Abstract
Objective: We conducted a case-control study to evaluate the diagnostic values of computed tomography (CT) and diffusion-weighted magnetic resonance imaging (DW-MRI) in differentiating malignancies from benign ovarian tumors and a meta-analysis to further confirm our results on DW-MRI. Methods: Totally 64 patients pathologically confirmed as ovarian cancer were included in this study. CT scan and DWI-MRI were performed and analyzed to get compared with pathological results, thereby assessing their accuracy, sensitivity and specificity. Meta-analysis was conducted by database searching and strict eligibility criteria, using STATA 12.0 (Stata Corp, College Station, TX, USA) software. Results: The accuracy, sensitivity, specificity, positive predictive value and negative predictive value for diagnosis of ovarian cancer in CT were 81.82%, 84.48%, 76.67%, 87.50% and 71.88%, respectively; those in DW-MRI were 89.77%, 93.10%, 83.33%, 91.53% and 86.21%, respectively. The Kappa coefficient of DW-MRI (K = 0.771) compared with pathological results was higher than CT (K = 0.602). The average apparent diffusion coefficient values of DW-MRI in diagnosis of benign and malignant ovarian tumors suggested statistically significant difference (1.325 ± 0.269×10-3 mm2/s vs. 0.878 ± 0.246×10-3 mm2/s, P < 0.001). Meta-analysis results showed that the combined sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio of DW-MRI in discriminating benign versus malignant ovarian tumors were 0.93, 0.88, 7.70, 0.08 and 101.24, respectively. The area under the summary receiver operating characteristic curve was 0.95. Conclusions: Both CT and DW-MRI were of great diagnostic value in differentiating malignancies from benign ovarian tumors, while DW-MRI was superior to CT with higher accuracy, sensitivity and specificity.
Keywords: Ovarian cancer, diffusion-weighted magnetic resonance imaging, computed tomography, apparent diffusion coefficient value, case-control, meta-analysis
Introduction
Ovarian cancer, which ranks third among the most common gynecologic cancers, is the 5th leading cause of death by cancer in women [1]. Although its five-year survival is much greater (90%) than localized diseases, the overall survival still remains less than 50% [2]. It has been well known that the disease stage at the time of first diagnosis is an essential prognostic factor of ovarian cancer [3]. However, due to the fact that early ovarian cancer is usually associated with nonspecific symptoms or clinically asymptomatic which could result in delayed presentation and diagnosis, nearly 2/3 of all ovarian carcinomas have progressed to International Federation of Gynecology and Obstetrics (FIGO) stage III or IV at the time of diagnosis [4,5].
At present, ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI) are usually used in the detection and characterization of ovarian tumors [6,7]. As a diagnostic method for ovarian cancer, CT scan has been proved to be more effective than ultrasonography in evaluating the nature of ovarian masses based on its combined advantages including meticulous technique, ready availability, and efficacy etc [1]. By this technique, masses could be characterized and features regarding benignity and malignancy could be observed [8]. MRI can specifically diagnose some certain pathologic types by providing accurate information on fat, collagen and hemorrhage [9]. Diffusion-weighted magnetic resonance imaging (DW-MRI), an emerging non-invasive MRI technique, is of the capability to evaluate the extent of microscopic diffusion which might exist in biologic tissues [10]. Typically, DW-MRI permits a quantitative evaluation by assessing apparent diffusion coefficient (ADC) values which measures the random motion rate of water molecules and decreases with increased tumor cellularity [11]. Recently, its usefulness in the diagnosis of gynecologic tumors has been reported in several studies [12-15]. DW-MRI has been considered as an effective tool to characterize epithelial ovarian tumors through helping discriminate benign, borderline and invasive tumors [16]. Evidence has shown that DWI-MRI and ADC were beneficial in differentiating malignant from benign ovarian lesions and may be helpful to predict suboptimal cytoreduction in ovarian cancer [17,18]. Nevertheless, there are also studies revealed that the characterization of ovarian masses as benign or malignant could not be achieved only based on DWI-MRI, thus its diagnostic capabilities in ovarian cancer still remains highly controversial [15,19].
In this regard, we conducted a case-control study to evaluate the diagnostic value of CT and DWI-MRI in discriminating benign versus malignant ovarian tumors and a meta-analysis to further confirm our results on DW-MRI.
Materials and methods
Subjects
Totally 64 patients hospitalized from October, 2012 to October, 2014 for ovarian cancer treatment at Yiwu Central Hospital were included in this study. The patients (average age: 46.7 years, range from 26 to 75 years) were examined by CT and DW-MRI and pathologically confirmed as ovarian cancer. This study was performed with the approval of the Ethical Committee of Yiwu Central Hospital. All participants signed written informed consents, and all the experimental procedures were performed in conformity with the Declaration of Helsinki [20].
CT scan
Two multi-detector scanners (16-slice and 64-slice, both from Toshiba, Otawara, Japan) were applied for CT examination. Enhanced CT scan of abdomen and pelvis was performed from the dome of the diaphragm to pubic symphysis, before which all patients were injected with non-ionic contrast medium (diatrizoate, 300 mg/ml). The images were acquired with 10 mm of slice thickness and interslice gap and the scan range was increased if huge ovarian masses were observed. No patients were excluded for contraindication to iodinated contrast media or radiation.
DW-MRI protocol
A GE Signa Excite Twin Speed 3.0T MR system (GE Medical Systems, Waukesha, WI, USA) was applied to perform DW-MRI using 8-channel torso phased array coil with the patients in the supine position. Coronal and axial T1-weighted spin-echo imaging (SE T1WI; Repetition Time (TR), 460 ms/Excitation Time (TE), 10 ms) and axial and sagittal T2-weighted fast SE imaging (SE T2WI; TR, 4060 ms/TE, 100 ms) were obtained. Further, dynamic contrast-enhanced axial and sagittal T1-weighted imaging (DCE T1WI) was performed with the following parameters: TR, 3.8 ms; TE, 1.7 ms; inversion time (TI), 15.0 ms; flip angle, 15°; receiver bandwidth, 62.5 kHz; and number of excitations (NEX), 0.75. DW-MRI parameters were as follows: slice thickness, 5 mm; gap, 1.5 mm; field of view (FOV), 32-36 cm; matrix, 256×192; and excitation, 5. Also, b values of 0, 500 and 1000 s/mm2 were applied in three orthogonal directions (Z, Y, and X), based on which the apparent diffusion coefficient (ADC) value was calculated.
Analysis of CT and DW-MRI images
Two experienced radiologists observed the images from CT and DW-MRI examinations without knowing the definitive pathological results. The imaging features of the ovarian tumors were analyzed, including tumor size, cystic lesions, cyst walls and septum, solid nodules, symptoms such as mesenteric or omental implant, ascites and lymphadenectasis etc., as well as the conditions of cystic liquid and solid masses. The results from detection of benign and malignant tumors and tumor stage were compared with the pathological results by surgery or laparoscopy, based on which the sensitivity, specificity and accuracy of CT scan, DW-MRI and pathological analysis in detecting ovarian cancer were respectively measured.
Statistical analysis
The software SPSS 19.0 (SPSS Inc., Chicago, IL, USA) was applied for statistical analysis. Diagnostic test was used for the evaluation of sensitivity, specificity and accuracy of CT scan and DW-MRI in detecting ovarian cancer. The difference of CT scan and DW-MRI with pathological analysis was evaluated by χ2 test and Kappa test (K ≥ 0.75 was considered as fair agreement; 0.4 ≤ K < 0.7 as moderate agreement; K < 0.4 as poor concordance). The difference of ADC values between benign and malignant groups was measured through t test. In addition, receiver operating characteristic (ROC) curve was applied to determine the optimal threshold in distinguishing malignancies from benign ovarian tumors. P < 0.05 was considered as statistically significant.
Meta-analysis of DW-MRI in distinguishing benign and malignant ovarian tumors
Chinese and English databases including PubMed, WanFang, Chinese National Knowledge Infrastructure (CNKI), and VIP were searched with combination of key words and their free words. Search terms included: Diffusion Magnetic Resonance Imaging, Diffusion MRI, Diffusion Weighted MRI, diffusion weighted imaging, diffusion, Ovarian Neoplasms, Ovary Neoplasms, Ovary Cancer, Ovarian Cancer, Cancer of Ovary, ovarian tumor, malignant tumor of ovary, ovarian carcinoma, ovarian epithelial carcinoma and OCE.
Studies were considered eligible if they: (1) were diagnostic studies investigating the role of DW-MRI in the differential diagnosis of benign and malignant ovarian tumors with pathological results as the gold standard of diagnosis; (2) provided complete fourfold tables, taking lesions of ovarian tumors as the unit. A unified data collection form was used by two investigators to independently extract data from included studies, and discussion was conducted if there was disagreement occurred. The meta-analyses were performed with the use of STATA 12.0 (Stata Corp, College Station, TX, USA) software. Fixed effects model or random effects model was applied to evaluate the diagnostic odds ratio (DOR), sensitivity, specificity as well as positive and negative likelihood ratio. Additionally, summary receiver operating characteristic (SROC) curve was used to measure the value of DW-MRI in the differential diagnosis of benign and malignant ovarian tumors by calculating the area under the curve (AUC). The existence of heterogeneity was evaluated using Bivariate Boxplot. The degree of heterogeneity was assessed via I 2 (range 0%~100%) with 100% indicating the maximum heterogeneity. If significant heterogeneity exists (P < 0.05 or I 2 > 50%), a random effects model was used, otherwise a fixed effects model was used [21]. If the angle between the straight line in Deeks’ funnel plot and the vertical axis (DOR) was closer to 90°, the probability of publication bias was smaller [22].
Results
Pathological results by surgery or laparoscopy
All ovarian tumors were pathologically confirmed by surgery or laparoscopy. Eighty-eight lesions (benign, n = 30; malignant, n = 58) were found in the 64 ovarian tumors, among which 40 were unilateral and 24 were bilateral. The pathological results after surgery or laparoscopy were shown in Table 1.
Table 1.
Pathological results of benign and malignant ovarian tumors after surgery or laparoscopy
| Masses | Pathological types | Number of cases |
|---|---|---|
| Benign | Thecoma | 2 |
| Benign | Serous cystadenoma | 5 |
| Benign | Mucinous cystadenoma | 5 |
| Benign | Ovarian endometriosis | 4 |
| Benign | Hemorrhagic corpus luteum cyst | 5 |
| Benign | Single cyst | 3 |
| Benign | Mature teratoma | 2 |
| Benign | Fibroma | 4 |
| Malignant | Serous papillary cystadenocarcinoma | 13 |
| Malignant | Mucinous cystadenocarcinoma | 11 |
| Malignant | Metastatic carcinoma | 10 |
| Malignant | Epithelioid sarcoma | 8 |
| Malignant | Immature teratoma | 5 |
| Malignant | Endometrioid adenocarcinoma | 6 |
| Malignant | Undifferentiated carcinoma | 5 |
CT and DW-MRI results
The CT and DW-MRI manifestations of benign ovarian tumors were: (1) well demarcated cystic mass from the surrounding tissue, round or oval in shape with thin and smooth cyst walls; (2) no enhancement or only mild enhancement in cyst walls; (3) presence of fat density/signal or punctate calcification, e.g. mature teratoma (Figure 1A); (4) presence of intracystic flaky fat or teeth-like bones; (5) no ascites. Benign ovarian tumors were identified if the results showed cystic mass and thin (< 3 mm) and smooth cyst walls.
Figure 1.
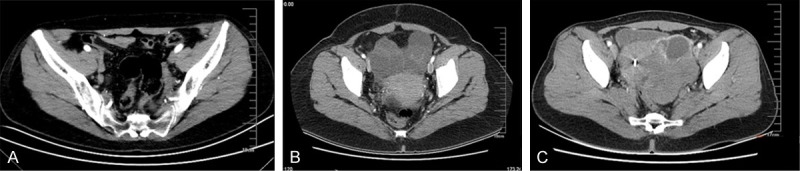
A. 52-year-old female suffered from mature teratoma (features: pelvic mass with soft tissue, fat and calcification); B. 61-year-old female with ovarian cancer (features: heterogeneous enhancement in solid and cystic mass); C. 45-year-old female with serous papillary cystadenocarcinoma (features: solid and cystic mass with massive ascites).
The features of malignant ovarian tumors by CT and DW-MRI were: (1) a large size (> 4 cm); (2) the presence of solid and cystic mass; (3) multiple thickened (> 3 mm) septations; (4) tumor necrosis; (5) nodularity; (6) heterogeneous enhancement in solid and cystic mass; (7) invasion of the surrounding organs or pelvic wall; (8) mesenteric or omental implant, ascites and lymphadenectasis; (9) lymphadenopathy. Figure 1B and 1C demonstrated the serous papillary cystadenocarcinoma for example.
Diagnostic values of CT and DW-MRI
The results of CT and DW-MRI examination of 88 ovarian lesions were analyzed taking pathological results as the gold standard of diagnosis and shown in Table 2. The sensitivity, specificity and accuracy of DW-MRI in diagnosing ovarian cancer were superior to CT scan. Kappa test revealed that both CT and DW-MRI were in agreement with the pathological results, with DW-MRI showing a higher coefficient than CT (DW-MRI, K = 0.771; CT, K = 0.602).
Table 2.
The sensitivity, specificity and accuracy of CT and DW-MRI in diagnosing ovarian cancer
| Techniques | Pathological types | Accuracy | Sensitivity | Specificity | Kappa test | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Benign | Malignant | K | P | |||||
| DW-MRI | Benign | 25 | 4 | 89.77% | 93.10% | 83.33% | 0.771 | < 0.001 |
| Malignant | 5 | 54 | ||||||
| CT | Benign | 23 | 9 | 81.82% | 84.48% | 76.67% | 0.602 | < 0.001 |
| Malignant | 7 | 49 | ||||||
Notes: Accuracy = (true positive + true negative)/(true positive + false negative + false positive + true negative); Sensitivity = true positive/(true positive + false negative); Specificity = true negative/(true negative + false positive); positive predictive value = true positive/(true positive + false positive); negative predictive value = true negative/(true negative + false negative); K, Kappa coefficient; P < 0.05, significant difference of Kappa coefficient between CT and DW-MRI.
The accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for diagnosis of ovarian cancer in CT were 81.82%, 84.48%, 76.67%, 87.50% and 71.88%, respectively; those in DW-MRI were 89.77%, 93.10%, 83.33%, 91.53% and 86.21%, respectively. The average ADC values of DW-MRI in diagnosis of benign and malignant ovarian tumors suggested statistically significant difference (1.325 ± 0.269×10-3 mm2/s vs. 0.878 ± 0.246×10-3 mm2/s, P < 0.001). The ROC curve (Figure 2) indicated that an ADC value of ≥ 1.063×10-3 mm2/s could be considered as the optimal threshold in distinguishing malignancies from benign ovarian tumors, where the sensitivity and specificity were 93.3% and 79.7%, respectively. The AUC value under ROC curve was 0.903.
Figure 2.
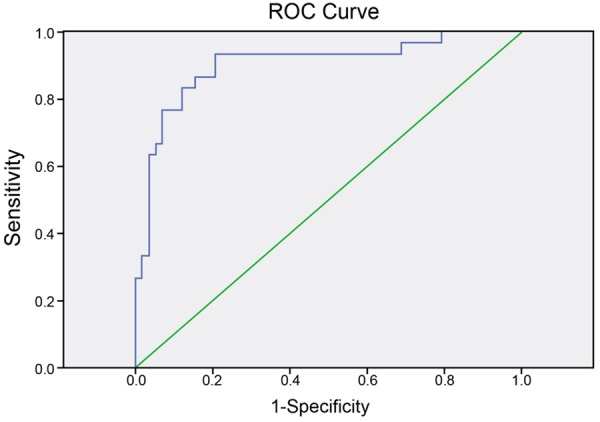
Receiver operating characteristic (ROC) curve for determination of the optimal threshold in distinguishing malignancies from benign ovarian tumors.
Results of meta-analysis
Totally 10 studies were enrolled in the meta-analysis [5,10,11,18,23-28]. According to Bivariate Boxplot (Figure 3), there was heterogeneity among the included studies with majority of observations in middle area. The I 2 values of sensitivity, specificity, positive and negative likelihood ratio and DOR were 61.00%, 30.07%, 0.00%, 59.49%, 30.2%, respectively. Since the I 2 values of sensitivity and negative likelihood ratio were more than 50%, which indicated the existence of heterogeneity, a random effects model was applied. In contrast, I 2 values of specificity, negative likelihood ratio and DOR were < 50%; therefore a fixed effects model was used.
Figure 3.
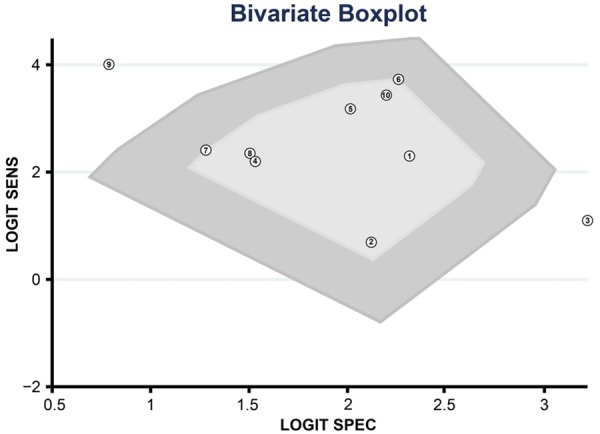
Bivariate Boxplot was used to evaluate the existence of heterogeneity among included studies in the meta-analysis.
The results of combined analyses on DW-MRI in discriminating benign versus malignant ovarian tumors were as follows: the combined sensitivity was 0.93 (95% CI: 0.89-0.96; Figure 4A), combined specificity 0.88 (95% CI: 0.84-0.91; Figure 4B), combined positive likelihood ratio 7.70 (95% CI: 5.71-10.38; Figure 5A), combined negative likelihood ratio 0.08 (95% CI: 0.05-0.12; Figure 4B), and combined DOR 101.24 (95% CI: 55.78-183.76; Figure 6). The AUC under SROC curve was 0.95 as shown in Figure 7. Deeks’ funnel plot showed that the vertical axis (i.e. DOR) was close to 90°, which suggested that the probability of publication bias was small (Figure 8).
Figure 4.
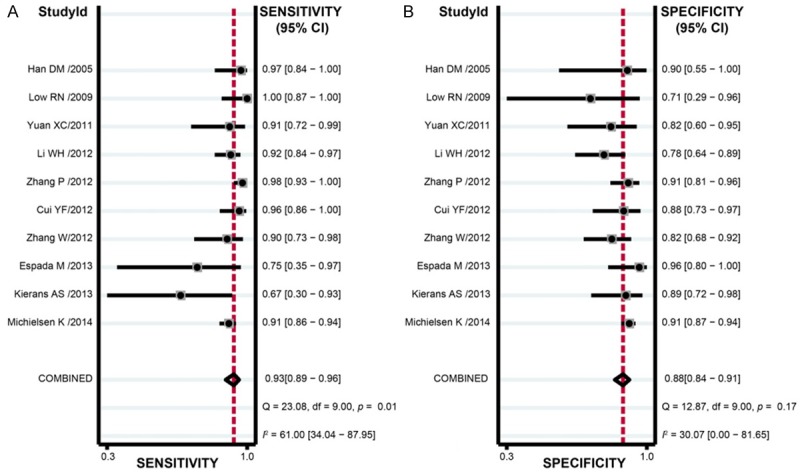
Sensitivity and specificity of diffusion-weighted magnetic resonance imaging (DW-MRI) in discriminating benign versus malignant ovarian tumors in the meta-analysis.
Figure 5.
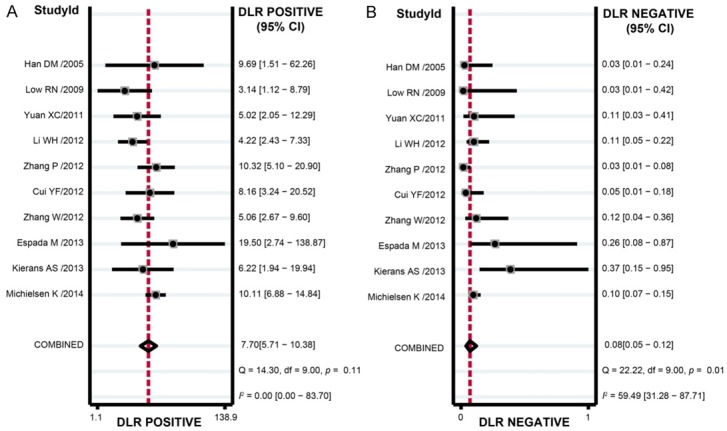
Positive and negative likelihood ratio of diffusion-weighted magnetic resonance imaging (DW-MRI) in discriminating benign versus malignant ovarian tumors in the meta-analysis.
Figure 6.
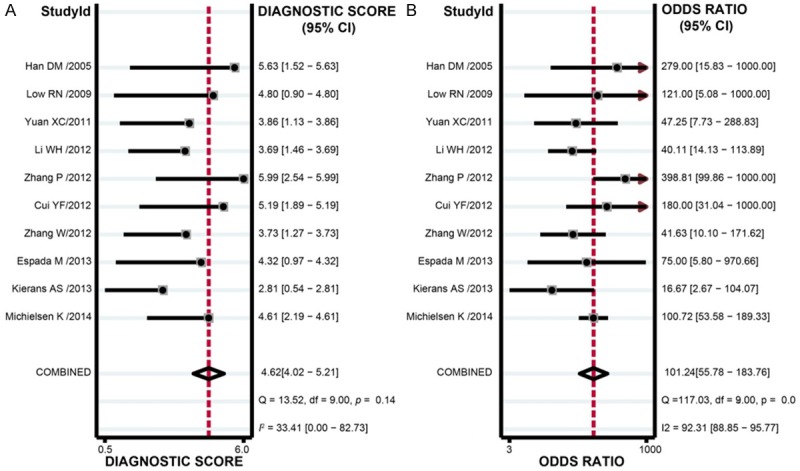
Diagnostic odds ratio of diffusion-weighted magnetic resonance imaging (DW-MRI) in discriminating benign versus malignant ovarian tumors in the meta-analysis.
Figure 7.
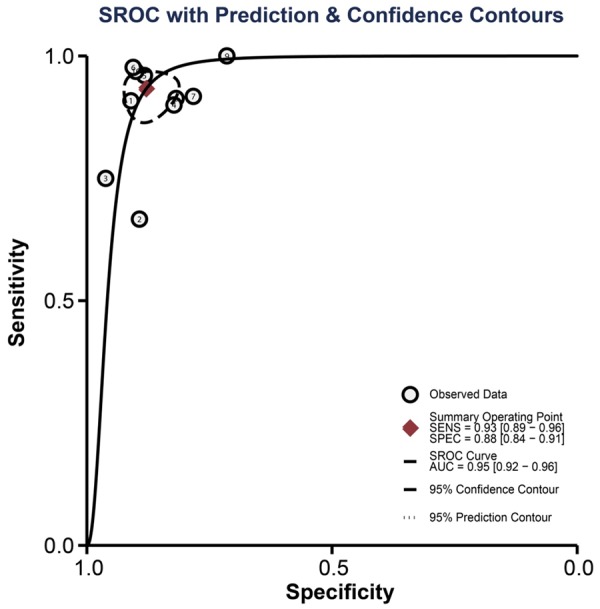
The AUC under SROC curve of diffusion-weighted magnetic resonance imaging (DW-MRI) in discriminating benign versus malignant ovarian tumors in the meta-analysis.
Figure 8.
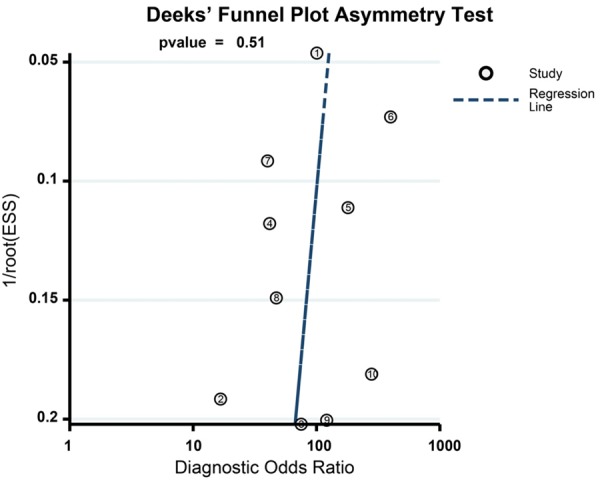
Deeks’ funnel plot of diffusion-weighted magnetic resonance imaging (DW-MRI) in discriminating benign versus malignant ovarian tumors in the meta-analysis.
Discussion
In this study, a case-control study was performed to evaluate the diagnostic value of CT and DWI-MRI in distinguishing malignancies from benign ovarian tumors, after which a meta-analysis was conducted to further confirm our results on DW-MRI. Results of our case-control study demonstrated that both CT and DW-MRI were of great diagnostic value in differentiating malignancies versus benign tumors, while DW-MRI was superior to CT with higher accuracy, sensitivity and specificity.
Ultrasonography is the most commonly used imaging modality in evaluating pelvic pathologies and adnexal masses; however, due to its variable specificity rate ranging 50-100%, CT and MRI are playing more and more important roles [29]. According to our results, the sensitivity and specificity of CT in distinguishing benign and malignant ovarian tumors were 84.48% and 76.67%, which were comparable to previous studies [30-32]. This might be caused by the difference of readers’ experience as well as the histological characteristics, e.g. tumor type. CT has advantage of fast acquisition, and can evaluate both abdomen and adnexa with its thin sections and high resolution to provide details of internal architecture of masses [1]. Therefore, as our results revealed, CT a reliable imaging technique in differential diagnosis of ovarian cancer.
We also found that DW-MRI was helpful in distinguishing malignancies from benign ovarian tumors, even superior to CT. MRI have been reported to distinguish benign and malignant ovarian tumors with an accuracy of 88-93% [6,33,34]. DW-MRI measures Brownian motions of water molecules in an anatomical district to reflect the biophysical tissue properties, including cellular density and organization, microcirculation as well as diffusivity of the water and so on [19]. It has been suggested that DW-MRI with ADC values are helpful in differentiating benign and malignant tissues in many regions, and malignant tissues, usually with high signal intensities, have low ADC values which reflect the restriction of water molecules mainly because of their higher cellularity, increased extracellular space tortuosity and tissue disorganization [35,36]. Therefore, DW-MRI may improve detection of malignancies by increase of their conspicuity, helping identify peritoneal metastases and recurrent disease [37,38]. Our results were further confirmed by the meta-analysis, and were in consistent with previous studies [17,18].
In this study, the accuracy, sensitivity and specificity of DW-MRI in distinguishing malignancies from benign ovarian tumors were 89.77%, 93.10% and 83.33%, respectively, higher than those of CT. This was in consistent with a study conducted by Manganaro et al. reporting that MRI was better than CT in evaluating spatial relations of pelvic masses and differentiating solid from cystic fluid components [39]. According to Tsili et al., MRI performed slightly better than CT, although no statistical significance was found [31]. DW-MRI requires no administration of exogenous contrast medium, and the restriction of water molecule movements can facilitate the incorporation of its sequences in imaging protocols, even in patients those with reduced renal function [36,40]. As for CT scan, it needs contrast media, and will expose patients to ionizing radiation, the ovaries in particular [41].
In conclusion, this study provided evidence that both CT and DW-MRI were of great diagnostic value in differentiating malignancies versus benign ovarian tumors, while DW-MRI was superior to CT with higher accuracy, sensitivity and specificity.
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments.
Disclosure of conflict of interest
None.
References
- 1.Khattak YJ, Hafeez S, Alam T, Beg M, Awais M, Masroor I. Ovarian masses: Is multi-detector computed tomography a reliable imaging modality? Asian Pac J Cancer Prev. 2013;14:2627–2630. doi: 10.7314/apjcp.2013.14.4.2627. [DOI] [PubMed] [Google Scholar]
- 2.Pickhardt PJ, Hanson ME. Incidental adnexal masses detected at low-dose unenhanced ct in asymptomatic women age 50 and older: Implications for clinical management and ovarian cancer screening. Radiology. 2010;257:144–150. doi: 10.1148/radiol.10100511. [DOI] [PubMed] [Google Scholar]
- 3.Zytoon AA, Murakami K, Eid H, El-Gammal M. High impact of fdg-pet/ct in diagnostic strategies for ovarian cancer. Acta Radiol. 2013;54:340–348. doi: 10.1258/ar.2012.120632. [DOI] [PubMed] [Google Scholar]
- 4.Mohaghegh P, Rockall AG. Imaging strategy for early ovarian cancer: Characterization of adnexal masses with conventional and advanced imaging techniques. Radiographics. 2012;32:1751–1773. doi: 10.1148/rg.326125520. [DOI] [PubMed] [Google Scholar]
- 5.Michielsen K, Vergote I, Op de Beeck K, Amant F, Leunen K, Moerman P, Deroose C, Souverijns G, Dymarkowski S, De Keyzer F, Vandecaveye V. Whole-body mri with diffusionweighted sequence for staging of patients with suspected ovarian cancer: A clinical feasibility study in comparison to ct and fdg-pet/ct. Eur Radiol. 2014;24:889–901. doi: 10.1007/s00330-013-3083-8. [DOI] [PubMed] [Google Scholar]
- 6.Valentini AL, Gui B, Micco M, Mingote MC, De Gaetano AM, Ninivaggi V, Bonomo L. Benign and suspicious ovarian masses-mr imaging criteria for characterization: Pictorial review. J Oncol. 2012;2012:481806. doi: 10.1155/2012/481806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka YO, Okada S, Satoh T, Matsumoto K, Saida T, Oki A, Yoshikawa H, Minami M. Solid non-invasive ovarian masses on mr: Histopathology and a diagnostic approach. Eur J Radiol. 2011;80:e91–97. doi: 10.1016/j.ejrad.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Mubarak F, Alam MS, Akhtar W, Hafeez S, Nizamuddin N. Role of multidetector computed tomography (mdct) in patients with ovarian masses. Int J Womens Health. 2011;3:123–126. doi: 10.2147/IJWH.S15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori M, Kim T, Onishi H, Nakamoto A, Tsuboyama T, Tatsumi M, Tomiyama N. Ovarian masses: Mr imaging with t1-weighted 3-dimensional gradient-echo ideal water-fat separation sequence at 3t. Magn Reson Med Sci. 2012;11:117–127. doi: 10.2463/mrms.11.117. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Chu C, Cui Y, Zhang P, Zhu M. Diffusion-weighted mri: A useful technique to discriminate benign versus malignant ovarian surface epithelial tumors with solid and cystic components. Abdom Imaging. 2012;37:897–903. doi: 10.1007/s00261-011-9814-x. [DOI] [PubMed] [Google Scholar]
- 11.Kierans AS, Bennett GL, Mussi TC, Babb JS, Rusinek H, Melamed J, Rosenkrantz AB. Characterization of malignancy of adnexal lesions using adc entropy: Comparison with mean adc and qualitative dwi assessment. J Magn Reson Imaging. 2013;37:164–171. doi: 10.1002/jmri.23794. [DOI] [PubMed] [Google Scholar]
- 12.Koyama T, Togashi K. Functional mr imaging of the female pelvis. J Magn Reson Imaging. 2007;25:1101–1112. doi: 10.1002/jmri.20913. [DOI] [PubMed] [Google Scholar]
- 13.Fujii S, Kakite S, Nishihara K, Kanasaki Y, Harada T, Kigawa J, Kaminou T, Ogawa T. Diagnostic accuracy of diffusion-weighted imaging in differentiating benign from malignant ovarian lesions. J Magn Reson Imaging. 2008;28:1149–1156. doi: 10.1002/jmri.21575. [DOI] [PubMed] [Google Scholar]
- 14.Thomassin-Naggara I, Darai E, Cuenod CA, Fournier L, Toussaint I, Marsault C, Bazot M. Contribution of diffusion-weighted mr imaging for predicting benignity of complex adnexal masses. Eur Radiol. 2009;19:1544–1552. doi: 10.1007/s00330-009-1299-4. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi M, Matsuzaki K, Nishitani H. Diffusion-weighted magnetic resonance imaging of ovarian tumors: Differentiation of benign and malignant solid components of ovarian masses. J Comput Assist Tomogr. 2010;34:173–176. doi: 10.1097/RCT.0b013e3181c2f0a2. [DOI] [PubMed] [Google Scholar]
- 16.Medeiros LR, Freitas LB, Rosa DD, Silva FR, Silva LS, Birtencourt LT, Edelweiss MI, Rosa MI. Accuracy of magnetic resonance imaging in ovarian tumor: A systematic quantitative review. Am J Obstet Gynecol. 2011;204:67, e1, 10. doi: 10.1016/j.ajog.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Zhang GF, He ZY, Li ZY, Zhu M, Zhang GX. Evaluation of primary adnexal masses by 3t mri: Categorization with conventional mr imaging and diffusion-weighted imaging. J Ovarian Res. 2012;5:33. doi: 10.1186/1757-2215-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espada M, Garcia-Flores JR, Jimenez M, Alvarez-Moreno E, De Haro M, Gonzalez-Cortijo L, Hernandez-Cortes G, Martinez-Vega V, Sainz De La Cuesta R. Diffusion-weighted magnetic resonance imaging evaluation of intraabdominal sites of implants to predict likelihood of suboptimal cytoreductive surgery in patients with ovarian carcinoma. Eur Radiol. 2013;23:2636–2642. doi: 10.1007/s00330-013-2837-7. [DOI] [PubMed] [Google Scholar]
- 19.Cappabianca S, Iaselli F, Reginelli A, D’Andrea A, Urraro F, Grassi R, Rotondo A. Value of diffusion-weighted magnetic resonance imaging in the characterization of complex adnexal masses. Tumori. 2013;99:210–217. doi: 10.1177/030089161309900215. [DOI] [PubMed] [Google Scholar]
- 20.M PN. World medical association publishes the revised declaration of helsinki. Natl Med J India. 2014;27:56. [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Zou S, Shen DH, Tong YX. The value of mr diffusion-weighted imaging combined with serum ca125 in the qualitative diagnosis of ovarian occupying lesion. Journal of Medical Imaging. 2012;22:1348–1353. [Google Scholar]
- 24.Zhang P, Cui Y, Li W, Ren G, Chu C, Wu X. Diagnostic accuracy of diffusion-weighted imaging with conventional mr imaging for differentiating complex solid and cystic ovarian tumors at 1.5t. World J Surg Oncol. 2012;10:237. doi: 10.1186/1477-7819-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui YF, Li WH, Zhu MJ, Wang DB, Zhang P, Zhang ZY, Chu CT. Clinical application and research of diffusion weighted mr imaging in complex ovarian tumors. Journal of China Clinic Medical Imaging. 2012;23:856–859. 869. [Google Scholar]
- 26.Yuan XC, Wang XF, Yao GH, Zheng LF, Hu YS, Zhang GX. The value of 3.0t mri for the diagnosis of the ovarian tumor. J Prac Radiol. 2011;27:1695–1698. 1712. [Google Scholar]
- 27.Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted mri of peritoneal tumors: Comparison with conventional mri and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol. 2009;193:461–470. doi: 10.2214/AJR.08.1753. [DOI] [PubMed] [Google Scholar]
- 28.Han DM, Yang XP, Wang HP, Li YX. Comparative study of ct and mri in the diagnosis of ovarian neoplasms. Journal of China Clinic Medical Imaging. 2005;16:441–443. [Google Scholar]
- 29.Santoso JT, Robinson A, Suganda S, Praservit S, Wan JY, Ueland F. Computed tomography adnexal mass score to estimate risk for ovarian cancer. Arch Gynecol Obstet. 2014;289:595–600. doi: 10.1007/s00404-013-3013-7. [DOI] [PubMed] [Google Scholar]
- 30.Gatreh-Samani F, Tarzamni MK, Olad-Sahebmadarek E, Dastranj A, Afrough A. Accuracy of 64-multidetector computed tomography in diagnosis of adnexal tumors. J Ovarian Res. 2011;4:15. doi: 10.1186/1757-2215-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsili AC, Tsampoulas C, Argyropoulou M, Navrozoglou I, Alamanos Y, Paraskevaidis E, Efremidis SC. Comparative evaluation of multidetector ct and mr imaging in the differentiation of adnexal masses. Eur Radiol. 2008;18:1049–1057. doi: 10.1007/s00330-007-0842-4. [DOI] [PubMed] [Google Scholar]
- 32.Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at us: Incremental value of second imaging test for characterization--meta-analysis and bayesian analysis. Radiology. 2005;236:85–94. doi: 10.1148/radiol.2361041618. [DOI] [PubMed] [Google Scholar]
- 33.Sohaib SA, Mills TD, Sahdev A, Webb JA, Vantrappen PO, Jacobs IJ, Reznek RH. The role of magnetic resonance imaging and ultrasound in patients with adnexal masses. Clin Radiol. 2005;60:340–348. doi: 10.1016/j.crad.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Bazot M, Darai E, Nassar-Slaba J, Lafont C, Thomassin-Naggara I. Value of magnetic resonance imaging for the diagnosis of ovarian tumors: A review. J Comput Assist Tomogr. 2008;32:712–723. doi: 10.1097/RCT.0b013e31815881ef. [DOI] [PubMed] [Google Scholar]
- 35.Morita S, Kojima S, Hirata M, Suzuki K, Ueno E. Perfusion fraction of diffusion-weighted mri for predicting the presence of blood supply in ovarian masses. J Magn Reson Imaging. 2011;34:1131–1136. doi: 10.1002/jmri.22695. [DOI] [PubMed] [Google Scholar]
- 36.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, Hammoud DA, Rustin GJ, Taouli B, Choyke PL. Diffusionweighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J, Sugihara S, Kigawa J, Terakawa N, Ogawa T. Detection of peritoneal dissemination in gynecological malignancy: Evaluation by diffusion-weighted mr imaging. Eur Radiol. 2008;18:18–23. doi: 10.1007/s00330-007-0732-9. [DOI] [PubMed] [Google Scholar]
- 38.Sala E, Priest AN, Kataoka M, Graves MJ, McLean MA, Joubert I, Griffiths JR, Crawford RA, Jimenez-Linan M, Earl HM, Brenton JD, Lomas DJ. Apparent diffusion coefficient and vascular signal fraction measurements with magnetic resonance imaging: Feasibility in metastatic ovarian cancer at 3 tesla: Technical development. Eur Radiol. 2010;20:491–496. doi: 10.1007/s00330-009-1543-y. [DOI] [PubMed] [Google Scholar]
- 39.Manganaro L, Bernardo S, Sergi ME, Sollazzo P, Vinci V, De Grazia A, Clerico A, Mollace MG, Saldari M. Burkitt’s lymphoma presented as advanced ovarian cancer without evidence of lymphadenopathy: Ct and mri findings. Case Rep Radiol. 2013;2013:940160. doi: 10.1155/2013/940160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vargas HA, Barrett T, Sala E. Mri of ovarian masses. J Magn Reson Imaging. 2013;37:265–281. doi: 10.1002/jmri.23721. [DOI] [PubMed] [Google Scholar]
- 41.Bekiesinska-Figatowska M, Bragoszewska H, Jurkiewicz E, Romaniuk-Doroszewska A, Uliasz M, Iwanowska B, Ceran A, Olszewski A. Magnetic resonance imaging as a diagnostic tool in case of ovarian masses in girls and young women. Med Sci Monit. 2007;13(Suppl 1):116–120. [PubMed] [Google Scholar]


