Abstract
Background: Patient-controlled sedation (PCS) has been suggested as an alternative method for sedative colonoscopy. However, as any new techniques, PCS introduction as a potential alternative to traditional intravenous sedation (IVS) has brought about challenges. To evaluate the advantages and disadvantages between PCS and IVS more comprehensively, we conducted a systematic review and meta-analysis of the published literature. Methods: Several databases were searched from inception to 1 April, 2015, for trials comparing PCS with IVS for colonoscopy. The outcomes of interest included time for cecal intubation, rate of complete colonoscopy, dose of sedative drugs used, pain scores, recovery time, complications. Inconsistency was quantified using I 2 statistics. Results: In all, 12 trials were finally selected (1091 patients, with 545 in the PCS group, and 546 in the IVS group). The total propofol used, time for cecal intubation, rate of complete colonoscopy and pain score had no statistical difference between the two groups. However, PCS showed a reduction in the recovery time, incidence of oxygen desaturation and hypotension. The rates of other complications and patients’ willingness to repeat the same sedation had no statistical difference between the two groups. Conclusion: PCS is as feasible and effective as traditional IVS for colonoscopy, and there is a tendency that PCS shows its superiority in recovery time, incidence for oxygen saturation and hypotension.
Keywords: Sedation, patient-controlled sedation, intravenous sedation, colonoscopy, meta-analysis
Introduction
Colonoscopy has become an essential part of the patient management, especially in the field of colorectal cancer prevention [1]. However, for most of the patients, colonoscopy is an unpleasant and tense procedure [2]. Thus, in some centers, sedative colonoscopy emerged at the right moment. There is latent concern about the complications associated with sedation, such as hypoxia and hypotension [3]. What’s more, the sedation was traditionally performed by an anesthetist, from an economic standpoint, it will substantially increase the staff cost [4]. Patient-controlled sedation (PCS) has been suggested as an alternative method for sedative colonoscopy [5]. PCS allows the patients themselves to match their sedation requirement to the stimulation during the colonoscopy [6], thereby avoiding overdose [7], and at the same time, cardiorespiratory complications related to sedative drugs are reduced. Some studies have endowed PCS as safe and with faster recovery [8,9]. However, as any new techniques, PCS introduction as a potential alternative to traditional intravenous sedation (IVS) has brought about challenges. To evaluate the advantages and disadvantages between PCS and IVS more comprehensively, we conducted a systematic review and meta-analysis of the published literature.
Methods
Search strategy and selection criteria
A thorough search was performed in the Medline via Pubmed, Embase, and Cochrane Controlled Register Databases of the articles published from inception of each database to 1 April, 2015. To obtain the relevant articles as sufficient as possible, we used the following keywords in combination as both Mesh Terms and text word: 1) ‘colonoscopy’; 2) ‘sedation’, ‘analgesia’, ‘anesthesia’, ‘narcotic’; 3) ‘randomized controlled trial’, ‘random’, ‘control groups’, ‘clinical trial’, ‘clinical study’, ‘controlled’, ‘randomized’. References from eligible articles have also been reviewed to avoid missing any pertinent trials.
Randomized, controlled studies comparing PCS with IVS for colonoscopy were enrolled. The inclusion criteria were: 1) clinical studies designed as prospective, randomized, and controlled trials; 2) trials comparing PCS with IVS performed by medical staff, regardless of performed by anesthetist, endoscopist, or nurse. The exclusion criteria were: a) case series or single-arm trials; b) non-randomized trials; c) conference abstract with which data could not be extracted; d) review or systematic review; e) repeated data (chose the one with better quality and more patients).
Study selection and data extraction
Two authors selected the articles independently. Firstly, they reviewed the titles and abstracts to identify eligible articles and remove duplicates. Secondly, full-text was assessed using the inclusion criteria and the exclusion criteria. Cochrane risk of bias assessment tool was used to evaluate the quality of each article. Any disagreement was resolved by discussion or by a third viewer. Two investigators used a standardized form to extract the data from the final selected articles about study type, publication year, time for cecal intubation, rate of complete colonoscopy, dose of sedative drugs used, pain scores, recovery time, complications and so forth.
In light of the fact that great heterogeneity exists, sensitivity analysis was performed. Funnel plot was used to investigate the publication bias.
Statistical analysis
Review Manager Version 5.1.0. (The Nordic Cochrane Centre, The Cochrane Collaboration, 2011, Copenhagen) was used for data analysis. Dichotomous variables were expressed as percentage and risk ratio (RR) with 95 percent confidence interval (95% CI). Continuous variables were calculated by mean values and standard deviation (SD), and the final results were expressed as mean difference with 95% CI. If mean values were not accessible, median values were used. For studies reporting the median and the quartiles, the median was treated as mean, and the distribution was assumed to be normal with a z-value of ± 0.68 SD corresponding to the 25th and 75th percentiles. SD was calculated in this manner [10]. If the studies were homogeneity, then fixed-effects model was reported, otherwise random-effects model or description of the results was used. I 2 was used to quantify heterogeneity [11], and if I 2 > 50%, heterogeneity should be explained or just describe the results. Statistical significance was defined as P < 0.05 (two-tailed).
Results
Search results
The initial research identified 1100 articles, and no new trials were identified by reference search. After removing the duplicated and reviewing the full-texts, 12 trials were finally selected (Figure 1 shows the flow chart of the selection process) [4-9,12-17]. In two of the final selected trials, the sedative method in the PCS group was inhalation of either methoxyflurane or a mixture of oxygen and nitrous oxide [5,12]. The remaining patients in the PCS group were all sedated through intravenous means. A total of 1091 patients were randomized either to the PCS group (545 patients), or the IVS group (546 patients). Table 1 describes the characteristics of the selected trials. Figure 2 shows the risk bias of the included studies using the Cochrane assessment tool.
Figure 1.

Flow chart of selection algorithm.
Table 1.
Characteristics of the selected studies
| Study | Country | Design | Control group | Number of patients | Age | Female % | Sedative drugs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| PCS | IVS | PCS | IVS | PCS | IVS | PCS | IVS | ||||
| Nguyen [12], 2013 | Australia | Multi-centered, RCT | Endoscopist | 125 | 126 | 51.4 | 54.9 | 44.0 | 42.9 | Methoxyflurane, inhaled | Midazolam + fentanyl |
| Mandel [13], 2010 | USA | Single-centered, RCT | Anesthetist | 25 | 25 | 58.0 | 59.1 | 36.0 | 44.0 | Propofol + remifentanil | Propofol + remifentanil |
| Crepeau [14], 2005 | France | Single-centered, RCT | Anesthetist | 35 | 35 | 56.8 | 58.4 | 28.6 | 40.0 | Propofol | Propofol |
| Stonell [6], 2006 | UK | Single-centered, RCT | Anesthetist | 20 | 20 | 46.0 | 47.0 | NG | NG | Propofol + fentanyl | Propofol + fentanyl |
| Heuss [4], 2004 | Switzerland | Single-centered, RCT | Nurse | 36 | 38 | 64.0 | 64.0 | 47.2 | 47.4 | Propofol + pethidine | Propofol + pethidine |
| Bright [15], 2003 | UK | Single-centered, RCT | Physician | 34 | 33 | 49.0 | 58.0 | 44.1 | 63.6 | Propofol + alfentanil | Midazolam + pethidine |
| Lee [7], 2002 | China | Single-centered, RCT | Endoscopist | 50 | 50 | 72.4 | 73.5 | 48.0 | 44.0 | Propofol + alfentanil | Diazemuls + meperidine |
| Ng [8], 2001 | Singapore | Single-centered, RCT | Anesthetist | 44 | 44 | 54.0 | 49.0 | 38.6 | 52.3 | Propofol | Midazolam |
| Kulling [9], 2001 | Switzerland | Single-centered, RCT | Nurse | 50 | 50 | 55.0 | 55.0 | 44.0 | 56.0 | Propofol + alfentanil | Propofol + alfentanil |
| Stermer [16], 2000 | Israel | Single-centered, RCT | Anesthetist | 25 | 25 | 68.1 | 66.3 | 36.0 | 48.0 | Midazolam + meperidine | Midazolam + meperidine |
| Roseveare [17], 1998 | USA | Single-centered, RCT | Endoscopist | 33 | 33 | 52.0 | 50.0 | NG | NG | Propofol + alfentanil | Diazepam |
| Saunders [5], 1994 | UK | Single-centered, RCT | Anesthetist | 30 | 29 | 46.0 | 42.0 | 53.3 | 62.1 | Nitrous oxide + oxygen, inhaled | Midazolam + pethidine |
Study was named using its first author, and publication year, and country. PCS, patient-controlled sedation; IVS, intravenous sedation; NG, not giving; RCT, randomized-controlled trial.
Figure 2.

Summary of risk bias of the included studies.
Outcomes
Sedation dose
The sedative drugs used in each trial are presented in Table 1. Seven trials reported about the total sedative drugs used [4,6-9,14,15], and in only 3 trials [4,6,9], the mean dose and SD could be extracted or calculated. The median propofol used in Crepeau et al.’s trial was 60 mg in the PCS group, while 248 mg in the IVS group (P < 0.001) [14]. And in Bright et al.’s trial, the amount in the PCS group and IVS group was 5.5 mg/kg/h, and 0.06 mg/kg, respectively [15]. Figure 3 shows the pooled results of the total propofol used. No statistical difference was found between both groups, and the mean difference was -5.26 (-20.75, 10.23) mg (P = 0.51). There was low-to-moderate heterogeneity among studies (I 2 = 41%).
Figure 3.

Meta-analysis of the total propofol used. PCS, patient-controlled sedation; IVS, intravenous sedation.
Procedure time
Four trials reported about the time for preparation [6,12,13,15]. Bright et al. gave a median of 15minutes in both PCS and IVS group (P = 0.2) [15]. The pooled result of the remaining 3 trials showed that, the preparation time in the PCS group was not statistically different from the IVS group [6,12,13]. The mean difference was 0.19 (-0.03, 0.42) minutes (P = 0.09) in fixed-effects model (Figure 4A), and 2.12 (0.36, 3.88) minutes (P = 0.02) in the random-effects model (Figure 4B). However, great heterogeneity existed (I 2 = 96%). Upon sensitivity analysis, heterogeneity still existed with removal of each study, possibly due to different means for timing. Hence, there was still debate towards time for preparation: Nguyen et al. found that the preparation time had no statistical difference between the two groups [12], while Mandel et al. and Stonell et al. supported that it was longer for PCS [6,13].
Figure 4.

Meta-analysis of the procedure time. A. Time for sedation preparation in fixed model; B. Time for sedation preparation in random model; C. Time for cecal intubation; D. Time for total procedure. PCS, patient-controlled sedation; IVS, intravenous sedation.
Four trials reported about the time for cecal intubation [4,5,12,14]. One showed mean time only: 12 minutes (PCS) VS. 8.2 minutes (IVS) (P = 0.004) [14], and one showed a median time of 13 minutes (PCS) VS. 14 minutes (IVS) [5]. The remaining 2 trials gave a mean difference of 0.00 (-0.24, -0.25) minutes (P = 0.98) (Figure 4C), without heterogeneity (I 2 = 0%).
Ten trials reported about the time for total procedure [4,6-8,12-17], 3 trials just provided the mean time or median time, and the time for total procedure was not statistically different between the two groups [14,15,17]. Seven trials showed the mean time and SD, which were put into meta-analysis. The mean difference was 0.02 (-0.22, 0.26) minutes (P = 0.87), with no heterogeneity (I 2 = 0%) (Figure 4D).
Rate of complete colonoscopy
Six trials reported about the rate of complete colonoscopy [7,12-15,17]. The rates in the PCS and the IVS group were similar (94.04% VS. 97.02%, P = 0.10), and the RR was 0.97 (0.93, 1.01) (Figure 5), with low-to-moderate heterogeneity (I 2 = 37%).
Figure 5.

Meta-analysis of the rate of complete colonoscopy. PCS, patient-controlled sedation; IVS, intravenous sedation.
Pain scores
Seven trials evaluated the pain scores [4,6-9,12,15]. Three trials used VAS pain score [6,9,12]. Two trials used the 5-point scale [4,15], 1 trial used the 4-point scale [8], and the remaining one used a 10-cm visual analog scale to evaluate the pain level [7]. It was impossible to pool the results of pain scores together, but all of the 7 trials proved that the PCS group and the IVS group had no statistical difference in pain scores.
Recovery time
Six trials evaluated the recovery time [5-7,12,15,17]. Stonell et al. concluded that, no difference existed between the two groups by using Aldrete Score [6]. While, for the remaining five trials, the conclusions were in consensus: there was an obvious reduction in the recovery time in the PCS group. Nguyen et al. showed a figure stating that the time to awake and time to discharge was shorter in the PCS group, without giving the exact time [12]. The remaining 4 trials showed us the median (and range) time for recovery (Table 2).
Table 2.
Recovery time in different studies
| Study | PCS | IVS | P-value |
|---|---|---|---|
| Bright [15], 2003 | 5 (0-25) | 35 (15-165) | < 0.0001 |
| Lee [7], 2002* | 0 (0-5) | 5 (5-10) | < 0.01 |
| Roseveare [17], 1998 | 15 (4-29) | 14 (5-40) | 0.0001 |
| Saunders [5], 1994 | 32 | 60 | < 0.0001 |
Numbers are expressed as median (range) in minutes.
Numbers are expressed as median (interquartile range) in minutes.
Study was named using its first author, and publication year, and country. PCS, patient-controlled sedation; IVS, intravenous sedation; NG, not giving.
Complications
The relatively common complications were oxygen desaturation, hypotension, arrhythmia, amnesia and after-effect.
Eight trials reported about the incidence of oxygen desaturation [4,5,7-9,12,14,17]. The rate of oxygen desaturation in the PCS group was lower than that in the IVS group (3.97% VS. 8.15%, P = 0.02), and the RR was 0.50 (0.28, 0.88) (Figure 6A). There was low heterogeneity among studies (I 2 = 10%).
Figure 6.

Meta-analysis of the complications. A. Rates for oxygen desaturation; B. Rates for hypotension; C. Rates for arrhythmia; D. Rates for amnesia and after-effect; E. Subgroup analysis of rates for amnesia and after-effect. PCS, patient-controlled sedation; IVS, intravenous sedation.
Four studies reported about the incidence of hypotension [5,7,12,15]. The rate of hypotension in the PCS was lower than that in the IVS group (4.60% VS. 11.34%, P = 0.01), and the RR was 0.42 (0.21, 0.81) (Figure 6B), with low-to-moderate heterogeneity (I 2 = 32%).
Two studies reported about the incidence of arrhythmia [12,14]. The rate of arrhythmia was similar in both groups (6.86% VS. 7.45%, P = 0.83), and the RR was 0.92 (0.43, 1.96) (Figure 6C), with low heterogeneity (I 2 = 15%).
Four studies reported about the incidence of amnesia [4,6,9,15]. The rate of amnesia in the PCS was not statistical different from that in the IVS group (29.29% VS. 41.84%, P = 0.22), and the RR was 0.71 (0.41, 1.23) in random-effects model (Figure 6D). There was great heterogeneity between studies (I 2 = 61%). Upon sensitivity analysis, still no statistical difference was found in the rate of amnesia (33.02% VS. 36.11%, P = 0.65, I 2 = 0%), with removal of one study [15] (Figure 6E). Bright et al. used propofol+ alfentanil in the PCS group, while midazolam+ pethidine in the IVS group, and they obtained a statistical difference in the rates of amnesia [15]. However, in the other 3 trials, they used the same sedative drug in both PCS group and IVS group [4,6,9]. Hence, the difference in Bright et al.’s trial may lie in the diverse sedative drug used in both groups.
Other complications such as dizziness, nausea, and vomiting have also been reported in 2 trials [5,7], and both supported that no statistical difference was found between the two groups. Two studies both agreed that the incidence of after-effects in the PCS group was lower than that in the IVS group [15,17].
Willingness to repeat
Four trials asked whether the patients were willing to use the same sedation method again [6,8,14,15]. The rate of patients willing to repeat the sedation method in the PCS was not statistical different from that in the IVS group (83.46% VS. 68.25%, P = 0.41), and the RR was 1.21 (0.77, 1.92) in random-effects model (Figure 7). There was great heterogeneity between studies (I 2 = 94%). Upon sensitivity analysis, great heterogeneity still existed with removal of each study. The heterogeneity might lie in who performed the sedation in the IVS group. In Bright et al.’s study, the sedation in the IVS group was performed by the physician [15], and they concluded no statistical difference existed between the groups. When we made a further subgroup analysis of the other 3 trials [6,8,14], the difference was not obvious (RR 1.29, 0.89-1.86 P = 0.18) in random-effects model, and heterogeneity was reduced (I 2 = 75%).
Figure 7.
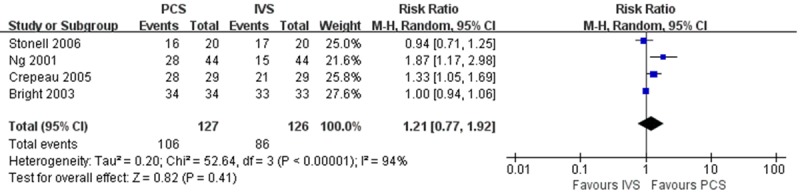
Meta-analysis of willingness to repeat the same sedation method. PCS, patient-controlled sedation; IVS, intravenous sedation.
Public bias
The funnel plots revealed evidence of bias, possibly publication bias, and the bias is not remarkable. Due to the reason that, most comparisons included a small number of trials, it is difficult to interpret the funnel plots.
Discussion
Sedative drugs are commonly used during colonoscopy so as to facilitate the procedure and relieve patient’s discomfort. However, it is reported that the use of sedative drugs would bring about cardiorespiratory complications in up to 20% of the patients [18]. The appearance of PCS seems to act as an alternative choice [19], as it allows the patients to adjust the dose of sedative drugs according to their own feeling towards consciousness and pain, thus excessive dose is avoided. Various studies have made comparisons between PCS and IVS, but up to now, a comprehensive summarization of these trials still lacks.
This is the first systematic review and meta-analysis to compare PCS with IVS for colonoscopy.
Patients experience various levels of discomfort during colonoscopy, depending on his/her tolerability, history of abdominal surgery and structure of the colon. Some patients may not require any form of sedation, while others absolutely need [20]. PCS provides the flexibility for varying the degree of sedation according to the patient’s own willing. Furthermore, individual pharmacodynamic differences exist among the patients, and PCS gives a chance to control the difference with a sense of autonomy [20].
PCS for colonoscopy can be achieved in a multiple combinations of sedative and analgesic drugs. The drugs can be administered through intravenous or inhalation. For intravenous sedation, propofol seems to be the preference choice due to its rapid on-set and off-set of action, and (at least) equivalent effectiveness to midazolam [21]. Sometimes, short acting opioids are also added to relieve patient’s pain. The sedative and analgesic drugs are likely to cause respiratory depression and hypotension. Sedative comedication and careful application of small doses help to avoid these sequelae [20]. The specialized pumps used in PCS allow for set of bolus dose and lockout time interval, and that the patient should be conscious enough to press the button, therefore, oversedation is prevented.
Not every patient suits for PCS; the patients qualified should comprehend the instructions, manipulate the device appropriately, and have a certain degree of cooperation. For those anticipating deep sedation and coming with too much anxiety, PCS may not be a wise choice. Maybe due to its high requirement of the patient, PCS is quite uncommon, but Vargo has faith that PCS will have platforms in the gastroenterology fields across many procedures [22].
The time of cecal intubation time and total procedure varied greatly in different trials, due to varying starting and ending points, different colonoscopy techniques, and endoscopist’s experience. The pooled results showed that, the use of PCS will not influence the efficiency of the total colonoscopy procedure. Though a minority of the patients might fail in administration the sedative drugs through PCS, and traditional IVS performed by anesthetist was needed, the proportion was really small, and had no effect on the cecal intubation rate.
The scales used to evaluate patient’s feeling of pain varied in different trials, but the eligible studies all achieved the same results: the pain score had no statistical difference between the two groups. Though one trial further evaluated the pain as follow-up parameter, and more patients in the PCS group reported to have pain, the authors believed that, the procedure did not influence the overall levels of satisfaction with the method of sedation. This may be a reflection of lighter sedation and lower level of amnesia in the PCS group [15].
In sedative colonoscopy, of most important clinical relevance is the time required for the patient to discharge from the endoscopy center, as this needs the workload of staff with respect to patient monitoring. Among the six selected trials evaluating the recovery time, only one trial proved that the two groups had no statistical difference in the time for Aldrete Score = 9 [6], while the left 5 trials all showed superiority of PCS over IVS in the recovery time, so the tendency is obvious that PCS needed less time for recovery.
The complication related to sedation is another vital issue we concern [23,24]. Actually, cardiorespiratory depression is the most commonly reported adverse events [25]. As the pooled results showed that, the rates of oxygen desaturation and hypotension in the PCS were both lower than those in the IVS group. This advantage of PCS may derive from the fact that, self administration of medication was drived by pain, while pain stimulates the cardiovascular and respiratory systems, which reduces the depressing effects of the sedative and analgesia drugs. On the other hand, PCS requires that the patient is conscious enough to depress the button. As a result, oversedation is unlikely if rapidly on-set drugs such as propofol and fentanyl are used [15].
Two trials have compared the direct costs in PCS and IVS [4,17], and the results showed that the costs in the PCS group were higher. But something should be taken into considerations, firstly, gains in efficiency throughout the procedure will offset the higher costs when compared with IVS; secondly, the fees paid to hire an anesthetist or nurse can be avoided; thirdly, additionally costs associated with the need for overnight admission, and lost work productivity is also saved [22]. Further studies are needed to answer this important question about the cost-effectiveness comparison between PCS and IVS.
Among the selected studies, two have slight difference from the others, as the method used in the PCS group was inhalation of either methoxyflurane through Penthrox or a mixture of oxygen and nitrous oxide through Entonox [5,12]. Nevertheless, the data in the two trials were both positive for PCS. Though oxygen and nitrous oxide has long been established as safe for patients, long-term exposure had drawn our attention, as it might cause bone marrow depression, megaloblastic changes and neurologic dysfunction [26,27]. These would happen only in an atmosphere with high concentration of nitrous oxide. With well-ventilation in the endoscopy center, there is little chance that the hazard would happen.
PCS has been widely investigated for nearly 30 years [28-31], and in the field of endoscopy, colonoscopy has taken a large proportion, while gastroduodenoscopy and prolonged procedures, such as endoscopic retrograde cholangiopancreatography and endoscopic ultrasound still need further research [32-35]. Meanwhile, the most appropriate bolus dose of medication, lockout time interval, the optimum of sedative and analgesia drugs and the ratio of them also needs more exploration [36-38].
In conclusion, PCS is as feasible and effective as the traditional IVS for colonoscopy, without prolonging the time of total procedure, reducing the cecal intubation rate, or influencing patient’s satisfaction, rather there seems to be a tendency that PCS shows its superiority in recovery time, incidence for oxygen saturation and hypotension. With appropriate patient selection, PCS may help to improve the workflow of endoscopy units.
Disclosure of conflict of interest
None.
References
- 1.Jover R, Herraiz M, Alarcon O, Brullet E, Bujanda L, Bustamante M, Campo R, Carreño R, Castells A, Cubiella J, García-Iglesias P, Hervás AJ, Menchén P, Ono A, Panadés A, Parra-Blanco A, Pellisé M, Ponce M, Quintero E, Reñé JM, Sánchez del Río A, Seoane A, Serradesanferm A, Soriano Izquierdo A, Vázquez Sequeiros E Spanish Society of Gastroenterology; Spanish Society of Gastrointestinal Endoscopy Working Group. Clinical practice guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012;44:444–451. doi: 10.1055/s-0032-1306690. [DOI] [PubMed] [Google Scholar]
- 2.Williams CB. Comfort and quality in colonoscopy. Gastrointest Endosc. 1994;40:769–770. [PubMed] [Google Scholar]
- 3.Iber FL, Sutberry M, Gupta R, Kruss D. Evaluation of complications during and after conscious sedation for endoscopy using pulse oximetry. Gastrointest Endosc. 1993;39:620–625. doi: 10.1016/s0016-5107(93)70211-4. [DOI] [PubMed] [Google Scholar]
- 4.Heuss LT, Drewe J, Schnieper P, Tapparelli CB, Pflimlin E, Beglinger C. Patient-controlled versus nurse-administered sedation with propofol during colonoscopy. A prospective randomized trial. AM J Gastroenterol. 2004;99:511–518. doi: 10.1111/j.1572-0241.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- 5.Saunders BP, Fukumoto M, Halligan S, Masaki T, Love S, Williams CB. Patient-administered nitrous oxide/oxygen inhalation provides effective sedation and analgesia for colonoscopy. Gastrointest Endosc. 1994;40:418–421. doi: 10.1016/s0016-5107(94)70203-9. [DOI] [PubMed] [Google Scholar]
- 6.Stonell CA, Leslie K, Absalom AR. Effect-site targeted patient-controlled sedation with propofol: comparison with anaesthetist administration for colonoscopy. Anaesthesia. 2006;61:240–247. doi: 10.1111/j.1365-2044.2005.04509.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee DW, Chan AC, Sze TS, Ko CW, Poon CM, Chan KC, Sin KS, Chung SC. Patient-controlled sedation versus intravenous sedation for colonoscopy in elderly patients: a prospective randomized controlled trial. Gastrointest Endosc. 2002;56:629–632. doi: 10.1067/mge.2002.128919. [DOI] [PubMed] [Google Scholar]
- 8.Ng JM, Kong CF, Nyam D. Patient-controlled sedation with propofol for colonoscopy. Gastrointest Endosc. 2001;54:8–13. doi: 10.1067/mge.2001.116110. [DOI] [PubMed] [Google Scholar]
- 9.Kulling D, Fantin AC, Biro P, Bauerfeind P, Fried M. Safer colonoscopy with patient-controlled analgesia and sedation with propofol and alfentanil. Gastrointest Endosc. 2001;54:1–7. doi: 10.1067/mge.2001.116174. [DOI] [PubMed] [Google Scholar]
- 10.Whitlock RP, Chan S, Devereaux PJ, Sun J, Rubens FD, Thorlund K, Teoh KH. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: a meta-analysis of randomized trials. Eur Heart J. 2008;29:2592–2600. doi: 10.1093/eurheartj/ehn333. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NQ, Toscano L, Lawrence M, Moore J, Holloway RH, Bartholomeusz D, Lidums I, Tam W, Roberts-Thomson IC, Mahesh VN, Debreceni TL, Schoeman MN. Patient-controlled analgesia with inhaled methoxyflurane versus conventional endoscopist-provided sedation for colonoscopy: A randomized multicenter trial. Gastrointest Endosc. 2013;78:892–901. doi: 10.1016/j.gie.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Mandel JE, Lichtenstein GR, Metz DC, Ginsberg GG, Kochman ML. A prospective, randomized, comparative trial evaluating respiratory depression during patient-controlled versus anesthesiologist-administered propofol-remifentanil sedation for elective colonoscopy. Gastrointest Endosc. 2010;72:112–117. doi: 10.1016/j.gie.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Crepeau T, Poincloux L, Bonny C, Lighetto S, Jaffeux P, Artigue F, Walleckx P, Bazin JE, Dapoigny M, Bommelaer G. Significance of patient-controlled sedation during colonoscopy. Results from a prospective randomized controlled study. Gastroenterol Clin Biol. 2005;29:1090–1096. doi: 10.1016/s0399-8320(05)82172-4. [DOI] [PubMed] [Google Scholar]
- 15.Bright E, Roseveare C, Dalgleish D, Kimble J, Elliott J, Shepherd H. Patient-controlled sedation for colonoscopy: a randomized trial comparing patient-controlled administration of propofol and alfentanil with physician-administered midazolam and pethidine. Endoscopy. 2003;35:683–687. doi: 10.1055/s-2003-41519. [DOI] [PubMed] [Google Scholar]
- 16.Stermer E, Gaitini L, Yudashkin M, Essaian G, Tamir A. Patient-controlled analgesia for conscious sedation during colonoscopy: a randomized controlled study. Gastrointest Endosc. 2000;51:278–281. doi: 10.1016/s0016-5107(00)70355-5. [DOI] [PubMed] [Google Scholar]
- 17.Roseveare C, Seavell C, Patel P, Criswell J, Kimble J, Jones C, Shepherd H. Patient-controlled sedation and analgesia, using propofol and alfentanil, during colonoscopy: A prospective randomized controlled trial. Endoscopy. 1998;30:768–773. doi: 10.1055/s-2007-1001419. [DOI] [PubMed] [Google Scholar]
- 18.Iber FL, Sutberry M, Gupta R, Kruss D. Evaluation of complications during and after conscious sedation for endoscopy using pulse oximetry. Gastrointest Endosc. 1993;39:620–625. doi: 10.1016/s0016-5107(93)70211-4. [DOI] [PubMed] [Google Scholar]
- 19.Graber RG. Propofol in the endoscopy suite: An anesthesiologist’s perspective. Gastrointest Endosc. 1999;49:803–806. doi: 10.1016/s0016-5107(99)70308-1. [DOI] [PubMed] [Google Scholar]
- 20.Kulling D, Biro P, Fried M, Bauerfeind P. A synopsis of patient-controlled analgesia and sedation for endoscopic procedures. Techniques in Gastrointestinal Endoscopy. 2004;6:65–68. [Google Scholar]
- 21.Reimann FM, Samson U, Derad I, Fuchs M, Schiefer B, Stange EF. Synergistic sedation with low-dose midazolam and propofol for colonoscopies. Endoscopy. 2000;32:239–244. doi: 10.1055/s-2000-134. [DOI] [PubMed] [Google Scholar]
- 22.Vargo J. Patient-controlled sedation for endoscopic procedures. Gastroenterol Hepatol (N Y) 2008;4:329–331. [PMC free article] [PubMed] [Google Scholar]
- 23.Bell GD, Charlton JE. Colonoscopy-is sedation necessary and is there any role for intravenous propofol? Endoscopy. 2000;32:264–267. doi: 10.1055/s-2000-97. [DOI] [PubMed] [Google Scholar]
- 24.Graber RG. Propofol in the endoscopy suite: an anesthesiologist’s perspective. Gastrointest EndosC. 1999;49:803–806. doi: 10.1016/s0016-5107(99)70308-1. [DOI] [PubMed] [Google Scholar]
- 25.Sumpelmann R, Busing H, Schroder D, Rekersbrink M, Krohn S, Strauss JM. [Patientcontrolled analgesia with clonidine and piritramide] . Anaesthesist. 1996;45:88–94. doi: 10.1007/s001010050245. [DOI] [PubMed] [Google Scholar]
- 26.Nunn JF. Clinical aspects of the interaction between nitrous oxide and vitamin B12. Br J Anaesth. 1987;59:3–13. doi: 10.1093/bja/59.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Koblin DD, Waskell L, Watson JE, Stokstad EL, Eger EN. Nitrous oxide inactivates methionine synthetase in human liver. Anesth Analg. 1982;61:75–78. [PubMed] [Google Scholar]
- 28.Lee DW, Chan AC, Wong SK, Fung TM, Li AC, Chan SK, Mui LM, Ng EK, Chung SC. Can visual distraction decrease the dose of patient-controlled sedation required during colonoscopy? A prospective randomized controlled trial. Endoscopy. 2004;36:197–201. doi: 10.1055/s-2004-814247. [DOI] [PubMed] [Google Scholar]
- 29.Campbell L, Imrie G, Doherty P, Porteous C, Millar K, Kenny GN, Fletcher G. Patient maintained sedation for colonoscopy using a target controlled infusion of propofol. Anaesthesia. 2004;59:127–132. doi: 10.1111/j.1365-2044.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 30.Loper KA, Ready LB, Brody M. Patient-controlled anxiolysis with midazolam. Anesth Analg. 1988;67:1118–1119. [PubMed] [Google Scholar]
- 31.Leitch JA, Anderson K, Gambhir S, Millar K, Robb ND, McHugh S, Kenny GN. A partially blinded randomised controlled trial of patientmaintained propofol sedation and operator controlled midazolam sedation in third molar extractions. Anaesthesia. 2004;59:853–860. doi: 10.1111/j.1365-2044.2004.03761.x. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson A, Grossmann B, Kullman E, Uustal E, Sjöberg F, Nilsson L. Sedation during endoscopic retrograde cholangiopancreatography: A randomized controlled study of patient-controlled propofol sedation and that given by a nurse anesthetist. Scand J Gastroenterol. 2015:1–8. doi: 10.3109/00365521.2015.1038848. [DOI] [PubMed] [Google Scholar]
- 33.Mazanikov M, Udd M, Kylanpaa L, Mustonen H, Lindström O, Färkkilä M, Halttunen J, Pöyhiä R. A randomized comparison of target-controlled propofol infusion and patient-controlled sedation during ERCP. Endoscopy. 2013;45:915–919. doi: 10.1055/s-0033-1344712. [DOI] [PubMed] [Google Scholar]
- 34.Mazanikov M, Udd M, Kylanpaa L, Lindström O, Aho P, Halttunen J, Färkkilä M, Pöyhiä R. Patient-controlled sedation with propofol and remifentanil for ERCP: A randomized, controlled study. Gastrointest Endosc. 2011;73:260–266. doi: 10.1016/j.gie.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Jowell PS, Eisen G, Onken J, Bute BP, Ginsberg B. Patient-controlled analgesia for conscious sedation during endoscopic retrograde cholangiopancreatography: a randomized controlled trial. Gastrointest Endosc. 1996;43:490–494. doi: 10.1016/s0016-5107(96)70292-4. [DOI] [PubMed] [Google Scholar]
- 36.Sultan SS. Patient-controlled sedation with propofol/remifentanil versus propofol/alfentanil for patients undergoing outpatient colonoscopy, a randomized, controlled doubleblind study. Saudi J Anaesth. 2014;8(Suppl 1):S36–S40. doi: 10.4103/1658-354X.144068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanti L, Agostoni M, Gemma M, Rossi G, Azzolini ML, Viale E, Guslandi M, Beretta L, Testoni PA. Two dosages of remifentanil for patient-controlled analgesia vs. meperidine during colonoscopy: A prospective randomized controlled trial. Dig Liver Dis. 2013;45:310–315. doi: 10.1016/j.dld.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Fanti L, Agostoni M, Gemma M, Gambino G, Facciorusso A, Guslandi M, Torri G, Testoni PA. Remifentanil vs. meperidine for patient-controlled analgesia during colonoscopy: A randomized double-blind trial. AM J Gastroenterol. 2009;104:1119–1124. doi: 10.1038/ajg.2009.53. [DOI] [PubMed] [Google Scholar]


