Abstract
To improve the understanding, diagnostic levels, and therapeutic levels of retroperitoneal hyaline vascular type LCD in the iliac vessel region. Diagnostic and therapeutic processes of 4 patients with retroperitoneal LCD in the iliac vessel region were retrospectively analyzed. The median ages of the research patients was 31.3 years old, Pelvic vascular dual-source computed tomography (CT) indicated an abnormal pelvic irregular cloddy intensity shadow with heterogeneous densities and punctate calcified lesions. The enhanced scanning showed significantly enhanced lesions and multiple tortuous vascular images inside and around the lesions. Patients’ preoperative diagnoses were all “pelvic mass with unknown characteristics”, and retroperitoneal masses were successfully stripped off after the laparoscopic surgery. Intra operative findings indicated 1 mass located at the left obturator nerve, 1 at the left internal iliac artery, and 2 at the right external iliac artery. The postoperative pathological reports suggest a diagnosis of Castleman’s disease. Retroperitoneal LCD in the iliac vessel region is generally asymptomatic. Preoperative imaging data may help with the diagnosis, but a confirmed diagnosis depends on the results of the pathological examination. Iliac artery embolization is performed prior to laparoscopic mass stripping if the masses have abundant blood supply, while lymphadenectomy is also applied to those with enlarged lymph nodes.
Keywords: Castleman’s disease, laparoscopic, diagnosis, treatment
Introduction
First reported and named by Castleman in 1956, Castleman’s disease (CD), also known as angio-follicular or giant lymph node hyperplasia, is the term used for a group of lymphoproliferative disorders that share diverse clinical manifestations and pathological characteristics resulting from unknown mechanisms and etiology [1]. CD can be classified as hyaline vascular (HV-CD), plasma cell (PC-CD), or mixed type (MT-CD) based on its pathological features. HV-CD, accounting for about 48% of all cases of CD, mainly manifests as follicular hyperplasia, germinal center atrophy with lymphopenia, dendritic cell dysplasia in the follicle, and extensive proliferation of hyaline capillaries (penetrating the follicular center) in the interfollicular areas. PC-CD, accounting for about 48% of all CD cases, is characterized by obvious germinal centers, numerous plasma cells, and unobvious angiogenesis in the interfollicular areas. MT-CD, with an estimated percentage of about 4%, shows features of a mixed hyaline vascular and plasma cell type [2].
CD can be both unicentric and multicentric. Multicentric CD usually has a rather poor prognosis and requires systemic treatment. Unicentric CD accounts for 47%-81% of all CD patients, and is also referred to as localized CD (LCD). LCD generally involves focal lesions and has a better prognosis for long-term survival after surgical treatment of the lesion resection when compared to multicentric CD. With HV-CD as the most common pathological type (76%-91%), LCD mainly involves lymph node enlargements at only a single site, and over 90% of LCD patients lack systemic symptoms. The most commonly involved sites are the mediastinal lymph nodes (60%-75%) [3] among other rare lesion sites including the eyes [4], pleura [5], pharynx [6], pancreas [7], liver [8], kidney [9]and the neurological system [10].
Here, with the goal of gaining a better understanding of this rare disease, we present the diagnostic and therapeutic processes of 4 patients with retroperitoneal LCD in the iliac vessel region that had been admitted at our hospitals.
Material and methods
The clinical data of 4 patients with pathologically confirmed retroperitoneal LCD in the iliac vessel region were collected between August 2007 and January 2015. With a median age of 36.3±14.3 years old, the 4 patients, and all female without complaint of discomfort, were preliminarily diagnosed with a B-type ultrasound. After admission, patients received routine blood tests and a comprehensive examination of coagulative functioning, hepatic functioning, and renal functioning in addition to biochemical tests, electrocardiograms, and chest X-rays, but no abnormalities were found. Pelvic magnetic resonance imaging (MRI) indicated an abnormal irregular cloddy intensity shadow, with isointensity on T1- and T2-weighted images and hyperintensity (slight) on diffusion-weighted images (Figures 1, 2). Enhanced scanning showed significantly enhanced lesions with mildly enhanced images of heterogeneous hypointensity inside, and vascular images inside and around the lesions (Figure 3). Pelvic vascular dual-source CT indicated an abnormal pelvic irregular cloddy intensity shadow with heterogeneous densities and punctate calcified lesions (Figure 4). The enhanced scanning showed significantly enhanced lesions and multiple tortuous vascular images inside and around the lesions (Figure 5). CTA shows that the internal iliac artery is a blood supply of the lesion. Multiple thickened vessels can be observed (Figure 6). The edge of the mass located at the left obturator nerve was not obvious and the remaining 3 masses were obvious. Internal and external iliac angiography and regional arterial embolization at the lesioned side were performed for all the patients 2-3 days before the laparoscopic surgery.
Figure 1.
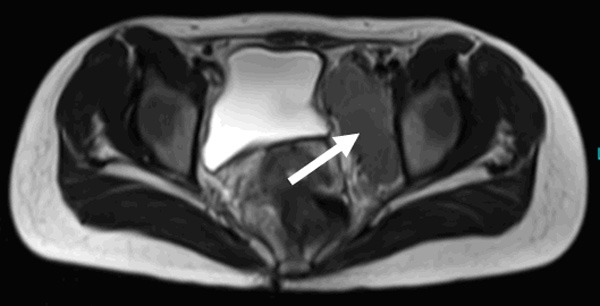
T2WI plain scanning indicates aquasi-oval low isointensity in the left pelvic cavity (white arrow).
Figure 2.
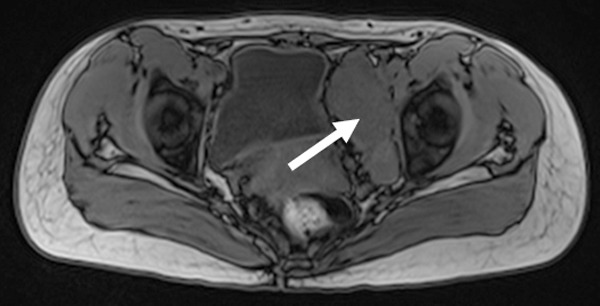
T1WI scanning indicates a quasi-oval low isointensity in the left pelvic cavity (white arrow).
Figure 3.
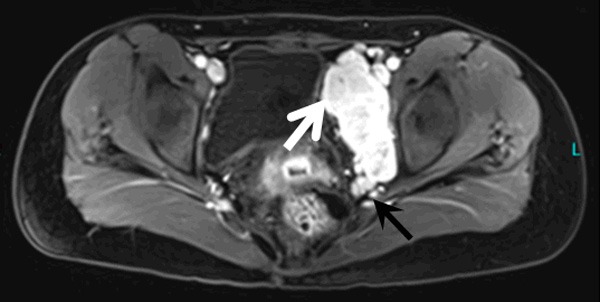
MRI enhanced scanning indicates that the lesion is significantly enhanced in the left pelvic cavity, demonstrating a slight hyperintensity in the diffused-weighted images (white arrow), while a mildly enhanced hypointense area can be found in the upper part of the lesion. Thickened vascular imaging is observed below the lesion (black arrow).
Figure 4.
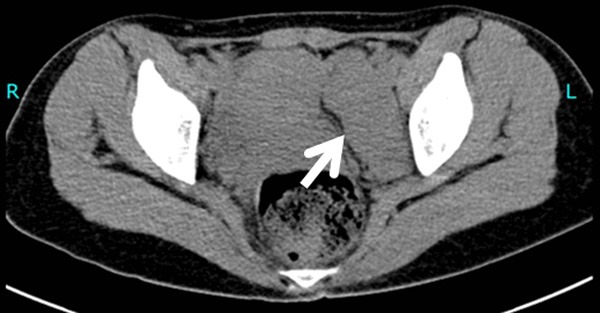
CT plain scanning shows a quasi-oval isointensity in the left pelvic cavity (white arrow).
Figure 5.
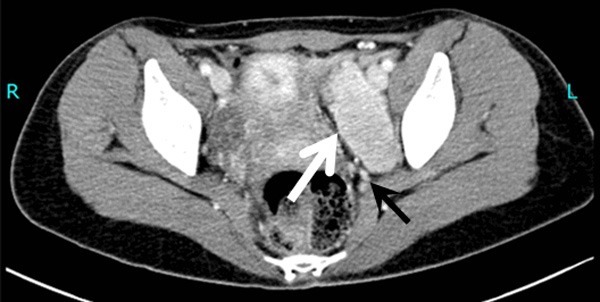
Enhanced CT scanning indicates obvious isointensity in the left pelvic cavity (white arrow) with multiple tortuous vascular images below (black arrow).
Figure 6.
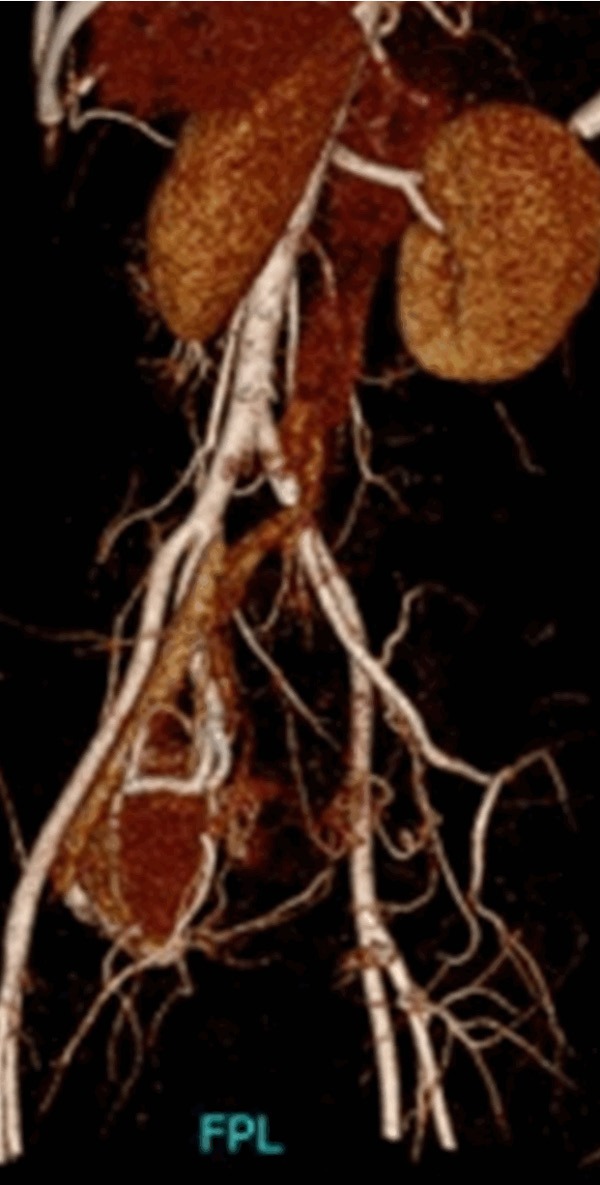
CTA shows that the internal iliac artery is a blood supply of the lesion. Multiple thickened vessels can be observed (white arrow).
Statistical analysis
SPSS 16.0 software (IBM, Armonk, NY, USA) was used for the statistical analysis. Quantitative data is presented as the mean ± SD. The significance of independent sample differences was tested using analysis of t-tests. Values of P<0.05 were considered statistically significant.
Results
Laparoscopic exploration was conducted under general anesthesia. In 1 case, the mass was located between the left iliac artery and vein; it extended vertically and inferiorly, enveloping the left obturator nerve and oppressing the internal iliac vessels to one side (Figure 7). The other 3 masses were located at the left internal iliac artery (1 case) and left external iliac artery (2 cases). The data was in table (Table 1), there was no statistical significance. Laparoscopic retroperitoneal mass striping plus ureter isolation at the lesioned side were applied to all the patients; additionally, a left pelvic lymphadenectomy was performed on the large lymph nodes for the 1 case of left obturator nerve involvement. Results of the frozen section indicated lymph node reactive hyperplasia and hyperplastic lesions of the retroperitoneal lymph nodes. After the operation, symptomatic treatments of antibiotics and infusions were administered. The pathological reports showed diagnoses of HV-CD (masses) and hyperplasia (enlarged lymph nodes) (Figure 8). Patients were discharged after healing. Follow-ups showed no local or distal recurrences.
Figure 7.
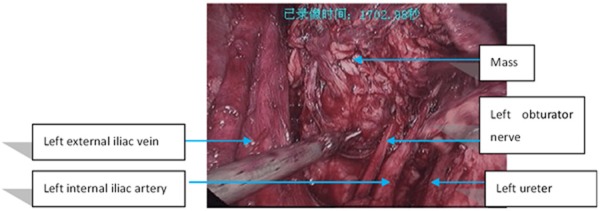
The mass is located at the left obturator nerve and between the left iliac artery and vein.
Table 1.
Clinic data
| Age (year) | Operation time (min) | Blood loss (ml) | BMI | Time to gas passage (h) | Hospital stay (day) | Diameter (cm) | |
|---|---|---|---|---|---|---|---|
| 31.3±5.6 | 55.4±8.9 | 87.8±12.5 | 19.6±3.1 | 14.6±8.2 | 7.8±2.3 | 6.8±2.3 | |
| P value | 0.672 | 0.752 | 0.681 | 0.545 | 0.819 | 0.286 | 0.435 |
Figure 8.
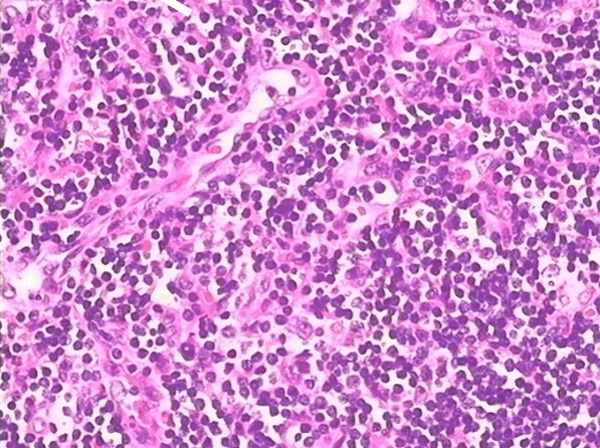
Hyperplasic lymph nodes and vascularity, with mesh-like local angiogenesis, proliferative and swelling vascular endothelial cells, and a few lymphoid follicles.
Discussion
The CD is a heterogeneous disorder that is usually complicated with multiple self-immune diseases. Although its etiology and mechanisms are still unknown, it may be associated with angiogenesis [11], viral infections [12], and dysregulation of the cytokines of HHV-8 and IL-6 [13,14]. Lacking significant symptoms, the preoperative diagnosis of localized CD (LCD) is mainly based on imaging results. Clinical symptoms of LCD are closely correlated with the pathological type. Hyaline vascular LCD is the most commonly observed type [15], and its imaging characteristics are as follows [16,17]: 1) Lesions located at the pelvic cavity and abdomen are usually quasi-circular, with shadows of isointensity during CT plain scanning, shadows of isointensity or slight hypointensity in T1WI imaging, and shadows of even hyperintensity in T2WI imaging during MRI plain scanning. Edges of the lesions are smooth and sharp with distinct boundaries from the adjacent structures, which is consistent with the intact capsules found in the gross pathological examinations. 2) Characteristic manifestations of punctate and bar-like branching calcifications can be found in parts of the masses. Pathological findings show that calcified tissues, indicating vascular degeneration and calcium deposits, are mainly located at the hyaline vascular sites. 3) Tumor edges are often clear, but cord-like and cotton-like shadows may be observed around some of the tumors. Lesions are significantly enhanced during the arterial phase and persistently enhanced during the portal and delayed phases, with an enhanced intensity similar to the artery. Bar-like shadows of hypointensity can be observed in parts of the lesions after enhancement. 4) Tortuous vessels can be found in parts of the lesions. Due to adhesions to the adjacent tissues, a mass edge in the case of the obturator nerve is unobvious, while the remaining 3 cases all demonstrate the features above. Positron emission computed tomography (PET-CT) is an effective means of examination for tumor diagnosis, and studies have proven that PET-CT is an efficacious means of diagnosing CD, since CD may have hypermetabolic features [18,19]. However, due to the expensive cost, it is hard to establish PET-CT as a routine form of examination.
For patients with either HV type or PC type LCD, surgical treatment can achieve a cure rate of approximately 100% [20]. The traditional open surgical excision of tumor before 2005. A report on the following laparoscopic resection of the tumor occurred after 2005. Laparoscopic exploration range is broader, thorough, and clearer. Local details can be carefully observed and operated in the enlarged view to ensure the fine anatomy and avoid accidental injury. Laparoscopic surgery has the following advantages: small trauma, quick recovery, short hospitalization time, et al. In the present study, the 4 patients all underwent the laparoscopic treatment. Their preoperative imaging results indicated that blood supports of the masses were abundant and that the masses were located in the iliac fossa; therefore, iliac embolism was performed in advance to reduce the intraoperative hemorrhage, while the obturator nerve, ureter, and iliac vessels were isolated to avoid injuries. Also, the enlarged lymph nodes were removed. Although the cure rate of surgical resection for LCD patients is high, patients with lesions that cannot be completely resected, in addition to patients who cannot receive operation due to physical problems, may be treated by single or combined radiotherapy. One study [21] from 29 LCD patients claimed that radiotherapy (total radiation dose of 40-50 Gy) was effective for 89.6% of the patients, and 44.8% of them were completely relieved. For patients who cannot take surgical treatment, the medication rituximab is also an alternative [22].
Preoperative imaging examination can provide information about the mass, such as the size, position, origin, and feature. This information is important for the laparoscopic treatment for retroperitoneal LCD in the iliac region. Preoperative iliac arterial embolism is recommended for masses with abundant blood supports. Laparoscopic retroperitoneal striping and ureter isolation at the lesioned side plus lymphadenectomy for enlarged lymph nodes are good choices. The resected retroperitoneal mass should be immediately processed for the routine pathological frozen section examination. Surgeons should be fully aware of the intra operative risks and be well-prepared for laparotomy whenever necessary.
Acknowledgements
We thank the staff members of this trial, our colleagues, and all the study staff for their enormous efforts in collecting and ensuring the accuracy and completeness of all the data.
Disclosure of conflict of interest
None.
References
- 1.Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer. 1956;9:822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A, Armitage JO, Loe MJ, Geyer SM, Allred J, Camoriano JK, Menke DM, Weisenburger DD, Ristow K, Dogan A, Habermann TM. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87:997–1002. doi: 10.1002/ajh.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eszes N, Tamási L, Csekeő A, Csomor J, Szepesi A, Varga G, Balázs G, Losonczy G, Müller V. Unicentric mixed variant Castleman disease associated with intrabronchial plasmacytoma. Diagn Pathol. 2014;9:64. doi: 10.1186/1746-1596-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshitari T, Kajita F, Tobe A, Itami M, Yotsukura J, Baba T, Yamamoto S. Refractory uveitis in patient with castleman disease successfully treated with tocilizumab. Case Rep Ophthalmol Med. 2012;2012:968180. doi: 10.1155/2012/968180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moloney F, Twomey M, Hinchion J, Maher M. Castleman disease: An unexpected cause of a solitary pleural mass. Case Rep Radiol. 2013;2013:130515. doi: 10.1155/2013/130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clain JB, Scherl S, Karle WE, Khorsandi A, Ghali V, Wang B, Urken ML. Castleman disease in the parapharyngeal space: a case report and review of the literature. Head Neck Pathol. 2013;7:389–392. doi: 10.1007/s12105-013-0449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecka F, Ferko A, Jon B, Subrt Z, Kasparova P, Repak R. Pancreatic Castleman disease treated with laparoscopic distal pancreatectomy. Hepatobiliary Pancreat Dis Int. 2013;12:332–334. doi: 10.1016/s1499-3872(13)60053-3. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi H, Mimura S, Nomura T, Tani J, Morishita A, Kobara H, Mori H, Yoneyama H, Deguchi A, Himoto T, Yamamoto N, Okano K, Suzuki Y, Masaki T. A rare case of hyaline-type Castleman disease in the liver. World J Hepatol. 2013;5:404–408. doi: 10.4254/wjh.v5.i7.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon JH, Min SK, Shin MK, Lee YS, Lee YG, Ko YH. Hyaline vascular castleman disease involving renal parenchyma and a lymph node: a case report. Korean J Pathol. 2012;46:79–82. doi: 10.4132/KoreanJPathol.2012.46.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turek G, Reszeć J, Kochanowicz J, Hermanowicz A, Mariak Z. A case of a solitary form of Castleman disease: ten-year follow-up. Neurol Neurochir Pol. 2013;47:179–183. doi: 10.5114/ninp.2013.34636. [DOI] [PubMed] [Google Scholar]
- 11.Fajgenbaum DC, Rosenbach M, van Rhee F, Nasir A, Reutter J. Eruptive cherry hemangiomatosis associated with multicentric Castleman disease: a case report and diagnostic clue. JAMA Dermatol. 2013;149:204–208. doi: 10.1001/jamadermatol.2013.1552. [DOI] [PubMed] [Google Scholar]
- 12.Venkataraman G, Uldrick TS, Aleman K, O’Mahony D, Karcher DS, Steinberg SM, Raffeld MA, Marshall V, Whitby D, Little RF, Yarchoan R, Pittaluga S, Maric I. Bone marrow findings in HIV-positive patients with Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Am J Clin Pathol. 2013;139:651–661. doi: 10.1309/AJCPKGF7U8AWQBVG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rhee F, Fayad L, Voorhees P, Furman R, Lonial S, Borghaei H, Sokol L, Crawford J, Cornfeld M, Qi M, Qin X, Herring J, Casper C, Kurzrock R. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J. Clin. Oncol. 2010;28:3701–3708. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- 14.Kawabata H, Kadowaki N, Nishikori M, Kitawaki T, Kondo T, Ishikawa T, Yoshifuji H, Yamakawa N, Imura Y, Mimori T, Matsumura Y, Miyachi Y, Matsubara T, Yanagita M, Haga H, Takaori-Kondo A. Clinical features and treatment of multicentric castleman’s disease: a retrospective study of 21 Japanese patients at a single institute. J Clin Exp Hematop. 2013;53:69–77. doi: 10.3960/jslrt.53.69. [DOI] [PubMed] [Google Scholar]
- 15.Zhou LP, Zhang B, Peng WJ, Yang WT, Guan YB, Zhou KR. Imaging findings of Castleman disease of the abdomen and pelvis. Abdom Imaging. 2008;33:482–488. doi: 10.1007/s00261-007-9282-5. [DOI] [PubMed] [Google Scholar]
- 16.Dong A, Dong H, Zuo C. Castleman disease of the porta hepatis mimicking exophytic hepatocellular carcinoma on CT, MRI, and FDG PET/CT. Clin Nucl Med. 2014;39:e69–72. doi: 10.1097/RLU.0b013e31827a25fd. [DOI] [PubMed] [Google Scholar]
- 17.Reddy D, Mitsuyasu R. HIV-associated multicentric Castleman disease. Curr Opin Oncol. 2011;23:475–481. doi: 10.1097/CCO.0b013e328349c233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossotti R, Moioli C, Schiantarelli C, Orcese C, Puoti M. FDG-PET imaging in the diagnosis of HIV-associated multicentric Castleman disease: something is still missing. Top Antivir Med. 2012;20:116–118. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee ES, Paeng JC, Park CM, Chang W, Lee WW, Kang KW, Chung JK, Lee DS. Metabolic characteristics of Castleman disease on 18F-FDG PET in relation to clinical implication. Clin Nucl Med. 2013;38:339–342. doi: 10.1097/RLU.0b013e3182816730. [DOI] [PubMed] [Google Scholar]
- 20.Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255:677–84. doi: 10.1097/SLA.0b013e318249dcdc. [DOI] [PubMed] [Google Scholar]
- 21.de Vries IA, van Acht MM, Demeyere T, Lybeert ML, de Zoete JP, Nieuwenhuijzen GA. Neoadjuvant radiotherapy of primary irresectable unicentric Castleman’s disease: A case report and review of the literature. Radiat Oncol. 2010;5:7. doi: 10.1186/1748-717X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandera B, Ainsworth C, Shikle J, Rupard E, Roach M. Treatment of Unicentric Castleman Disease With Neoadjuvant Rituximab. Chest. 2010;138:1239–1241. doi: 10.1378/chest.09-2084. [DOI] [PubMed] [Google Scholar]


