Abstract
Background: The risk of bradykinin B2 receptor (BDKRB2) -58T/C gene polymorphism on hypertension remains controversial. Materials and methods: The Cochrane Library, Chinese Biomedical Database, EBSCO, Embase, ISI, MEDLINE, and PubMed were retrieved, and relevant articles were selected. Results: The significant association between BDKRB2-58T/C gene polymorphism and risk of hypertension were found under C-allele comparison [odds ratio (OR): 1.22, 95% confidential intervals (CI): 1.05-1.42, recessive model (OR: 1.32, 95% CI: 1.07-1.64), dominant model (OR: 0.74, 95% CI: 0.58-0.94), homozygote model (OR: 1.66, 95% CI: 1.11-2.47) and heterozygote model (OR: 1.23, 95% CI: 1.06-1.43). The magnitude of the association between the BDKRB2-58T/C gene polymorphism and risk of hypertension was substantiated in Asians under C-allele comparison (OR: 1.24, 95% CI: 1.04-1.49), recessive model (OR: 1.39, 95% CI: 1.04-1.86), dominant model (OR: 0.72, 95% CI: 0.56-0.93), homozygote model (OR: 1.78, 95% CI: 1.09-2.90) and heterozygote model (OR: 1.26, 95% CI: 1.07-1.49). No publication bias was found in the meta-analysis. Conclusions: The meta-analysis suggested -58C allele and -58CC genotype increase the risk of hypertension. Inversely, -58TT genotype decreases the risk of hypertension.
Keywords: Bradykinin B2 receptor, polymorphism, hypertension, meta-analysis
Introduction
Earlier studies suggested that hypertension is a complex multifactorial disorder with genetic heritability averaging approximately 30% [1]. The kallikrein-kinin system (KKS) plays critical roles in the cardiovascular system, affecting blood pressure regulation [2]. KKS is implicated in hypertension regulation via the potent vasoactive peptide bradykinin. Bradykinin is released from the kininogens via the proteolytic activity of kallikreins and acts on two bradykinin receptors known as bradykinin B1 receptor (BDKRB1) and bradykinin B2 receptor (BDKRB2) [3-5]. Bradykinin induces vasodilation, releases of nitric oxide and promotes water and sodium excretion [5]. Two bradykinin receptors (BDKRB1 and BDKRB2), predominantly the BDKRB2, mediate the main physiological activities and cardiovascular actions [3,6]. The human BDKRB2 is implicated as a candidate in the complex genetic underpinning of hypertension [7].
The risk of BDKRB2-58T/C gene polymorphism on hypertension remains controversial. Two published meta-analyses reported the association between BDKRB-58T/C gene polymorphism and hypertension [8,9]. Niu et al. [8] included only four studies with 823 hypertensive subjects and 916 healthy volunteers. In contrast to Li et al. [9], we included two more new large-sample case-control studies, thus we enrolled 13 studies with 2369 hypertensive subjects and 2294 healthy volunteers. Additionally, sensitivity analyses and publication bias had not been performed in these two articles.
The purpose of present meta-analysis is to provide an updated quantitative assessment of the association between BDKRB2-58T/C gene polymorphism and risk of hypertension. In this study, we followed the inclusion and firstly the exclusion criteria to reduce the possible selection bias. Secondly, the included studies in the present article were conducted around the world (China, Japan, India, United States, Canada, and Italy), which are certainly representatives of ethnicities.
Methods
Searching strategies
The Cochrane Library, Chinese Biomedical Database, EBSCO, Embase, ISI, MEDLINE, and PubMed were retrieved, and relevant articles were selected from the building of the database to 31st, January 2015. The keywords include (“bradykinin B2 receptor” OR “BDKRB2”) AND “polymorphism” AND (“hypertension” OR “blood pressure”). There were no language restrictions. Related research references were reviewed at the same time.
Study selection
Inclusion criteria of studies
In the present meta-analysis, the studies were included under the following inclusion criteria: (a) case-control studies described the association between BDKRB2-58T/C gene polymorphism and risk of hypertension, (b) studies defined diagnostic criteria for hypertension: systolic pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg, (c) studies reported the genotype frequency of BDKRB2-58T/C gene polymorphism, and (d) studies provided information about odds ratio (OR) and 95% confidence intervals (CI) of the association between BDKRB2-58T/C gene polymorphism and risk of hypertension.
Exclusion criteria of studies
Studies were excluded under the following criteria: (a) no case-control studies, (b) patients with secondary hypertension, coronary heart disease, renal failure or diabetes mellitus, (c) studies did not provide available genotype data for BDKRB2-58T/C gene polymorphism, and (d) data or articles published repeatedly.
Data extraction and quality
Two researchers (Kaiping Luo & Wenling Kang) independently searched the electronic databases listed above. The title, abstract and full-text of all potentially relevant literatures were screened to evaluate their relevance. Disagreements were resolved by discussion. The following information was extracted from the included articles: first author, year of publication, ethnicity, country, study design, numbers of cases and controls, sample source, diagnostic criteria, gender, the genotype and allele counts of BDKRB2-58T/C gene polymorphism. The Newcastle-Ottawa Scale (NOS) was used to evaluate the study quality [10]. For case-control studies, the star evaluates three broad perspectives: selection, comparability, and exposure. The study can be allotted a maximum of one star for each numbered item within the selection and exposure categories. A maximum of two stars can be allotted for comparability. Newcastle-Ottawa quality assessment scale ranges from 0 to 9 stars. A total score of five or more stars was indicated a high-quality study.
Statistical analysis
Odds ratio (OR), with 95% confidential intervals (CI) was calculated by Cochrane RevMan software version 5.2 (Cochrane Library, UK). OR was calculated to examine the contrast of C-allele comparison (C vs T), dominant model (TT vs CT + CC), recessive model (CC vs CT + TT), homozygote model (CC vs TT) and heterozygote model (CT vs TT), respectively. A chi-square test was used to estimate whether or not the observed frequencies of genotypes in the controls conformed to Hardy-Weinberg expectations (HWE). P-value < 0.05 was considered statistically significant.
Heterogeneity among studies was evaluated by the Cochrane’s Q statistic and I 2 statistic, P-value < 0.10 or I 2 score > 50% was considered statistically significant [11]. When no heterogeneity was detected among studies, the fixed effects model (Mantel-Haenszel method) was applied; otherwise, the random effects model (Dersimonian-Laird method) was used [12]. The subgroup analysis was conducted to deal with heterogeneity. Subgroups were defined by continents and races.
Sensitivity analysis was conducted to evaluate the stability of the main meta-analysis results. Publication bias was evaluated by constructing a funnel plot and using Egger’s linear regression tests (Egger’s tests) and Begg’s rank correlation tests (Begg’s tests) [13].
Results
Characteristics of included studies
Of 194 articles identified in the initial search, 132 were retrieved for more detailed evaluation, 120 studies were excluded (trials only reported BDKRB2-58T/C gene but not including hypertension, not relevant to BDKRB2-58T/C gene polymorphism, duplicated articles, no explicit data, off topic, etc.), then 13 trials [14-26] were finally included in the analyses, enrolling with 4663 subjects (Figure 1). According to NOS, all articles were of high quality. Except one study (Dong et al.), the frequencies of genotypes in the controls of the rest of the included studies were conformed to HWE. Deviation from HWE in the controls can be attributed to the limited sample sizes of the studies, but can also reflect an effect of the genetic polymorphism on hypertension. The characteristics of the articles were summarized in Table 1.
Figure 1.
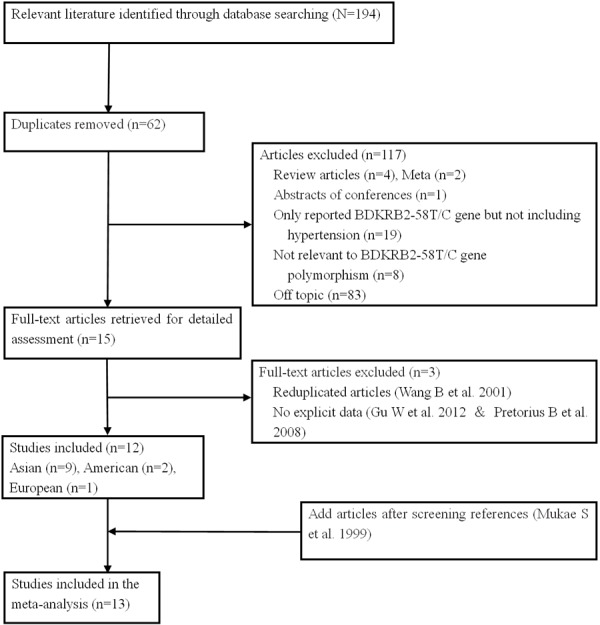
Flow diagram of the selection of studies for inclusion in the meta-analysis.
Table 1.
The different genotype distribution of BDKRB-58T/C gene polymorphism for cases and controls
| Cases | Controls | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Study | Year | Country | Distribution of genotype | Allele frequency | HWE | Distribution of genotype | Allele frequency | HWE | NOS score | ||||||
| P-value | P-value | ||||||||||||||
|
|
|||||||||||||||
| TT | CT | CC | T | C | TT | CT | CC | T | C | ||||||
| Mukae S | 1999 | Japan | 13 | 59 | 28 | 85 | 115 | 0.04 | 25 | 57 | 18 | 107 | 93 | 0.15 | 5 |
| Gainer JV | 2000 | American | 2 | 34 | 41 | 83 | 116 | 0.10 | 17 | 58 | 45 | 92 | 148 | 0.81 | 6 |
| Aoki S | 2001 | Japan | 19 | 88 | 43 | 126 | 174 | 0.01 | 38 | 84 | 28 | 160 | 140 | 0.13 | 5 |
| Wang B | 2001 | China | 15 | 74 | 31 | 104 | 136 | 0.01 | 24 | 57 | 17 | 105 | 91 | 0.09 | 5 |
| Fu Y | 2004 | Japan | 70 | 139 | 66 | 279 | 271 | 0.85 | 116 | 227 | 98 | 459 | 423 | 0.51 | 5 |
| Cui J-1 | 2005 | A-C | 27 | 55 | 37 | 109 | 129 | 0.45 | 16 | 47 | 27 | 79 | 101 | 0.56 | 5 |
| Cui J-2 | 2005 | G-C | 25 | 46 | 25 | 96 | 96 | 0.68 | 17 | 43 | 32 | 77 | 107 | 0.70 | 5 |
| Cui J-3 | 2005 | A-A | 10 | 44 | 59 | 64 | 162 | 0.66 | 20 | 32 | 43 | 72 | 118 | 0.01 | 5 |
| Cui J | 2005 | American | 62 | 145 | 121 | 269 | 387 | 0.12 | 53 | 122 | 102 | 228 | 326 | 0.13 | 5 |
| Milan A | 2005 | Italy | 21 | 59 | 49 | 101 | 157 | 0.65 | 15 | 52 | 28 | 82 | 108 | 0.26 | 6 |
| Dong HY | 2006 | China | 15 | 47 | 35 | 78 | 116 | 0.91 | 24 | 51 | 11 | 98 | 74 | 0.05 | 6 |
| Li NF | 2008 | China | 102 | 199 | 53 | 403 | 305 | 0.06 | 62 | 106 | 48 | 230 | 202 | 0.83 | 6 |
| Hang LW | 2009 | China | 10 | 50 | 35 | 70 | 120 | 0.20 | 36 | 85 | 36 | 157 | 157 | 0.30 | 5 |
| Bhupatiraju C | 2011 | India | 39 | 101 | 74 | 179 | 249 | 0.66 | 53 | 122 | 74 | 228 | 270 | 0.84 | 5 |
| Zou L | 2011 | China | 30 | 49 | 24 | 109 | 97 | 0.65 | 25 | 48 | 30 | 98 | 108 | 0.50 | 5 |
| Song J | 2012 | China | 96 | 157 | 74 | 349 | 305 | 0.52 | 60 | 88 | 54 | 216 | 188 | 0.07 | 5 |
HWE: Hardy-Weinberg expectations, NOS: Newcastle-Ottawa Scale, A-C: American Caucasian, G-C: Greek Caucasian, A-A: African American.
Meta-analysis results
The meta-analysis of overall studies
OR with 95% CI were used to assess the association between BDKRB2-58T/C gene polymorphism and risk of hypertension. Significant heterogeneities were observed across the studies for C-allele comparison (P < 0.01 and I 2 = 68%), recessive model (P < 0.01 and I 2 = 55%), dominant model (P < 0.01 and I 2 = 60%), homozygote model (P < 0.01 and I 2 = 79%), except heterozygote model (P = 0.06 and I 2 = 39%), as described in Figures 2, 3, 4, 5 and 6. Therefore, the random effects model or the fixed effects model was used to assess the overall OR. The significant association between BDKRB2-58T/C gene polymorphism and risk of hypertension was found under C-allele comparison (OR = 1.22; 95% CI = 1.05-1.42; P = 0.01), recessive model (OR = 1.32; 95% CI = 1.07-1.64; P = 0.01), dominant model (OR = 0.74; 95% CI = 0.58-0.94; P = 0.02), homozygote model (OR = 1.66; 95% CI = 1.11-2.47; P = 0.01) and heterozygote model (OR = 1.23; 95% CI = 1.06-1.43; P = 0.007), respectively.
Figure 2.
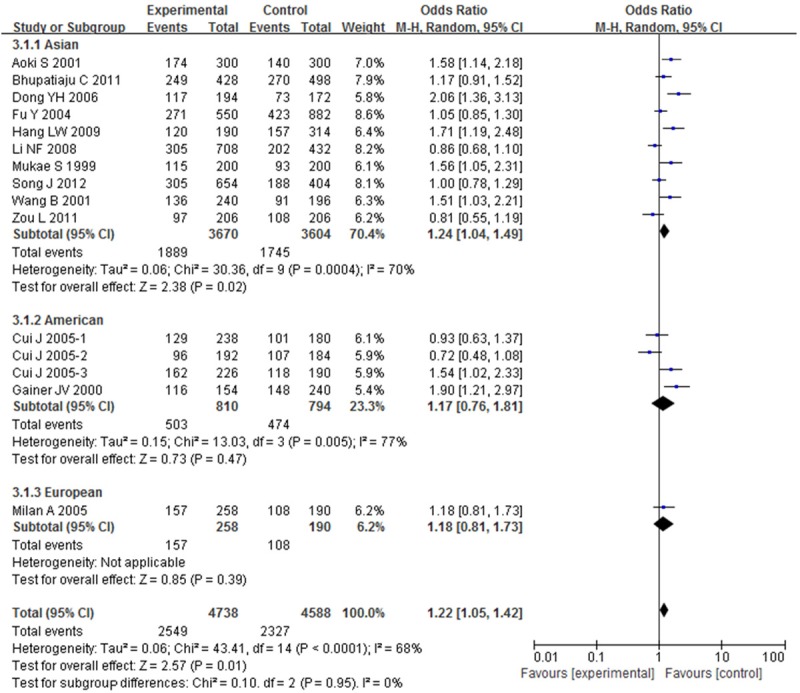
Overall effect estimates of the BDKRB2-58T/C gene polymorphism for hypertension risk under C-allele comparison.
Figure 3.
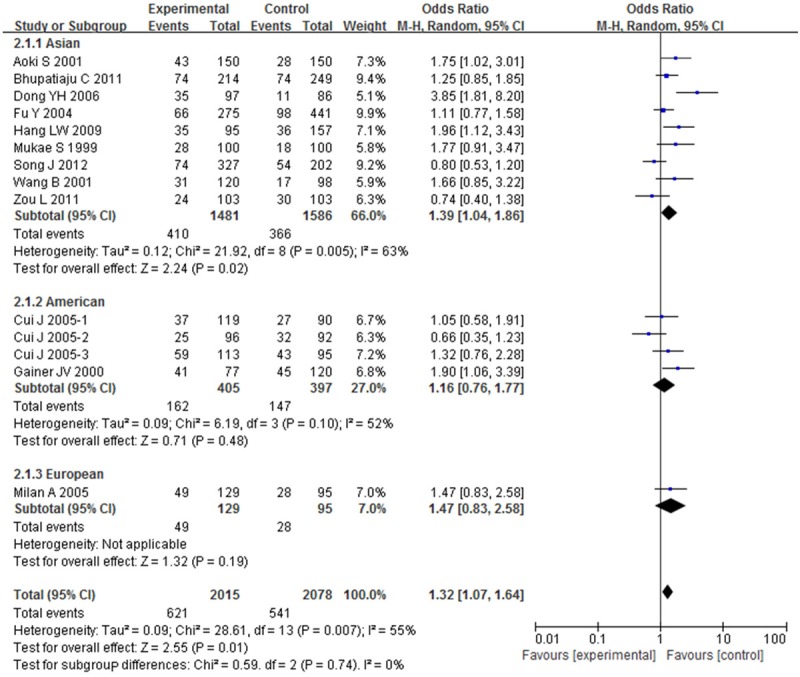
Overall effect estimates of the BDKRB2-58T/C gene polymorphism for hypertension risk under recessive model.
Figure 4.
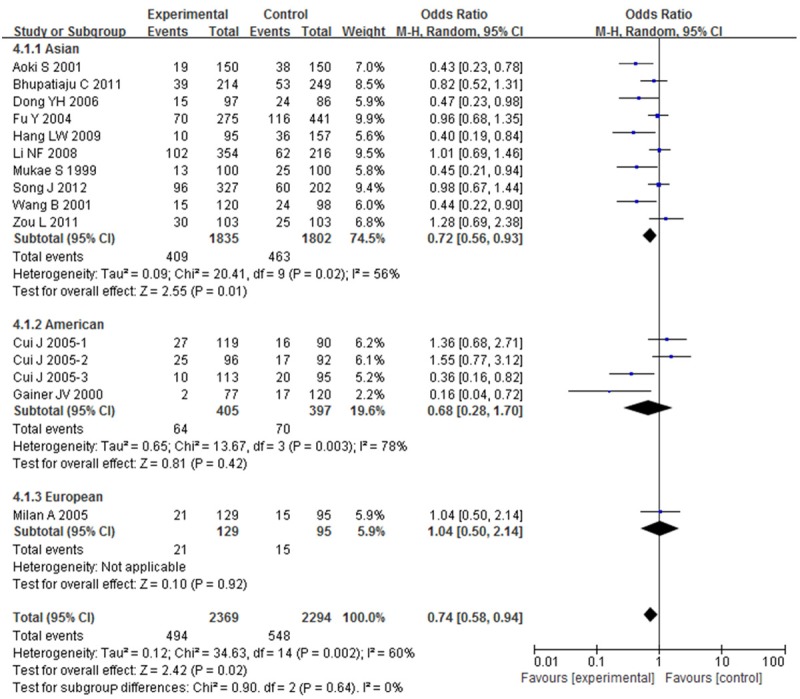
Overall effect estimates of the BDKRB2-58T/C gene polymorphism for hypertension risk under dominant model.
Figure 5.
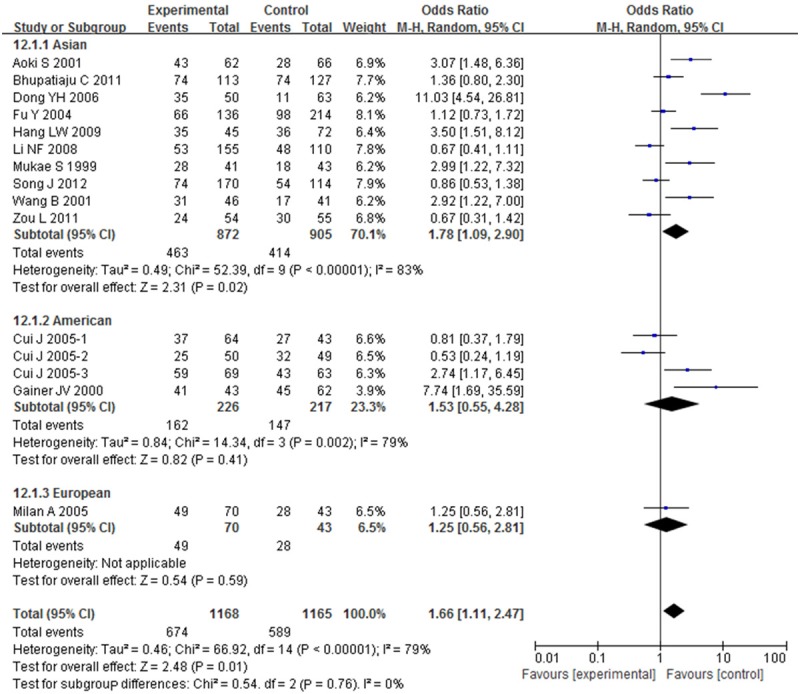
Overall effect estimates of the BDKRB2-58T/C gene polymorphism for hypertension risk under homozygote model.
Figure 6.
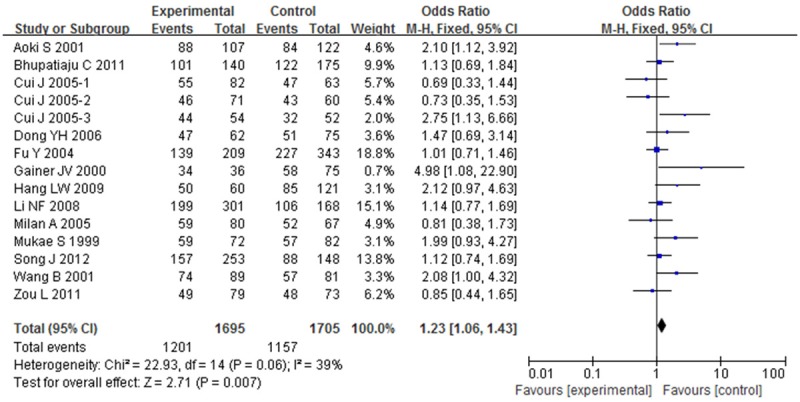
Overall effect estimates of the BDKRB2-58T/C gene polymorphism for hypertension risk under heterozygote model.
The meta-analysis of subgroups
In order to evaluate the impact of ethnicity differences on the association between BDKRB2-58T/C gene polymorphism and risk of hypertension, we performed a subgroup analysis stratified by continents. The magnitude of the association between BDKRB2-58T/C gene polymorphism and hypertension was substantiated in Asian under C-allele comparison (OR = 1.24; 95% CI = 1.04-1.49; P = 0.02), recessive model (OR = 1.39; 95% CI = 1.04-1.86; P = 0.02), dominant model (OR = 0.72; 95% CI = 0.56-0.93; P = 0.01), homozygote model (OR = 1.78; 95% CI = 1.09-2.90; P = 0.02) and heterozygote model (OR = 1.26; 95% CI = 1.07-1.49; P = 0.007) with heterogeneity (I 2 = 70%, 63%, 56%, 83% and 19%, respectively). The magnitude of this association in American and European were weakened to nonsignificance.
We also performed a subgroup analysis stratified by races. The study was separated into five subgroups by races (Chinese, Japanese, Indian, Caucasian, and African-American). A significant correlation between BDKRB2-58T/C gene polymorphism and risk of hypertension was found in African-American under C-allele comparison (OR; 95% CI: 1.70; 1.25-2.30), recessive model (OR; 95% CI: 1.57; 1.05-2.33), dominant model (OR; 95% CI: 0.30; 0.15-0.62), homozygote model (OR; 95% CI: 3.79; 1.82-7.85) and heterozygote model (OR; 95% CI: 3.32; 1.56-7.10), but no correlation was found between BDKRB2-58T/C gene polymorphism and risk of hypertension in other races, as shown in Figure 7.
Figure 7.
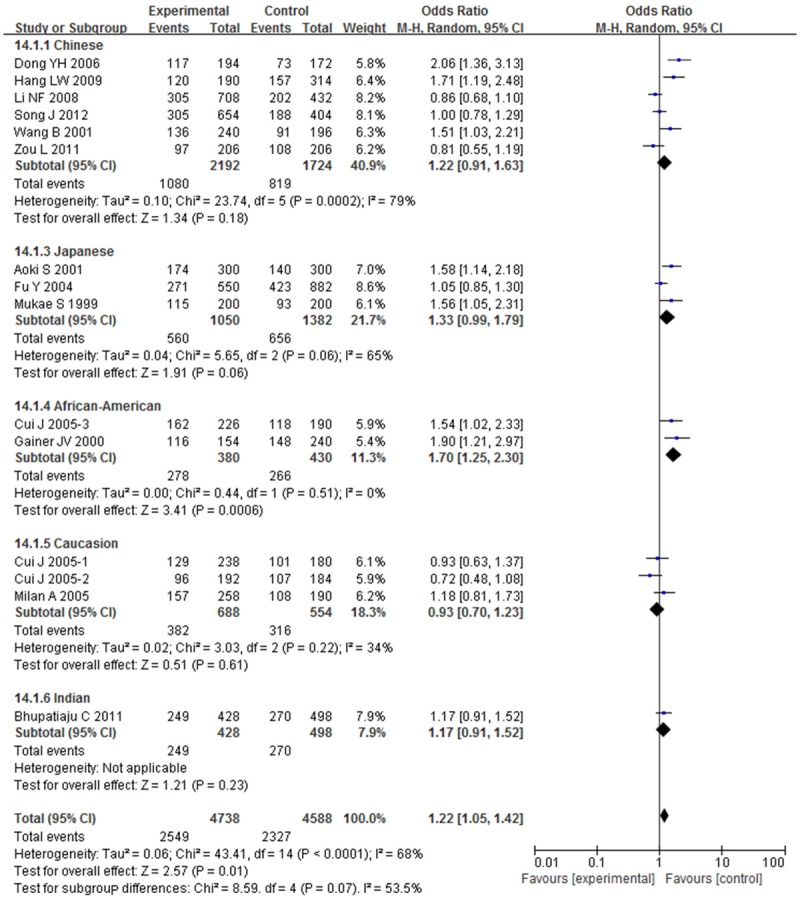
Overall effect estimates of the BDKRB2-58T/C gene polymorphism for hypertension risk under C-allele comparison stratified by races.
Sensitivity analyses and publication bias
To estimate the stability of the results in the meta-analysis, sensitivity analysis was performed through sequentially omitting each study. Apart from recessive model, none of the results were materially changed, which indicated the stability of the findings. Sensitivity analyses indicated that Li et al. [22] was the main source of heterogeneity under recessive model, then the heterogeneity was significantly decreased by omitting the study. The corresponding OR was conspicuously altered with Li et al. [22] (recessive model: OR = 1.25; 95% CI = 1.00-1.57) or without the study (recessive model: OR = 1.32; 95% CI = 1.07-1.64). Thus, in the present meta-analyses, Li et al. [22] was excluded under recessive model. It showed that the present meta-analyses had high sensitivity and poor stability. The Egger’s test was performed to assess the publication bias of the included studies (Figure 8). No publication bias was found in the present meta-analysis (Egger’s: t = 1.93; P = 0.076, Begg’s: z = 1.58; P = 0.113).
Figure 8.
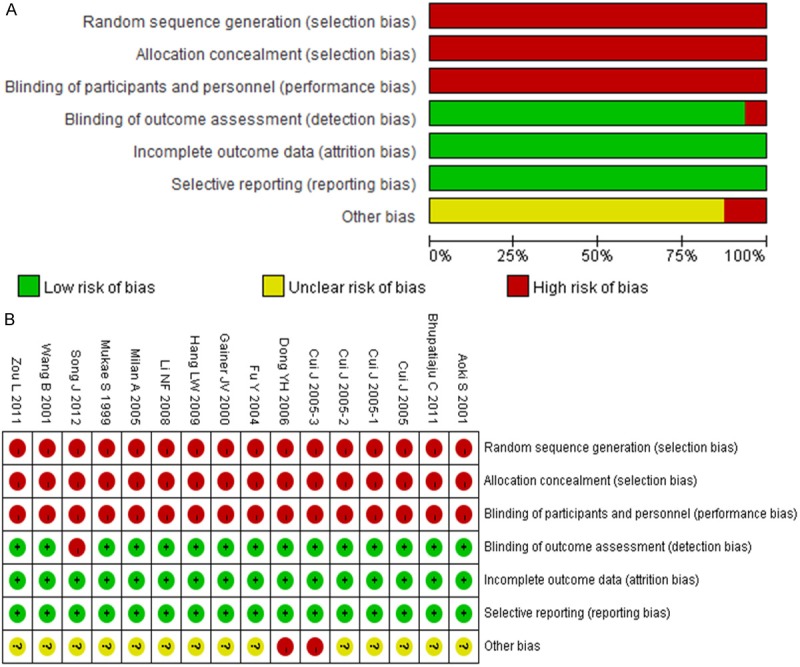
Risk of bias graph (A) and risk of bias summary (B).
Discussion
The kallikrein-kinin system (KKS) is involved in the development of human hypertension, and the BDKRB2 mediates the majority of bradykinin effects. The BDKRB2 gene is known to be highly polymorphic and has been proposed as one of the candidate genes in hypertension. A study of its genomic structure has reported that BDKRB2 gene is characterized by three well-defined polymorphisms located in each of the three exons and one polymorphism located in the promoter region [27]. Braun et al. reported that the polymorphism of BDKRB2 may influence the transcription rate of the gene. They found that the luciferase promoter assay of -58T was higher than that of -58C in a healthy German population. Mukae et al. [14] reported an increased frequency of the -58C allele in hypertensive Japanese subjects compared with normotensives. Wang B et al. [17] reported -58CC genotype distribution and -58C allele frequency were higher in hypertensive Chinese subjects than normotensives. Aoki et al. [16] found significant differences of -58CC genotype and -58C allele frequencies in BDKRB2 gene polymorphism between subjects with essential hypertension (EH) and controls in Japanese. The low transcriptional activity of BDKRB2 promoter polymorphism might be involved in the development of hypertension [14].
Although many articles have analyzed the research results about the BDKRB2-58T/C gene polymorphism and risk of hypertension, definite conclusions cannot be drawn. Therefore, we performed a meta-analysis of published studies to estimate relationships between BDKRB2-58T/C gene polymorphism and risk of hypertension, attempted to establish a comprehensive picture of this gene-disease association. Interestingly, the BDKRB2-58T/C gene polymorphism is significantly associated with the risk of hypertension. The -58C allele (OR: 1.22, 95% CI: 1.05-1.42) and -58CC genotype (OR: 1.32, 95% CI: 1.07-1.64) increased the risk of hypertension. On the contrary, -58TT genotype (OR: 0.74, 95% CI: 0.58-0.94) decreased the risk of hypertension.
To our knowledge, there are two published meta-analyses regarding BDKRB2-58T/C gene polymorphism and hypertension [8,9]. Niu et al. [8] included only four studies with 823 hypertensive subjects and 916 healthy volunteers. In contrast to Li et al. [9], we included two more new large-sample case-control studies, thus we enrolled 13 studies with 2369 hypertensive subjects and 2294 healthy volunteers. The key findings of the present meta-analysis for the first time demonstrated that -58CC genotype increased the risk of hypertension, and inversely -58TT genotype decreased the risk of hypertension. Additionally, sensitivity analyses and publication bias had not been performed in these two articles. In the present study, we followed the inclusion and firstly the exclusion criteria to decline the possible selection bias. Furthermore, the included studies in the present article were conducted around the world (China, Japan, India, United States, Canada, and Italy), which are certainly representatives of ethnicities. And thirdly, sensitivity analyses and publication bias were performed. Therefore, the results in the present meta-analysis are more convincing.
The subgroup analysis was conducted to explore the source of heterogeneity. One possibility might be the issue of genetic heterogeneity across different continents groups. There are significant ethnic differences between BDKRB2-58T/C gene polymorphism and risk of hypertension. In the subgroup analysis stratified by continents, the -58C allele and firstly, the -58CC genotype of the BDKRB2 gene were observed to confer an increased risk of hypertension in Asian, but not in American and European. For the first time, the -58TT genotype of the BDKRB2 gene decreased the risk of hypertension in Asian, but not in American and European. In the subgroup analysis stratified by races, the -58C allele and firstly, -58CC genotype were observed to confer an increased risk of hypertension in African-American. Inversely, -58TT genotype decreased the risk of hypertension in African American, but not in other races.
Sensitivity analyses showed that Li et al. [22] was the main source of heterogeneity. Thus, the study was excluded under recessive model, considering of the limitation of evidence, we could not further explore the origin of heterogeneity. More case-control studies are required to provide a more representative statistical analysis.
Potential limitations of the present meta-analysis should be acknowledged. Firstly, thirteen studies included in our meta-analysis were limited to improve the accuracy of the results, especially in the subgroup analysis. Therefore, high-quality case-control studies performed in various ethnic groups are required to provide a more robust analysis. Secondly, there was significant heterogeneity in the present study. Much of this is likely due to the differences in ethnicity, study design, study population, and sample source. Thirdly, as with all meta-analyses, we can’t exclude the possibility of publication bias, because studies without statistically significant results would not be published.
Conclusion
The meta-analysis provides the evidence that BDKRB2-58T/C gene polymorphism is significantly associated with the risk of hypertension. The -58C allele and -58CC genotype increase the risk of hypertension. Inversely, -58TT genotype decreases the risk of hypertension.
Disclosure of conflict of interest
None.
References
- 1.Kurtz TW, Spence MA. Genetics of essential hypertension. Am J Med. 1993;94:77–84. doi: 10.1016/0002-9343(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 2.Linz W, Wiemer G, Gohlke P, Unger T, Scholkens BA. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- 3.Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- 4.Seguin L, Widdowson PS, Giesen-Crouse E. Existence of three subtypes of bradykinin B2 receptors in guinea pig. J Neurochem. 1992;59:2125–33. doi: 10.1111/j.1471-4159.1992.tb10103.x. [DOI] [PubMed] [Google Scholar]
- 5.Mattson DL, Cowley AJ. Kinin actions on renal papillary blood flow and sodium excretion. Hypertension. 1993;21:961–5. doi: 10.1161/01.hyp.21.6.961. [DOI] [PubMed] [Google Scholar]
- 6.Katori M, Majima M. Pivotal role of renal kallikrein-kinin system in the development of hypertension and approaches to new drugs based on this relationship. Jpn J Pharmacol. 1996;70:95–128. doi: 10.1254/jjp.70.95. [DOI] [PubMed] [Google Scholar]
- 7.Wang DZ, Chao L, Chao J. Hypotension in transgenic mice overexpressing human bradykinin B2 receptor. Hypertension. 1997;29:488–93. doi: 10.1161/01.hyp.29.1.488. [DOI] [PubMed] [Google Scholar]
- 8.Niu W, Qi Y, Gao P, Zhu D. A meta-analysis of the bradykinin B2 receptor gene --58C/T polymorphism with hypertension. Clin Chim Acta. 2010;411:324–8. doi: 10.1016/j.cca.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Li YY, Zhang H, Xu J, Qian Y, Lu XZ, Yang B, Chen M, Yang ZJ, Cao KJ. Bradykinin beta2 receptor -58T/C gene polymorphism and essential hypertension: a meta-analysis. PLoS One. 2012;7:e43068. doi: 10.1371/journal.pone.0043068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukae S, Aoki S, Itoh S, Nishio K, Iwata T, Ueda H, Geshi E, Fuzimaki T, Katagiri T. Promoter polymorphism of the beta2 bradykinin receptor gene is associated with essential hypertension. Jpn Circ J. 1999;63:759–62. doi: 10.1253/jcj.63.759. [DOI] [PubMed] [Google Scholar]
- 15.Gainer JV, Brown NJ, Bachvarova M, Bastien L, Maltais I, Marceau F, Bachvarov DR. Altered frequency of a promoter polymorphism of the kinin B2 receptor gene in hypertensive African-Americans. Am J Hypertens. 2000;13:1268–73. doi: 10.1016/s0895-7061(00)01215-2. [DOI] [PubMed] [Google Scholar]
- 16.Aoki S, Mukae S, Itoh S, Sato R, Nishio K, Iwata T, Katagiri T. The genetic factor in acute myocardial infarction with hypertension. Jpn Circ J. 2001;65:621–6. doi: 10.1253/jcj.65.621. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Dang A, Liu G. Genetic variation in the promoter region of the beta2 bradykinin receptor gene is associated with essential hypertension in a Chinese Han population. Hypertens Res. 2001;24:299–302. doi: 10.1291/hypres.24.299. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Katsuya T, Matsuo A, Yamamoto K, Akasaka H, Takami Y, Iwashima Y, Sugimoto K, Ishikawa K, Ohishi M, Rakugi H, Ogihara T. Relationship of bradykinin B2 receptor gene polymorphism with essential hypertension and left ventricular hypertrophy. Hypertens Res. 2004;27:933–8. doi: 10.1291/hypres.27.933. [DOI] [PubMed] [Google Scholar]
- 19.Cui J, Melista E, Chazaro I, Zhang Y, Zhou X, Manolis AJ, Baldwin CT, Destefano AL, Gavras H. Sequence variation of bradykinin receptors B1 and B2 and association with hypertension. J Hypertens. 2005;23:55–62. doi: 10.1097/00004872-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Milan A, Mulatero P, Williams TA, Carra R, Schiavone D, Martuzzi R, Rabbia F, Veglio F. Bradykinin B2 receptor gene (-58T/C) polymorphism influences baroreflex sensitivity in never-treated hypertensive patients. J Hypertens. 2005;23:63–9. doi: 10.1097/00004872-200501000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Dong HY, Li QR, Wang Q, Luo ZG. Association of genetic polymorphism of b2-bradykinin receptor gene and AGT gene with essential hypertension in Shenzhen population. Acta Academiae Medicinae Militaris Tertiae. 2006:1252–1254. [Google Scholar]
- 22.Li NF, Wang YC, Zhou L, Li T, Wang XL, Zhu YF, et al. Association of genetic variants in the promoter region of the b2 bradykinin receptor gene and essential hypertension in Kazakans of Xinjiang. Journal of Medical Molecular Biology. 2008:110–113. [Google Scholar]
- 23.Zou L, Li FQ, Zhou P, Chen DL. Relationship between plasma kallikrein B1 and bradykinin receptor B2 gene polymorphism, body mass index and geriatric essential hypertension. Chinese Journal of Gerontology. 2011:2609–2612. [Google Scholar]
- 24.Bhupatiraju C, Patkar S, Pandharpurkar D, Joshi S, Tirunilai P. Association and interaction of -58C>T and +/-9 bp polymorphisms of BDKRB2 gene causing susceptibility to essential hypertension. Clin Exp Hypertens. 2012;34:230–5. doi: 10.3109/10641963.2011.631653. [DOI] [PubMed] [Google Scholar]
- 25.Song J, Sun K, Zhang SJ, Lu S, Li CP, Han YL, et al. Relationship of bradykinin B2 receptor gene polymorphism with hypertension in Korean Mudanjiang region. Chinese Journal of Gerontology. 2012:462–464. [Google Scholar]
- 26.Hang LW. Association between genetic polymorphism with essential hypertension. Chongqing Medical University. 2009 [Google Scholar]
- 27.Braun A, Kammerer S, Bohme E, Muller B, Roscher AA. Identification of polymorphic sites of the human bradykinin B2 receptor gene. Biochem Biophys Res Commun. 1995;211:234–40. doi: 10.1006/bbrc.1995.1801. [DOI] [PubMed] [Google Scholar]


