Abstract
Head and neck squamous cell cancer (HNSCC) is the sixth most common cancer in the world. Effective therapeutic modalities such as surgery, radiation, chemotherapy and combinations of each are used in the management of the disease. In most cases, treatment fails to obtain total cancer cure. In recent years, it appears that one of the key determinants of treatment failure may be the presence of cancer stem cells (CSCs) that escape currently available therapies. CSCs form a small portion of the total tumor burden but may play a disproportionately important role in determining outcomes. CSCs have stem features such as self-renewal, high migration capacity, drug resistance, high proliferation abilities. A large body of evidence points to the fact that CSCs are particularly resistant to radiotherapy and chemotherapy. In HNSCC, CSCs have been increasingly shown to have an integral role in tumor initiation, disease progression, metastasis and treatment resistance. In the light of such observations, the present review summarizes biological characteristics of CSCs in HNSCC, outlines targeted strategies for the successful eradication of CSCs in HNSCC including targeting the self-renewal controlling pathways, blocking epithelial mesenchymal transition, niche targeting, immunotherapy approaches and highlights the need to better understand CSCs biology for new treatments modalities.
Keywords: Biology, Head and neck neoplasms, Oral cancer, Neoplastic stem cells, Molecular targeted therapy, Radiation therapy, Chemotherapy
Core tip: The cancer stem cells (CSCs) theory offers an insight into why currently available therapies for head and neck cancer fail so often. Eradication of cancers may require the targeting and elimination of CSCs, especially for head and neck squamous cell cancer (HNSCC). This represents a challenge because many pathways, such as those involved in self-renewal, are shared by CSCs and their normal counterparts and might lead to major toxicities. Developing radio sensitizing strategies is investigated and appears to eliminate CSCs. Overcoming chemo resistance, radio resistance and immune evasion mechanisms of CSCs remains a cornerstone of novel adjuvant therapies specifically targeting CSCs in HNSCC.
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) remains a major health problem throughout the world, with an estimated 500000 new cases diagnosed yearly[1]. HNSCC refers to a group of cancers that originate in the epithelium of the oral cavity, pharynx and larynx. Currently, therapeutic strategies for HNSCC include surgery, radiotherapy, chemotherapy, concurrent chemoradiation and monoclonal antibodies. Despite progress in the field of oncology, the overall 5-year survival rate of HNSCC is below 50%, unchanged in the last 30 years[2]. Local recurrence affects about 60% of patients and metastases develop in 20% of cases. Locoregional failure is linked to unfavorable outcome[3,4]. A new, more strategic approach is needed for the treatment of recurrent head and neck squamous cell carcinoma, as most cases cannot be cured with current therapeutic modalities. The presence of a peculiar subpopulation of cells has been identified in several tumors, including HNSCC: This small population of cancer cells possesses the capability to self-renewal, is highly tumorigenic, and behaves as tumor progenitor cells. Such characteristics are consistent with the features of cancer stem cells (CSCs)[5,6]. The role of these cells in HNSCC progression and metastasis is a significant point to be further emphasized on for eliminating the disease. Indeed, in addition to their ability for self-renewal, differentiation, and regeneration, CSCs possess significant resistance to radiochemotherapy[7,8]. Furthermore, by being able to do epithelial mesenchymal transition (EMT), which is a key step in embryogenesis, CSCs might facilitate the metastatic characteristics of tumors[9-11]. Therefore, targeted elimination of these CSCs could define new therapeutic strategies for head and neck cancer treatment. If the most common method for identifying CSCs relies on the expression of specific cell surface antigens that enrich for cells with CSC properties, their detection within the total tumor bulk remains a challenge. Indeed, the development of new CSC targeting therapeutic strategies is currently obstructed by the lack of trustworthy markers for the identification of CSCs[12-14]. Besides, molecular mechanisms at the basis of CSCs origin are yet not fully understood. Nonetheless, targeting self-renewal pathways in CSCs, such as the Wnt, Notch, and Hedgehog pathways, or specific CSC markers, such as CD133, CXCR1, and CD44 may offer therapeutic benefits to head and neck cancer therapy[13]. In addition to CSC biomarkers, micro environmental factors, such as niche-specific properties constitute obvious potential targets in order to eradicate high-risk HNSCC cells; to abolish the crosstalk between endothelial cells and CSCs in a targeted manner might be relevant for the treatment of head and neck cancer patients[15]. This review discusses the properties of head and neck tumoral stem cells, outlines initial targeted therapeutic strategies against them, and presents challenges for the future (Figure 1).
Figure 1.
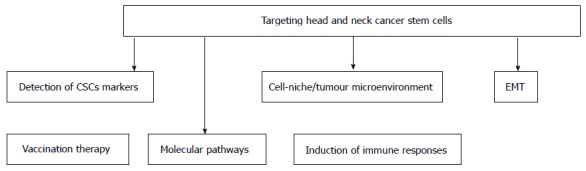
Therapeutic perspectives through a multistrategic approach. CSCs: Cancer stem cells; EMT: Epithelial mesenchymal transition.
CSCS IN HSNCC: IDENTIFICATION, CHARACTERIZATION AND PROPERTIES
Role of stem cell molecular markers
HNSCC are solid tumors with heterogeneous content. Indeed, into the tumor, not all cells possess the capacity for self-renewal and unlimited growth. In tumor architecture, it is widely agreed that CSCs are held accountable for tumor growth whereas differentiated cells usually contribute to the tumor bulk[5]. CSC populations are defined by four key features: Only a small portion of intratumoral cancer cells can form a new tumor in an in vivo xenograft assay, particular cell surface markers allow to identify CSC populations from non-CSC populations, the ability to generate endless copies of themselves through self-renewal, and the potential to give rise to differentiated non-stem cell cancer progeny[16]. As all chemotherapy regimens often damage normal, rapidly dividing cells, CSC-like populations, with low turnover and infrequent cell cycling, may escape treatment[17]. Thus, there is an urgent need for early detection of CSCs in the tumor cell population. Identification of CSCs based on increased expression of certain markers in cancerous tissue is the basis of the target therapy which is described later in this review. It is more clear that the development of novel therapeutic strategies will come about through identification of HNSCC CSC populations that regulate tumor growth, metastasis, and treatment resistance. Thanks to the development of immunofluorescence tools, it is possible to more easily isolate CSCs using their surface proteins. The main molecular markers implicated in HNSCC CSC detection are summarized in Table 1.
Table 1.
Main molecular markers implicated in head and neck squamous cell cancer cancer stem cell detection
| Ref. | Stem cell marker | Cancer cell lines studied |
| Prince et al[5] | CD44, BMI 1 | HNSCC generated in immunodeficient mouse model |
| Wei et al[25] | CD133 | HNSCC cell lines (hep-2) |
| Chen et al[21] | ALDH 1 | Immunodeficient mouse model |
| Krishnamurthy et al[15] | ALDH | Head and neck squamous cell carcinoma |
HNSCC: Head and neck squamous cell cancer; ALDH: Aldehyde dehydrogenase.
Role of the CD44 marker
One of the first studies of CSCs in HNSCC using an immunodeficient mouse as model demonstrated that a minor population of CD44+ cancer cells, which account for less than 10% of the cells in a HNSCC primary tumor could give rise to new tumors in vivo and displayed the ability of self-renewal and differentiation. The CD44 protein is a cell surface glycoprotein that is responsible for cell adhesion, migration and homing. It is a receptor for hyaluronic acid and can also interact with other ligands such as collagen species and matrix metalloproteases[5]. Takahashi et al[18] demonstrated that cell-cell dissociation and actin remodeling in tumor necrosis factor-induced EMT were mediated by specific interaction between CD44 and hyaluronan; another result was an enhanced motility. CD44+CD24- CSCs play a critical role in tumor progression and metastasis[19]. Some of HNSCC with CD44s (standard form) and CD44 v6 (alternative splice variant) expressions are associated with a poorer disease-free survival, in laryngeal cancers particularly[20]. Also, high levels of nuclear BMI-1 were found in CD44+CD24- cells of the tumor population. BMI-1 is a stem cell-related gene involved in the mechanisms of carcinogenesis in head and neck cancers[5]. By simultaneous evaluating both CD44 and BMI-1, it could lead to precise characterization of the CSC population within the tumor cellular architecture.
Aldehyde dehydrogenase activity
Aldehyde dehydrogenase (ALDH) has also been considered to be a marker for identifying HNSCC CSCs. The ALDH family, of which ALDH1 is a member, is a family of cytosolic isoenzymes, which are highly ex pressed in many stem and progenitor cells. These enzymes are responsible for oxidizing intracellular aldehydes and contribute to the oxidation of retinol to retinoic acid, in stem cell differentiation notably; moreover, ALDH1 is involved in the resistance of progenitor cells to chemotherapeutic agents. Many studies have proved the role of ALDH1+ cells in tumorigenesis, metastasis and chemo resistance in HNSCC. For instance, Chen et al[21] showed that ALDH1+ CD44+ cells resist radiotherapy and maintain CSC-like properties in HNSCC cells which allow them to promote tumor propagation[22]. Recently, Krishnamurthy et al[15] found that the combined use of ALDH1 and CD44 is more relevant for identifying CSC-like populations as it is more selective than any other marker used alone. It is clear that only one marker is not sufficient to identify a pure CSC population in HSNCC. The best chance of developing targeted identification and treatment goes through a panel of markers with a more narrowly definition of CSCs.
Other markers and role of side population cells
Several studies evidenced the abilities of CD133+ stem-like cells: They possess higher clonogenicity, higher tumourigenic potential and are more invasive, in comparison with CD133- cells. CD133+ cells play a crucial role in the resistance to standard chemotherapy with paclitaxel[23]. CD133 antigen also known as prominin-1 is a glycoprotein that is encoded by the PROM1 gene. It is a member of pentaspan transmembrane glycoproteins (5-transmembrane, 5-TM), which specifically localize to cellular protrusions. If it was initially considered as a marker for hematopoietic stem cells[24], it has been then identified as a CSC marker in several cancers and particularly in the laryngeal cancer, using the Hep-2 cell line. Indeed, in an in vivo study, CD133+ cells sorted from the Hep-2 cell line had higher tumorigenic potential than CD133- cells[25]. Higher CD133 levels are found in CD44+ cancer stem-like cells in comparison with CD44- cells in HNSCC, which support the putative role of CD133+ as a CSC marker. Using CD133 might serve to identify head and neck cancer patients that are resistant to conventional chemotherapy[26]. Furthermore, side population cells have shown to express stem cell properties when isolated from cancer samples. Their identification does not rely on the relative binding of antibodies but is based on their ability to efflux a fluorescent dye that binds to DNA[27,28]. Side population cells are more tumorigenic, chemoresistant and have displayed self-renewal in vivo. Besides, side population cells show a more aggressive schema of tumour growth (in vitro)[29]. New strategies to target these cells need to be designed. Above all, further research on the exact role of side population cells and their implication in tumourigenesis is required as the exact mechanisms are not yet fully understood.
MOLECULAR STRATEGIES TO TARGET CSCS IN HSNCC
CSCs and therapeutic resistance
CSCs have important implications regarding cancer treatment and may lead to new perspectives on therapeutic strategies with a rethink of actual treatment paradigm. Indeed, indiscriminate cytoreduction is the aim of current chemotherapy and radiation treatment for HNSCC whereas the CSC hypothesis suggests that the elimination of CSCs is the only way to treat cancer effectively. Thus, significant reductions in the tumor volume are not enough to prevent tumor recurrence in HNSCC. Moreover, evidence suggests that CSCs have inherent drug and radiation resistance, rendering most conventional therapies ineffective. Radio resistance of CSCs has been attributed to their self-renewal capacity, DNA repair capacity, free-radical scavenging, upregulation of cell cycle control mechanisms and specific interactions with the stromal microenvironment. Chemotherapy resistance is frequently related to accelerated drug transport and to drug metabolism[30,31]. Bmi-1 and CD44 knockdowns have led to an improvement of CSCs chemosensitivity in HNSCC. In particular, knockdown of CD44 increased the sensitivity of HNSCC cells to cisplatin, underlying the crucial of CSCs in the response to chemotherapy[32]. Concerning Bmi-1, a stem-cell-related gene, which participates in the self-renewal of hematopoietic and neuronal stem cells, and has been implicated in the tumorigenesis of various malignancies the experiment showed that that knockdown of Bmi-1 increased the effectiveness of radiotherapy and resulted in inhibition of tumor growth in nude mice transplanted with ALDH1+ CSCs[33]. Moreover, Chen et al[32] focused on the Snail superfamily of zinc-finger transcription factors, implicated in the regulation of EMT during embryonic development. The importance of SNAI1 in the growth of cancer cells and their metastatic potential has been shown in various malignancies[34]. Chen et al[32] found that the endogenous co-expression of ALDH1+ and Snail resulted in decreased ALDH1 expression, inhibition of CSC-like properties, and decreased tumorigenesis in ALDH1+ CD44+ cells. By regulating the EMT, Snail is a key factor in maintaining CSC properties, and could be used as a therapeutic measure for the treatment of HNSCC. Besides, Snail small interfering RNA could reduce resistance to chemo radiotherapy in ALDH1+ cells[32]. Ultimately, the expression of drug efflux pumps by CSCs, another mechanism of chemo resistance remains to be explored in HNSCC. A better understanding of resistance mechanisms in HNSCC CSCs will require future studies and constitutes a prerequisite for improving therapy and possibly preventing tumor spread or recurrence. The main determinants of CSC radioresistance are summarized in Table 2.
Table 2.
Main determinants of cancer stem cell radioresistance
| Molecular determinants of radioresistance | Mechanism |
| Intrinsic determinants | Enhanced DNA repair capability |
| Protection from oxidative DNA damage | |
| Activation of the cell survival pathways | |
| (PI3K/Akt, WNT/β-catenin, notch) | |
| Expression of drug efflux pumps | |
| Extrinsic determinants | Hypoxic environment |
Targeting stem cell niches
Beyond intrinsic factors, the unique CSC microenvironment could play a crucial role in the radio resistance of CSCSs. Indeed, it has been showed that stromal environment and CSC niche play a vital role in the behavior of cancer cells. As the vast majority of the stem cells are found within a 100 μm-radius of a blood vessel in HNSCC, the existence of a perivascular niche was suggested. Using the SCID mouse model of human tumor angiogenesis, it was observed that specific ablation of tumor-associated endothelial cells with an inducible Caspase-9 result in the decrease of the fraction of head and neck CSCs[15]. Thus, targeting the stem cell niche directly can weaken the source of nutrition and change the essential signals needed by CSCs to proliferate. Therapeutic strategies as suggested by Tang et al[35] included targeting candidate CSCs and their microenvironment niche, which contributes to self-renewal of these cells along with the reactive oxygen species status of these cells, and tweaking their intracellular milieu to facilitate apoptotic death signals over proliferative effects may facilitate a new prospective towards target therapy in HNSCC. Similarly, Krishnamurthy and al showed that targeting CSCs either directly or via their niche could lead to a more durable response in HNSCC, hence the emergence of a new concept using both conventional chemotherapy and CSC-targeted therapy[36]. The niche provides the soil for CSC self-renewal and maintenance, stimulating essential signaling pathways in CSCs and leading to secretion of factors that promote angiogenesis and long-term growth of CSCs. Hence, the role of targeting “vascular niche” in treatment of HNSCC cannot be neglected. The use of anti-angiogenic agents, such as bevacizumab, could be a therapeutic strategy in HNSCC; if it mediates CSC depletion in gliomas it could prove useful in reducing the proportion of HNSCC CSCs. Exploiting the functional interdependence of CSCs and vascular endothelial cannot be neglected in order to reduce the rate of HNSCC recurrence and metastasis[37-46].
EMT and molecular pathways
EMT is the process that allows a polarized epithelial cell to assume a mesenchymal cell phenotype, which is characterized by enhanced motility and invasiveness. The crosstalk between HNSCC cells and other cells of the tumor microenvironment could lead to EMT, which enhances the motility of carcinoma cells and endows them with stem cell properties. The invasive phenotype of cells that have undergone EMT allows them to penetrate the lymphatic and/or angiogenic vasculature. Blocking the crosstalk between tumor and stromal cells, and thus inhibiting EMT might be a therapeutic strategy in HNSCC. The activation of the EMT program has been shown in HNSCC populations thanks to microarray analysis; moreover, in these cells, the molecular characterization of gene expression also allowed to show the activation of Wnt/beta-catenin signaling pathway, usually involved in the maintenance of pluripotency, differentiation and proliferation. Inhibitors of this pathway are in clinical trials in several cancers[47-49]. Numerous molecules targeting the Wnt pathway are either in the discovery stage or early phase 1 trials directed variously against Wnt/Receptor interactions and cytosolic and nuclear signaling[50,51]. Furthermore, others implicated molecular pathways are still under investigation in HNSCC, including the promising JAK/STAT pathway. In HNSCC-CD44+ALDH1+ transplanted immunodeficient mice, an inhibitor of STAT3 combined with radiotherapy significantly suppressed tumorigenesis and improved the survival rate[52]. Other drugs have been formulated to target other pathways in CSC formation such as Notch or Hedgehog but the ability of these drugs to selectively target CSCs while preserving normal stem cells remains a challenge. In nasopharyngeal carcinomas, targeting beta-catenin signaling pathway through E-cadherin repressor ZEB2 by using miR200a, allowed to induce stem-like traits, including CD133+ side population, sphere formation capacity, increased Oct4 and ALDH expression in tumor spheres, and tumorigenicity in vivo[53]. TrκB, a 145-KDa receptor tyrosine kinase is supposed to be both involved in EMT and invasion process of cancer cells in HNSCC. Studies showed that downregulation of TrκB, suppressed tumor growth[54]. Ultimately, recent studies have reported the role of hypoxia or overexpression of HIF-1α in the induction of EMT and metastasis in head and neck cancer cells. HIF-1-α regulates the expression of Twist by binding to the hypoxia-response element. Co-expression of HIF-1-α, Twist in human head and neck tumors correlates with metastasis and poor prognosis[55]. It is undeniable that EMT is a central process in the acquisition of stem-like properties and ultimately contributes to local invasion and metastatic spread frequently observed in patients with head and neck cancer.
IMMUNOTHERAPEUTIC APPROACHES TARGETING HEAD AND NECK TUMORAL STEM CELLS
CSC-induced immune responses
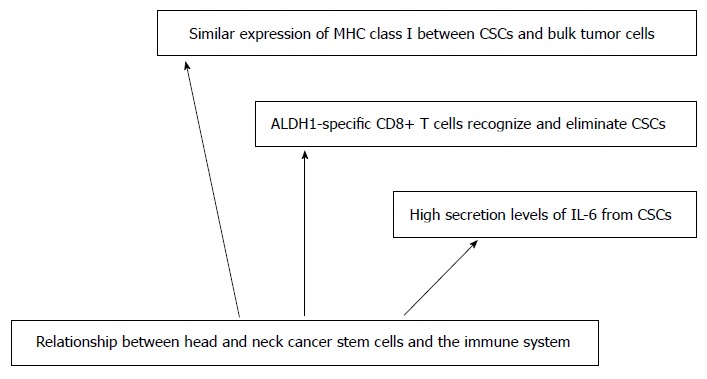
Beyond chemo resistance and radio resistance, emerging CSC targeted therapies in HNSCC have to overcome another major hindrance: Immune-escape-mechanisms of CSC. Indeed, current immunotherapy is mainly based on antigens presented to effector T cells by dendritic cells. Or, generally, these antigens are selected and derived from bulk tumor cells; they are not derived of CSCs that may not express immunogenic differentiation antigens[38]. CSCs also may be defective in antigen presentation due to the downregulation of human leukocyte antigen (HLA) surface expression[39]. Therefore, in a heterogeneous tumor entity, CSCs may lead to a treatment failure and disease progression, escaping from the attack of current immunotherapy. Concerning HNSCC, a better knowledge of the crosstalk between CSCs and the immune system is crucial in order to develop specific targeted therapies, the immunogenicity of HNSCC-CSCs having been observed recently. Recently, a CD8 defined T-cell epitope of ALDH1 was identified as a potential target[22]. Among reported CSCs markers, ALDH1 is the most specific CSC marker used to identify highly tumorigenic cells present in HNSCC[21]. ALDH1 has been recognized as an antigen-source eliciting a humoral immune response in HNSCC. Visus et al[22] showed that ALDH1A1 peptide was an HLA-A2-restricted, naturally presented, CD8+ T cell-defined tumor-antigen. ALDH1 peptide-specific CD8+ T cells could only recognize HLA-A2+ HNSCC cell lines overexpressing ALDH1 but not a human fibroblast cell line. Moreover, the data presented by Liao et al[40] have shown that the host immune system is able to recognize and distinguish CSCs with ALDH1 phenotype from non-CSC cells. In addition to ALDH1, other cancer antigens were found to be preferentially expressed in CSCs: Cyclin A1 was reported in leukemic stem cells of acute myeloid leukemia whereas DNAJB8 was identified as novel cancer antigen in renal CSCs[56,57]. This specific expression of cancer antigens may enable us to target CSCs specifically. Moreover, development of ALDH1A1 peptide-based vaccines for therapy represents a novel area for future research in HNSCC.
ALDH1A1: A potential target for vaccination therapy
Another attractive approach to target CSCs is to develop antitumor T-cell vaccines. Studies on vaccination against antigen ALDH1A1+ of CSCs have been performed and have achieved significant progress. Visus et al[41] have demonstrated the ability in vivo of generated ALDH1A1-specific cytotoxic T lymphocytes to eliminate ALDH (bright) cells present in HLA-A2+ HNSCC carcinoma cell lines. They also found antitumor activity by adoptive immunotherapy with ALDH1A1-specific cytotoxic T lymphocytes in vivo. The elimination of ALDH(bright) cells thanks to ALDH1A1-specific CD8+ T cells could inhibit tumor growth and metastases[41]. Ning et al[42] investigated immunogenicity induced by murine ALDH (high) CSC used as a source of antigen to prime derived-cells as a vaccine for malignant squamous cell carcinoma in immunocompetent mice used as hosts. High immunogenicity was found among ALDH(high) CSCs with a most effective role as an antigen source in comparison with unselected tumor cells. A high level of IgG produced by splenocytes subjected to CSC-tumor-lysate-pulsed derived-cells and the binding of the antibody from CSC-vaccinated murine hosts to CSCs which resulted in the CSCs lysis via complement-dependent cytotoxicity have been observed. Studies showed that cytotoxic T lymphocytes generated from peripheral blood mononuclear cells or splenocytes harvested from CSC-vaccinated hosts had the ability to kill CSCs in vitro[42]. Consistent with the findings of Ning group, Duarte et al[43] first demonstrated an ALDH(high) CSC-based vaccine could drastically reduce both tumor volume and occurrence in a rat colon carcinoma syngeneic model: 50% of the CSC-based vaccinated animals became resistant to tumor development and a 99.5% reduction in tumor volume compared to the control group occurred. Beyond the fact that these studies provide a greater view of the immune biology of CSCs, vaccination with CSCs has proved to be effective in killing head and neck CSCs specifically, reducing tumor volume and preventing tumor recurrence.
Immune suppressive role of CSCs
Immunotherapeutic approaches for HNSCC are complicated due to the deep immune suppression induced by this disease. Mechanisms such as increased apoptosis of tumor-specific CD8+ T-cells and increased tumor-infiltrating T regulatory cells in peripheral blood and at the tumor site have been demonstrated[58]. Krishnamurthy et al[15] showed that the location of CSCs was in close proximity to blood vessels. Clinically, patients with recurrent HNSCC showed an increased concentration of IL-6 in serum in comparison with patients with primary HNSCC[44]. Elevated IL-6 levels could independently predict tumor recurrence, poor survival, and tumor metastasis[45]. Yu et al[44] demonstrated that secretion levels of IL-6 from CSCs were crucial to maintain the self-renewal and tumorigenic properties of CSCs in HNSCC. On the one hand, CSCs can be recognized and inhibited in their outgrowth by the immune system and on the other hand, CSCs can promote tumor progression either by immunoediting for CSCs that are more suitable to survive in an immunocompetent host or by establishing conditions that facilitate tumor outgrowth within the tumor immune-microenvironment. Tumor associated macrophages may play a critical role in tumor progression by interacting with the tumor microenvironment and tregs are thought to promote tumor progression[59]. In a study concerning primary human gliomas, the distribution of TAM at the invasive tumor front was correlated with the presence of CD133+ glioma CSCs. Tumor associated macrophages could significantly enhance the invasive capability of glioma stem cells through paracrine production of TGF-B1[60]. The role of tumor associated macrophages in the regulation of CSCs drug resistance has been identified by Jinsuhi et al[61] They found a large amount of tumor associated macrophages in CD44+ ALDH+ colon tumor and CD133+ ALDH+ lung cancer cells: Those macrophages allow activating Sonic Hedgehog pathways in CSCs in cooperation with IL-6. Targeting tumor associated macrophages by inhibiting either the myeloid cell receptors colony-stimulating factor-1 receptor or chemokine receptor improves chemotherapeutic efficacy, inhibits metastasis and increases antitumor T cell responses in pancreatic ductal adenocarcinoma[62]. All these findings validate the interplay between CSCs and the tumor immune microenvironment. Therefore, specific targeting of head and neck tumoral stem cells by immunotherapeutic approaches may lead to more efficacious and lasting therapeutic results in the future. Nonetheless, it seems necessary to address several points before immunotherapeutic approaches targeting CSCs can be brought into clinical trials. These include the effective isolation of CSCs from bulk tumor mass to measure potential immunotherapeutic effects on CSC, to determine the antigen-profile presented on CSCs specifically to identify specific CSC targets as well as the induction and enhancement of antigen processing and presentation of CSC epitopes. A lot of work remains to be done to get a better understanding of the immune suppressive role of CSCs in HNSCC. The various immunotherapeutic approaches are displayed in Figure 2.
Figure 2.
Immunotherapeutic approaches. CSCs: Cancer stem cells; ALDH: Aldehyde dehydrogenase.
CONCLUSION
The CSC theory provides new opening for the treatment of HNSCC. This theory also helps to explain why currently available therapies for head and neck cancer so often fail. Eradication of cancers may require the targeting and elimination of CSCs, especially for HNSCC and thus, there is an urgent need to alter the current paradigm in drug development. Efforts are still advocated to determine specific markers and methods to specifically target these cells, towards a more specific tumor treatment. To date, no antibody selectively targeting CSC has been described in HNSCC yet, but candidates are under investigation. For instance, CD44v6 antibodies either radiolabeled or coupled with a cytotoxic drug entered phase I clinical testing in patients with HNSCC. In a phase I dose escalation study, the treatment with a radiolabeled antibody showed promising anti-tumor effects[63]. Clearly, huge variety of approaches to eradicate CSCs is being explored, and particularly in vitro assays; there still remains the issue of how to avoid unwanted toxicity in vivo. Developing radio sensitizing strategies is also being investigated and appears to eliminate CSCs. Overcoming chemo resistance, radio resistance and immune evasion mechanisms of CSCs remains a cornerstone of novel adjuvant therapies specifically targeting CSCs in HNSCC. Bertrand et al[64] demonstrated that the combination of UCN-01 (a checkpoint kinase inhibitor) and ATRA (all-trans retinoic acid) with irradiation decreased the survival fraction of CSCs and could be used as a powerful radio sensitizing strategy in HNSCC. Furthermore, advances in nanotechnology could allow a better understanding of the regulatory mechanisms that govern CSC biology in vivo.
Footnotes
Conflict-of-interest statement: Authors declare they have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 28, 2015
First decision: October 27, 2015
Article in press: December 14, 2015
P- Reviewer: Economescu MC S- Editor: Qi Y L- Editor: A E- Editor: Jiao XK
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26:2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Sjögren EV, Wiggenraad RG, Le Cessie S, Snijder S, Pomp J, Baatenburg de Jong RJ. Outcome of radiotherapy in T1 glottic carcinoma: a population-based study. Eur Arch Otorhinolaryngol. 2009;266:735–744. doi: 10.1007/s00405-008-0803-9. [DOI] [PubMed] [Google Scholar]
- 5.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890; discussion 1883-1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 7.Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98:1755–1757. doi: 10.1093/jnci/djj505. [DOI] [PubMed] [Google Scholar]
- 8.Damek-Poprawa M, Volgina A, Korostoff J, Sollecito TP, Brose MS, O’Malley BW, Akintoye SO, DiRienzo JM. Targeted inhibition of CD133+ cells in oral cancer cell lines. J Dent Res. 2011;90:638–645. doi: 10.1177/0022034510393511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One. 2011;6:e16466. doi: 10.1371/journal.pone.0016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis SJ, Divi V, Owen JH, Bradford CR, Carey TE, Papagerakis S, Prince ME. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1260–1266. doi: 10.1001/archoto.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S, Wang Z. Head neck squamous cell carcinoma c-Met+ cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129:2337–2348. doi: 10.1002/ijc.25927. [DOI] [PubMed] [Google Scholar]
- 12.Harper LJ, Piper K, Common J, Fortune F, Mackenzie IC. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J Oral Pathol Med. 2007;36:594–603. doi: 10.1111/j.1600-0714.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 13.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 14.Graziano A, d’Aquino R, Tirino V, Desiderio V, Rossi A, Pirozzi G. The stem cell hypothesis in head and neck cancer. J Cell Biochem. 2008;103:408–412. doi: 10.1002/jcb.21436. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nör JE. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 17.Hermann PC, Bhaskar S, Cioffi M, Heeschen C. Cancer stem cells in solid tumors. Semin Cancer Biol. 2010;20:77–84. doi: 10.1016/j.semcancer.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S, Koga H, et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem. 2010;285:4060–4073. doi: 10.1074/jbc.M109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack B, Gires O. CD44s and CD44v6 expression in head and neck epithelia. PLoS One. 2008;3:e3360. doi: 10.1371/journal.pone.0003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staibano S, Merolla F, Testa D, Iovine R, Mascolo M, Guarino V, Castellone MD, Di Benedetto M, Galli V, Motta S, et al. OPN/CD44v6 overexpression in laryngeal dysplasia and correlation with clinical outcome. Br J Cancer. 2007;97:1545–1551. doi: 10.1038/sj.bjc.6604070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Visus C, Ito D, Amoscato A, Maciejewska-Franczak M, Abdelsalem A, Dhir R, Shin DM, Donnenberg VS, Whiteside TL, DeLeo AB. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumor antigen in squamous cell carcinoma of the head and neck. Cancer Res. 2007;67:10538–10545. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010;289:151–160. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 25.Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ, Maccallum J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck. 2009;31:94–101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto A, Chikamatsu K, Sakakura K, Hatsushika K, Takahashi G, Masuyama K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45:633–639. doi: 10.1016/j.oraloncology.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Wan G, Zhou L, Xie M, Chen H, Tian J. Characterization of side population cells from laryngeal cancer cell lines. Head Neck. 2010;32:1302–1309. doi: 10.1002/hed.21325. [DOI] [PubMed] [Google Scholar]
- 28.Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, Prince ME. Head and neck cancer stem cells: the side population. Laryngoscope. 2011;121:527–533. doi: 10.1002/lary.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5:e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, Chung CH, Lu B. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol. 2011;2011:941876. doi: 10.1155/2011/941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa K, Yoshioka Y, Isohashi F, Seo Y, Yoshida K, Yamazaki H. Radiotherapy targeting cancer stem cells: current views and future perspectives. Anticancer Res. 2013;33:747–754. [PubMed] [Google Scholar]
- 32.Chen YC, Chang CJ, Hsu HS, Chen YW, Tai LK, Tseng LM, Chiou GY, Chang SC, Kao SY, Chiou SH, et al. Inhibition of tumorigenicity and enhancement of radiochemosensitivity in head and neck squamous cell cancer-derived ALDH1-positive cells by knockdown of Bmi-1. Oral Oncol. 2010;46:158–165. doi: 10.1016/j.oraloncology.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 35.Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J. 2007;21:3777–3785. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- 36.Krishnamurthy S, Nör JE. Head and neck cancer stem cells. J Dent Res. 2012;91:334–340. doi: 10.1177/0022034511423393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Bao Q, Renner A, Camaj P, Eichhorn M, Ischenko I, Angele M, Kleespies A, Jauch KW, Bruns C. Cancer stem cells and angiogenesis. Int J Dev Biol. 2011;55:477–482. doi: 10.1387/ijdb.103225yz. [DOI] [PubMed] [Google Scholar]
- 38.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busse A, Letsch A, Fusi A, Nonnenmacher A, Stather D, Ochsenreither S, Regenbrecht CR, Keilholz U. Characterization of small spheres derived from various solid tumor cell lines: are they suitable targets for T cells? Clin Exp Metastasis. 2013;30:781–791. doi: 10.1007/s10585-013-9578-5. [DOI] [PubMed] [Google Scholar]
- 40.Liao T, Kaufmann AM, Qian X, Sangvatanakul V, Chen C, Kube T, Zhang G, Albers AE. Susceptibility to cytotoxic T cell lysis of cancer stem cells derived from cervical and head and neck tumor cell lines. J Cancer Res Clin Oncol. 2013;139:159–170. doi: 10.1007/s00432-012-1311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visus C, Wang Y, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, Brand RE, Ferrone CR, Whiteside TL, Ferrone S, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8+ T cells. Clin Cancer Res. 2011;17:6174–6184. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, Li M, Ginestier C, Wicha MS, Moyer JS, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012;72:1853–1864. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duarte S, Momier D, Baqué P, Casanova V, Loubat A, Samson M, Guigonis JM, Staccini P, Saint-Paul MC, De Lima MP, et al. Preventive cancer stem cell-based vaccination reduces liver metastasis development in a rat colon carcinoma syngeneic model. Stem Cells. 2013;31:423–432. doi: 10.1002/stem.1292. [DOI] [PubMed] [Google Scholar]
- 44.Yu CC, Tsai LL, Wang ML, Yu CH, Lo WL, Chang YC, Chiou GY, Chou MY, Chiou SH. miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer. Cancer Res. 2013;73:3425–3440. doi: 10.1158/0008-5472.CAN-12-3840. [DOI] [PubMed] [Google Scholar]
- 45.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, Wolf GT, Teknos TN. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 46.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 47.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 48.Zechner D, Fujita Y, Hülsken J, Müller T, Walther I, Taketo MM, Crenshaw EB, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 50.Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1:552–566. doi: 10.18632/oncotarget.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takebe N, Ivy SP. Controversies in cancer stem cells: targeting embryonic signaling pathways. Clin Cancer Res. 2010;16:3106–3112. doi: 10.1158/1078-0432.CCR-09-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YW, Chen KH, Huang PI, Chen YC, Chiou GY, Lo WL, Tseng LM, Hsu HS, Chang KW, Chiou SH. Cucurbitacin I suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma--derived CD44(+)ALDH1(+) cells. Mol Cancer Ther. 2010;9:2879–2892. doi: 10.1158/1535-7163.MCT-10-0504. [DOI] [PubMed] [Google Scholar]
- 53.Xia H, Cheung WK, Sze J, Lu G, Jiang S, Yao H, Bian XW, Poon WS, Kung HF, Lin MC. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and beta-catenin signaling. J Biol Chem. 2010;285:36995–37004. doi: 10.1074/jbc.M110.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D, et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29:2047–2059. doi: 10.1038/onc.2009.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 56.Ochsenreither S, Majeti R, Schmitt T, Stirewalt D, Keilholz U, Loeb KR, Wood B, Choi YE, Bleakley M, Warren EH, et al. Cyclin-A1 represents a new immunogenic targetable antigen expressed in acute myeloid leukemia stem cells with characteristics of a cancer-testis antigen. Blood. 2012;119:5492–5501. doi: 10.1182/blood-2011-07-365890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishizawa S, Hirohashi Y, Torigoe T, Takahashi A, Tamura Y, Mori T, Kanaseki T, Kamiguchi K, Asanuma H, Morita R, et al. HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells. Cancer Res. 2012;72:2844–2854. doi: 10.1158/0008-5472.CAN-11-3062. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 59.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, Wang B, Yi L, Wang QL, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189:444–453. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 61.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colnot DR, Quak JJ, Roos JC, van Lingen A, Wilhelm AJ, van Kamp GJ, Huijgens PC, Snow GB, van Dongen GA. Phase I therapy study of 186Re-labeled chimeric monoclonal antibody U36 in patients with squamous cell carcinoma of the head and neck. J Nucl Med. 2000;41:1999–2010. [PubMed] [Google Scholar]
- 64.Bertrand G, Maalouf M, Boivin A, Battiston-Montagne P, Beuve M, Levy A, Jalade P, Fournier C, Ardail D, Magné N, et al. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev. 2014;10:114–126. doi: 10.1007/s12015-013-9467-y. [DOI] [PubMed] [Google Scholar]


