp. 112–114. [Google Scholar]
- 7.Lichtman D.M., Swafford A.W., Kerr D.M. Calcified abscess in the ulnar nerve in a patient with leprosy. J Bone Joint Surg. 1979;61:620–621. [PubMed] [Google Scholar]
- 8.Salafia A., Chauhan G. Nerve abscess in children and adult leprosy patients: analysis of 145 cases and review of the literature. Acta Leprol. 1996;10:45–50. [PubMed] [Google Scholar]
- 9.Martinoli C., Bianchi S., Dahmane M., Pugliese F., Bianchi-Zamorani M.P., Valle M. Ultrasound of tendons and nerves. Eur Radiol. 2002;12:44–55. doi: 10.1007/s00330-001-1161-9. [DOI] [PubMed] [Google Scholar]
- 10.Hari S., Subramanian S., Sharma R. Magnetic resonance imaging of ulnar nerve abscess in leprosy: a case report. Lepr Rev. 2007;78:155–159. [PubMed] [Google Scholar]
- 11.Luyckx G., Vanhoenacker F.M., Parizel P.M. Exotic pathology of the hand and foot. A pictorial review. JBR–BTR. 2008;91:160–165. [PubMed] [Google Scholar]