Abstract
Signal transducer and activator of transcription 3 (STAT3) and phospho-STAT3 (pSTAT3) play important roles in the development of gastric cancer. STAT3 is often associated with cell survival, proliferation, and transformation. The prognostic value of STAT3/pSTAT3 in patients with gastric cancer remains controversial in numerous published studies. The aim of this study was to summarize recent findings relevant to the prognostic role of STAT3 and pSTAT3 in patients with gastric cancer. A meta-analysis was performed by searching Web of Knowledge, EMBASE, and PubMed to identify studies on the prognostic impact of STAT3/pSTAT3 in gastric cancers in August 2014. In all, 10 studies were included in the analysis. Data were collected for comparing survival rates in patients with high STAT3 levels compared to those with low levels. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Sensitivity analysis was conducted, and publication bias was evaluated. Eventually, 1667 cases of gastric cancer were subjected to the final analysis. Among patients with gastric cancer, poor survival was predicted by higher expressions of STAT3 (HR=2.30; 95% CI=1.13-4.68; P=0.02) and pSTAT3 (HR=1.75; 95% CI=1.17-2.61; P=0.006). Moreover, overexpression of STAT3 was associated with poor tumor stage. Additionally, our analysis did not show any statistically significant effect of publication bias regarding STAT3 or pSTAT3. The results of this meta-analysis demonstrated that overexpression of STAT3 and pSTAT3 was associated with poor prognosis in gastric cancer.
Keywords: Gastric cancer, signal transducer and activator of transcription 3, prognosis, meta-analysisjr
Introduction
Gastric cancer is the fourth most common cancer worldwide and the second most lethal neoplasm overall [1]. Despite progress in multimodality therapy, the five-year survival rate remains low. Therefore, identification of factors that affect patient survival is critical for novel therapy development.
Signal transducer and activator of transcription 3 (STAT3) plays an important role in many pathophysiologic processes, such as differentiation, proliferation, survival, inflammation, angiogenesis, and immune function [2-4]. As an oncogenic transcriptional factor, the role of STAT3 in tumorigenesis has been demonstrated in various human cancers, including breast, pancreatic, prostate, nasopharyngeal, and gastric cancers [5-7]. STAT3 is the most important member of the STAT family. It can be activated via phosphorylation by many cytokines and growth factor receptors [8], such as EGFR, c-Met, and the IL-6 receptor. The activated STAT3 complex then translocates into the nucleus, where it initiates transcription of STAT3 target genes (including cyclin D1, Bcl-xL, survivin, and VEGF) [9]. STAT3 is transiently phosphorylated in normal cells. However, persistent STAT3 phosphorylation is observed in various cancer cells [10].
Recent evidence indicates that STAT3 is aberrantly active in gastric cancers and gastric cancer cell lines [11,12]. However, the prognostic value of STAT3 or phospho-STAT3 (pSTAT3) in gastric cancer is controversial. Several studies have reported that STAT3 activation is associated with poor prognosis for gastric cancer patients [13,14]. On the contrary, another study found no significant relationship between STAT3 activation and patient survival [15]. Thus, we conducted this meta-analysis to explore the biological significance of STAT3/pSTAT3 in gastric cancer.
Materials and methods
Literature search
We searched the PubMed, EMBASE, and ISI Web of Knowledge databases in August 2014, for all eligible studies. The search was conducted using the following search terms: “STAT3” OR “signal transducer and activator of transcription 3”, and “gastric OR stomach” and “cancer OR carcinoma”. In an effort to broaden the search, reference lists from identified primary studies were then searched carefully to include some eligible studies that would have been missed by electronic searches alone.
Study selection
Titles and abstracts of all candidate papers were screened by two reviewers (G Liao and S He). Papers that could not be categorized based on titles and abstracts alone were retrieved for full-text review. Two reviewers screened and checked these papers individually according to the inclusion criteria. Disagreements between reviewers were resolved through consensus with a third reviewer (L Chen).
Inclusion criteria for the primary studies were as follows: (i) Diagnosis of gastric cancer in humans was proven, (ii) STAT3 or pSTAT3 evaluation was performed, (iii) data reported was related to the survival of patients with gastric cancer, and (iv) if an author published the same group of cases in different journals, the most recent and completed study was included. In addition, the language was restricted to English. Reviews, letters to the editors, and meeting abstracts were excluded.
Data extraction
Two authors carefully reviewed all eligible studies and extracted the following data: first author, publication year and country, the number of patients evaluated, follow-up times, detection methods, cut-off values, and survival data. Any disagreements in the data extraction were resolved by discussion among authors.
Methodological assessment
Methodological assessment was conducted according to REMARK guidelines [16] for each eligible study by independent reviewers (G Liao and S He). The REMARK guidelines are widely used for evaluating the quality of studies on prognostic markers of cancers that were included in meta-analyses [17,18]. Briefly, the scale contained 18 items (Table 1), and each item was scored according to an ordinal scale (possible values 1 and 0): 1 represented the complete description or partly matched description, whereas 0 represented no matched description. The total scored was ranged from 0 to 18.
Table 1.
Definitions of 18 items of study reporting quality
| Study design |
| 1. Objectives or prespecified hypothesis: state the study objectives, prespecified hypothesis or study protocol |
| 2. Sample size: state a statistical sample size or power calculation |
| 3. Follow-up description: state the follow-up period or the median follow-up time |
| 4. Population source: state health care setting from which patients were recruited |
| 5. Population selection criteria: state inclusion or exclusion |
| 6. Population characteristics: state the population characteristics (e.g., age, gender, and disease stage) |
| 7. Number of patients included in each stage of the analysis and reason for dropout: description of number of patients at different stage, including the number of patients who participate in the study, who met the inclusion criteria, and who followed up and reason for dropout |
| Assay method |
| 1. Sample handling: state the method of storage |
| 2. Assay method: state the type of assay method used to measure Stat3/p-Stat3 |
| 3. Manufacturer: state the name of company which makes the assay for Stat3/p-Stat3 |
| 4. Cut off point determination: state methods used for cut-off point determination |
| Confounders |
| 1. Conventional risk factors: state the conventional risk factors (e.g., age, gender, depth of tumor, lymph node metastasis) relating with the Stat3/p-Stat3 expression |
| 2. Other biomarkers (e.g., p53, PCNA, VEGF, and microvessel density): state other biologic marker relating with the disease |
| Outcome |
| 1. Clinical endpoint: define the clinical endpoint |
| 2. Validation: state the outcome events checked by independent source (e.g., medical records, outpatient visits, by letter, and by telephone) |
| Analysis |
| 1. Univariate estimate: report the effect of Stat3/p-Stat3 on outcome |
| 2. Multivariate estimate: adjusted for risk factors or other biomarkers (list above) |
| 3. Missing value: state the number of patients with missing value for Stat3/p-Stat3 or confounders and how to deal with it |
Statistical analyses
Hazard ratios (HRs) and 95% confidence intervals (CIs) were applied to estimate the association between STAT3/pSTAT3 expression and survival (including disease specific survival and overall survival) in patients with gastric cancer. The most accurate results were found in studies that reported the exact HR values and its 95% CIs. However, for some of the studies that did not report HR and 95% CIs directly in the study, mathematical HR approximation was estimated using Parmar’s methods [19]. If the available survival data were only in the form of figures, we read Kaplan-Meier curves by using Engauge Digitizer version 4.1 (free software down-loaded from http://sourceforge.net) and extracted survival rates to calculate the HR and standard error (SE). Odds ratio was applied for evaluating the relationship between STAT3 expression and tumor stage.
The meta-analysis was conducted by Review Manager Software (version 5.2, The Nordic Cochrane Centre, Copenhagen, Denmark). Statistical heterogeneity was evaluated by Cochrane’s Q test (Chi squared test; χ2) and by measuring inconsistency (I2) [20,21]. I2>50% represents significant heterogeneity. Given that data were gathered from distinctly different populations with potential heterogeneity [22], a random effects model was adopted for the pooled analysis of HRs with 95% CIs. In addition, risk of publication bias was assessed using a funnel plot for overall survival, and the exact statistical value was assessed by using a method reported by Egger M [23]. Sensitivity analysis was performed by sequential omission of any single study. P values of less than 0.05 were considered statistically significant.
Results
The primary search retrieved 1404 studies; 148, 657, and 617 articles were retrieved from PubMed, EMBASE, and Web of Knowledge, respectively. First, 728 duplicated studies were removed by Endnote software; then, the remaining 676 studies were assessed by reading their titles and abstracts. At this stage, studies that did not meet the criteria for our meta-analysis were excluded. Thus, 19 papers were identified for full-text evaluation. After carefully reading the entire papers, nine studies were excluded because they did not evaluate STAT3 expression on survival or survival data could not obtain in the study. Finally, 10 studies that met our inclusion criteria were included in our meta-analysis [13,14,24-31]. These included four studies that analyzed STAT3 as a prognostic marker and eight studies that analyzed pSTAT3 as a prognostic marker. The selection chart is listed in Figure 1. The major baseline characteristics of the included studies and quality assessments are reported in Table 2. The details of each study quality assessment are provided in Table S1. Quality scores ranged from 11 to 15, with a median value of 13.7. All of the included studies mentioned study objectives, sample sizes, population sources, population characteristics, assay methods, the antibody manufacturers, cut-off values, clinical endpoints, and univariate survival analyses. However, fewer studies provided information on number of patients included in each stage of the analysis and reason for dropout, confounders of other biological markers, and missing values.
Figure 1.
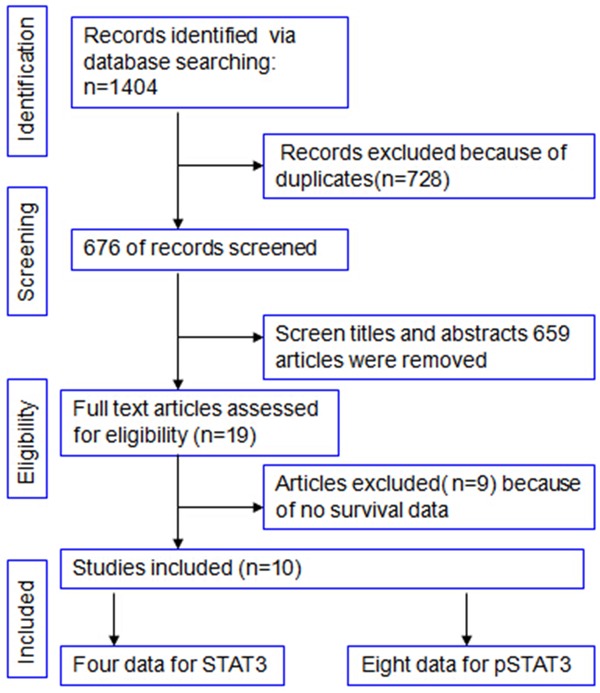
Flow diagram of study selection.
Table 2.
Basic characteristics of included studies evaluating survival in gastric cancer patients
| First author | Year | Country | Cases | HR | Survival analysis | Follow up time (month) | Technique | Cut-off value | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| STAT3 | |||||||||
| Chatrerje D | 2008 | USA | 143 | 3.00 (1.82-4.95) | Univariate | 34 (12-180) | IHC | ≥4 | 13 |
| Deng J | 2010 | China | 53 | 4.44 (1.80-10.93) | Univariate | 35 (4-85) | IHC | >3 | 15 |
| Kim DY | 2008 | Korea | 100 | 2.73 (0.83-9.05) | Univariate | NA | IHC | >10% | 13 |
| Xiong H | 2012 | China | 305 | 0.66 (0.24-1.80) | Multivariate | 39.7 (4-84) | IHC | ≥5% | 13 |
| pSTAT3 | |||||||||
| Deng J | 2010 | China | 53 | 9.605 (3.11-29.69) | Multivariate | 35 (4-85) | IHC | >3 | 15 |
| Deng J | 2013 | China | 114 | 2.49 (1.41-4.39) | Multivariate | 38 (2-108) | IHC | >25% | 15 |
| Gong W | 2005 | USA | 86 | 1.36 (0.79-2.36) | Multivariate | 25.7 | IHC | >3 | 15 |
| Inokuchi M | 2011 | Japan | 126 | 2.00 (0.91-4.5) | Multivariate | 73 (2-135) | IHC | >10% | 12 |
| Lee J | 2009 | Korea | 311 | 1.51 (1.03-2.21) | Multivariate | NA | IHC | ≥1% | 15 |
| Woo S | 2011 | Korea | 285 | 0.57 (0.36-0.91) | Univariate | 51 (1-72) | IHC | >1% | 15 |
| Xiong H | 2012 | China | 305 | 2.10 (1.53-2.89) | Multivariate | 39.7 (4-84) | IHC | ≥5% | 13 |
| Yakata Y | 2006 | Japan | 111 | 1.63 (0.73-3.65) | Univariate | NA | IHC | >10% | 11 |
NA, not available, STAT3, signal transducer and activator of transcription 3, pSTAT3, phospho-STAT3, IHC: immunohistochemistry.
All of the studies were published from 2005 to 2013. The sample size ranged from 53 to 311 cases, with most studies (8/10) reporting more than 100 cases. All of the included studies detected STAT3 and pSTAT3 expression by immunohistochemistry.
Meta-analysis of STAT3 expression in gastric cancer
Pooled analyses of the four studies that evaluated STAT3 expression in gastric cancer was shown in Figure 2. The results indicate that STAT3 overexpression was positively associated with poor survival in patients with gastric cancer (HR=2.30; 95% CI=1.13-4.68; P=0.02). Of note, the heterogeneity was statistically significant (χ2=8.95; P=0.03; I2=66%).
Figure 2.
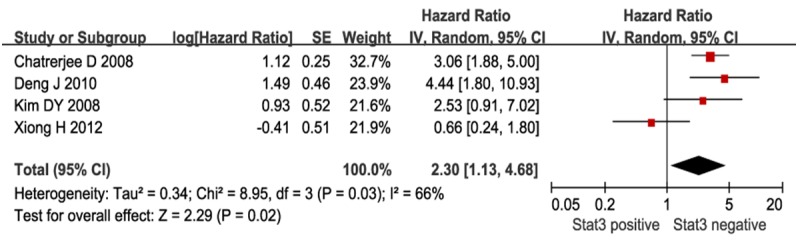
Meta-analysis of STAT3 expression and prognosis in patients with gastric cancer. Results are presented as individual and pooled HR, and 95% CI.
In order to evaluate the effect of STAT3 expression on the clinical characteristics of gastric cancer, we next studied the relationship between STAT3 expression and tumor node metastasis (TNM) stage. As shown in Figure 3, STAT3 overexpression correlated with higher TNM stage (Odds ratio =0.31; 95% CI=0.15-0.66; P=0.002).
Figure 3.
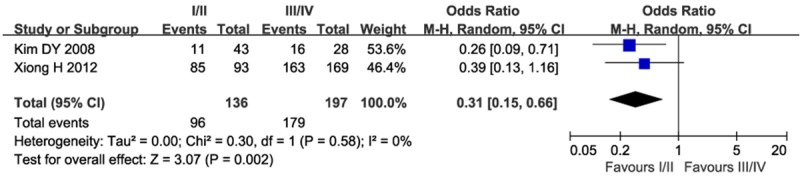
Meta-analysis of STAT3 expression and TNM stage in patients with gastric cancer.
Meta-analysis of pSTAT3 expression in gastric cancer
Increased levels of pSTAT3 was associated with a significant increase of mortality risk as compared to low pSTAT3 levels in the random effects model (HR=1.75; 95% CI=1.17-2.61; P=0.006), although significant heterogeneity (χ2=26.49; P=0.0004; I2=74%) was present in Figure 4.
Figure 4.
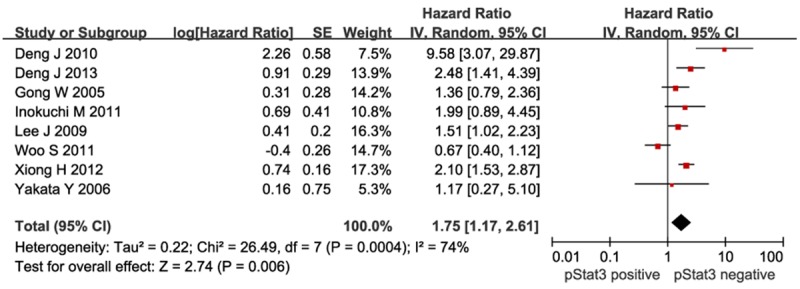
Meta-analysis of pSTAT3 expression and prognosis in patients with gastric cancer. Results are presented as individual and pooled HR, and 95% CI.
To investigate the cause of the heterogeneity, meta-regression and subgroup analyses were performed to evaluate the following factors: study country, publication year, survival analysis, quality score, and number of patients. The results showed that none of these factors significantly influenced the heterogeneity (Table 3). We performed a leave-one-out sensitivity analyses by excluding one study at a time and recalculating HR and 95% CI values. The results of this analysis confirmed that pSTAT3 over expression was association with mortality risk (Figure 5). Moreover, by excluding the study reported by Woo S [28], increased pSTAT3 indicated poor survival (HR=2.00; 95% CI=1.45-2.75; P<0.0001), and there was no significant heterogeneity among the remaining studies (χ2=12.17; P=0.06; I2=51%).
Table 3.
Subgroup analyses and meta-regression the association between pSTAT3 and survival in gastric cancer
| Subgroup | No. of studies | No. of patients | Pooled HR (Random) | Meta-regression P value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | I2 (%) | P value | ||||
| Country | 0.579 | ||||||
| Asian country | 7 | 1352 | 1.63 | 1.11-2.38 | 66 | 0.008 | |
| Non-Aisan country | 2 | 229 | 2.06 | 0.93-4.56 | 79 | 0.03 | |
| Year | 0.708 | ||||||
| <2010 | 5 | 731 | 1.86 | 1.27-2.70 | 43 | 0.13 | |
| >2010 | 4 | 830 | 1.61 | 0.88-2.94 | 82 | 0.0009 | |
| Survival analysis | 0.687 | ||||||
| Multivariate | 5 | 942 | 1.85 | 1.51-2.26 | 0 | 0.42 | |
| Univariate | 4 | 619 | 1.58 | 0.62-4.01 | 84 | 0.0003 | |
| Number of patients | 0.214 | ||||||
| <200 | 6 | 660 | 2.16 | 1.61-2.91 | 12 | 0.34 | |
| >200 | 3 | 901 | 1.32 | 0.72-2.42 | 86 | 0.0009 | |
| Antibody used | 0.406 | ||||||
| Stat3 | 3 | 329 | 2.16 | 1.22-3.83 | 58 | 0.09 | |
| p-Stat3 | 6 | 1232 | 1.56 | 1.03-2.34 | 70 | 0.005 | |
| Quality score | 0.118 | ||||||
| ≤14 | 5 | 765 | 2.28 | 1.79-2.89 | 0 | 0.63 | |
| >14 | 4 | 796 | 1.35 | 0.82-2.23 | 75 | 0.007 | |
Figure 5.
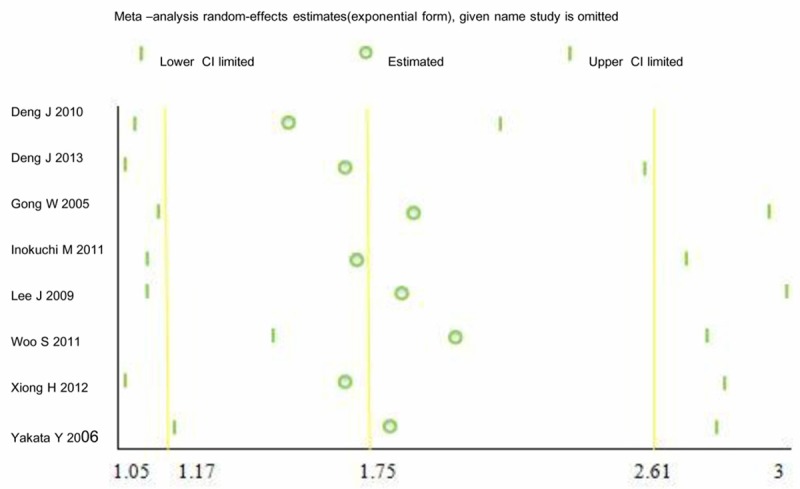
Sensitivity analysis results.
Publication bias
For STAT3, a funnel plot of the HR showed no obvious publication bias (Figure 6A) and the assessment of publication bias by Egger’s tests showed no significance (P=0.541) for studies included in the analysis of survival. For pSTAT3, there was visual symmetry in the funnel plot graph (Figure 6B), and Egger’s tests showed no statistical significance of publication bias (P=0.726).
Figure 6.
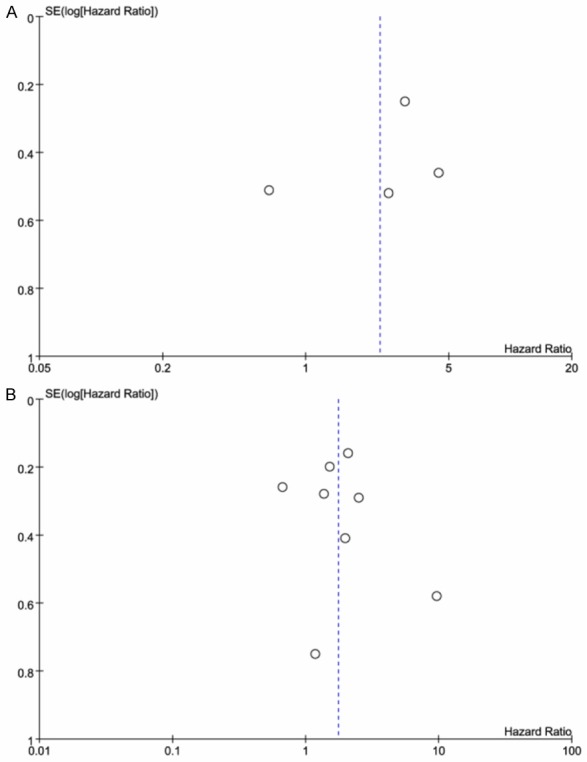
Funnel plot of the HR for publication bias, A. For STAT3; B. For pSTAT3.
Discussion
STAT3 proteins are involved in various cellular processes and are major effectors of cytokine and chemokine receptor signaling. Moreover, STAT3 proteins are key modulators of a variety of human cancers [32], regulating cell proliferation, apoptosis, survival, metastasis, and angiogenesis [33]. In normal cells, STAT3 phosphorylation occurs only transiently [34]. By contrast, almost 70% of human cancers display persistent STAT3 phosphorylation. Phosphorylated STAT3 initiates transcription of multiple cancer-associated genes, such as Cyclin D1, Bcl-xl, survivin, IL-6, and MMP-9. As such, STAT3 is a molecular hub for diverse signaling pathways, and is considered as a novel molecular target for cancer drugs [35]. A previous meta-analysis reported that over expression of STAT3 or pSTAT3 is associated with poor prognosis in patients with non-small-cell lung cancer [36]. However, STAT3/pSTAT3 as a prognostic marker of overall survival in patients with gastric cancer needs to be fully explored.
This meta-analysis was conducted to elucidate the prognostic role of STAT3/pSTAT3 expression in patients with gastric cancer. Our study combined results from four individual studies that indicated STAT3 was significantly associated with poor survival in patients with gastric cancer (HR=1.73; 95% CI=1.25-2.39; P=0.0009). Our study also analyzed results from eight individual studies that demonstrated pSTAT3 was associated with an unfavorable prognosis in patients with gastric cancer (HR=1.75; 95% CI=1.17-2.61; P=0.006). To our knowledge, this is the first meta-analysis to investigate STAT3/pSTAT3 as a prognostic marker in gastric cancer.
To gain further insights into the role of STAT3/pSTAT3 as a biological marker, we next investigated the association of STAT3/pSTAT3 expression with TNM stage, a classification currently used for the prognostic judgment for patients affected with solid cancers. TNM staging is regarded as the most reliable factor to predict the prognosis of gastric cancer. With appropriate treatment, up to 90% of gastric cancer patients will reach the 5-year survival mark [37]. On the contrary, in cases of advanced gastric cancer, the response to treatment rates and survival rates are poor. Specially, lymph node status and lymph node resection are both effective prognostic factors [38,39]. Our study suggested that high expression of STAT3, but not pSTAT3, significantly correlated with TNM. Due to the limited number of studies, the correlation is required to be further investigated.
The prognosis of gastric cancer is also affected by the race of patients. In Western countries, the incidence of gastric cancer is declining; however, patients who are diagnosed with gastric cancer are presenting with advanced TNM stages and have a poor prognosis. In contrast, in Japan, where the incidence of gastric cancer is still high, the percentage of cases diagnosed at the stage of “early gastric cancer” has greatly increased, and thus, the prognosis has also improved [40]. One study reported that in patients with gastric cancer, when grouped by ethnicity, Asians have improved survival compared to other ethnic categories (Caucasian, African-origin, American Indians, or others) [41].
Due to the important role of STAT3 in cancer, STAT3 has been validated as an anti-cancer target in several studies [42], and many studies have shown inhibition of STAT3 activation can be effective for cancer prevention and treatment [43,44]. A previous study indicated that combined use of NF-κB and STAT3 inhibitors may enhance the efficacy of the anti-metastatic treatment of gastric cancer [45]. Another study demonstrated that STAT3 inhibition leads to profound sensitization to both chemotherapy and radiotherapy in a cancer mouse model [46].
We should consider the following limitations in this meta-analysis. First, this meta-analysis did not address heterogeneity issues, and a notable heterogeneity was revealed amongst these studies. However, in terms of pSTAT3, when we conducted a sensitivity analysis by omitting the study reported by Woo S [28], no statistically significant heterogeneity was noted among the remaining studies (χ2=12.17; P=0.06; I2=51%). Furthermore, to identify potential sources of heterogeneity among the pSTAT3 data, meta-regression and subgroup analyses were conducted. The results of meta-regression revealed that study location, year of publication, quality score, and number of patients had no significant correlation to heterogeneity (Table 3). Therefore, these results suggested that the study reported by Woo S [28] contributed significantly to the heterogeneity. Since there were only four studies regarding STAT3 as a prognostic marker in gastric cancer, we did not perform subgroup analysis and meta-regression analysis for STAT3.
Second, most included studies were of a retrospective nature; the patients were selected randomly only in one study [25]. This fact could contribute to possible publication bias. However, our statistical test indicated no significant publication bias amongst the studies. To date, high-quality double-blind randomized controlled trials to evaluate the relationship between STAT3 and patient survival have not been reported. Future studies with well-designed should assess the prognostic role of STAT3 in gastric cancer.
Third, the included studies in our meta-analysis are all published in English that might contribute to the possibility of language bias. In addition, we excluded unpublished studies and conference abstracts because they did not have sufficient information. Those unpublished studies might have more frequently refuted our hypotheses.
Fourth, although we found a positive correlation between STAT3 expression and TNM stage, the definition of TNM stage was in accordance with different version of edition and the number of studies was limited; thus, future studies should pay attention to these issues. Finally, according to the quality assessment, few studies had included the number of patients in each stage, few had missing value, as well as had confounders of other biological markers; thus, future studies should account for these biases.
In summary, our data suggest that STAT3 or pSTAT3 could be a valuable prognostic marker in gastric cancer, with higher STAT3 protein expression significantly associating with higher TNM stage and increased pSTAT3 associating with increased mortality risk. Owing to the limited scope of the study, future studies should be performed with prospective, well-designed, randomized controlled trials to fully elaborate the prognostic value of STAT3/pSTAT3 in gastric cancer.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review) Int J Oncol. 2012;41:1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 3.Yeh JE, Toniolo PA, Frank DA. Targeting transcription factors: promising new strategies for cancer therapy. Curr Opin Oncol. 2013;25:652–658. doi: 10.1097/01.cco.0000432528.88101.1a. [DOI] [PubMed] [Google Scholar]
- 4.Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho Y, Tsao SW, Zeng M, Lui VW. STAT3 as a therapeutic target for Epstein-Barr virus (EBV): associated nasopharyngeal carcinoma. Cancer Lett. 2013;330:141–149. doi: 10.1016/j.canlet.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Chung SS, Giehl N, Wu Y, Vadgama JV. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J Oncol. 2014;44:403–411. doi: 10.3892/ijo.2013.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo K, Ma Q, Li J, Wang Z, Shan T, Li W, Xu Q, Xie K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol Cancer Ther. 2013;12:264–273. doi: 10.1158/1535-7163.MCT-12-0809. [DOI] [PubMed] [Google Scholar]
- 8.Luwor RB, Stylli SS, Kaye AH. The role of Stat3 in glioblastoma multiforme. J Clin Neurosci. 2013;20:907–911. doi: 10.1016/j.jocn.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Giraud AS, Menheniott TR, Judd LM. Targeting STAT3 in gastric cancer. Expert Opin Ther Targets. 2012;16:889–901. doi: 10.1517/14728222.2012.709238. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Deng J, Wang L, Lee H, Armstrong B, Scuto A, Kowolik C, Weiss LM, Forman S, Yu H. S1PR1 is an effective target to block STAT3 signaling in activated B cell-like diffuse large B-cell lymphoma. Blood. 2012;120:1458–1465. doi: 10.1182/blood-2011-12-399030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai AH, Lo AW, Chu SH, Tong JH, Lo KW, Sung JJ, Chan FK. Constitutional activation of IL-6-mediated JAK/STAT pathway through hypermethylation of SOCS-1 in human gastric cancer cell line. Br J Cancer. 2004;91:1335–1341. doi: 10.1038/sj.bjc.6602133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YC, Wang JL, Kong X, Sun TT, Chen HY, Hong J, Fang JY. CD24 mediates gastric carcinogenesis and promotes gastric cancer progression via STAT3 activation. Apoptosis. 2014;19:643–656. doi: 10.1007/s10495-013-0949-9. [DOI] [PubMed] [Google Scholar]
- 13.Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010;16:5380–5387. doi: 10.3748/wjg.v16.i42.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646–651. doi: 10.1111/j.1440-1746.2008.05671.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi JH, Ahn MJ, Park CK, Han HX, Kwon SJ, Lee YY, Kim IS. Phospho-Stat3 expression and correlation with VEGF, p53, and Bcl-2 in gastric carcinoma using tissue microarray. APMIS. 2006;114:619–625. doi: 10.1111/j.1600-0463.2006.apm_401.x. [DOI] [PubMed] [Google Scholar]
- 16.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100:229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 17.Steels E, Paesmans M, Berghmans T, Branle F, Lemaitre F, Mascaux C, Meert AP, Vallot F, Lafitte JJ, Sculier JP. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a metaanalysis. Eur Respir J. 2001;18:705–719. doi: 10.1183/09031936.01.00062201. [DOI] [PubMed] [Google Scholar]
- 18.Ruys AT, Groot KB, Wiggers JK, Klumpen HJ, Ten KF, van Gulik TM. Prognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:487–500. doi: 10.1245/s10434-013-3286-x. [DOI] [PubMed] [Google Scholar]
- 19.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng J, Liang H, Zhang R, Sun D, Pan Y, Liu Y, Zhang L, Hao X. STAT3 is associated with lymph node metastasis in gastric cancer. Tumour Biol. 2013;34:2791–2800. doi: 10.1007/s13277-013-0837-5. [DOI] [PubMed] [Google Scholar]
- 25.Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD, Xie K, Sawaya R, Huang S. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386–1393. doi: 10.1158/1078-0432.CCR-04-0487. [DOI] [PubMed] [Google Scholar]
- 26.Inokuchi M, Murayama T, Hayashi M, Takagi Y, Kato K, Enjoji M, Kojima K, Kumagai J, Sugihara K. Prognostic value of co-expression of STAT3, mTOR and EGFR in gastric cancer. Exp Ther Med. 2011;2:251–256. doi: 10.3892/etm.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Kang WK, Park JO, Park SH, Park YS, Lim HY, Kim J, Kong J, Choi MG, Sohn TS, Noh JH, Bae JM, Kim S, Lim DH, Kim KM, Park CK. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS. 2009;117:598–606. doi: 10.1111/j.1600-0463.2009.02512.x. [DOI] [PubMed] [Google Scholar]
- 28.Woo S, Lee BL, Yoon J, Cho SJ, Baik TK, Chang MS, Lee HE, Park JW, Kim YH, Kim WH. Constitutive activation of signal transducers and activators of transcription 3 correlates with better prognosis, cell proliferation and hypoxia-inducible factor-1alpha in human gastric cancer. Pathobiology. 2011;78:295–301. doi: 10.1159/000321696. [DOI] [PubMed] [Google Scholar]
- 29.Xiong H, Du W, Wang JL, Wang YC, Tang JT, Hong J, Fang JY. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med (Berl) 2012;90:1037–1046. doi: 10.1007/s00109-012-0869-0. [DOI] [PubMed] [Google Scholar]
- 30.Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- 31.Chatterjee D, Sabo E, Tavares R, Resnick MB. Inverse association between Raf Kinase Inhibitory Protein and signal transducers and activators of transcription 3 expression in gastric adenocarcinoma patients: implications for clinical outcome. Clin Cancer Res. 2008;14:2994–3001. doi: 10.1158/1078-0432.CCR-07-4496. [DOI] [PubMed] [Google Scholar]
- 32.Peyser ND, Grandis JR. Critical analysis of the potential for targeting STAT3 in human malignancy. Onco Targets Ther. 2013;6:999–1010. doi: 10.2147/OTT.S47903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajendran P, Li F, Shanmugam MK, Kannaiyan R, Goh JN, Wong KF, Wang W, Khin E, Tergaonkar V, Kumar AP, Luk JM, Sethi G. Celastrol suppresses growth and induces apoptosis of human hepatocellular carcinoma through the modulation of STAT3/JAK2 signaling cascade in vitro and in vivo. Cancer Prev Res (Phila) 2012;5:631–643. doi: 10.1158/1940-6207.CAPR-11-0420. [DOI] [PubMed] [Google Scholar]
- 34.Zammarchi F, de Stanchina E, Bournazou E, Supakorndej T, Martires K, Riedel E, Corben AD, Bromberg JF, Cartegni L. Antitumorigenic potential of STAT3 alternative splicing modulation. Proc Natl Acad Sci U S A. 2011;108:17779–17784. doi: 10.1073/pnas.1108482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Xu YH, Lu S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur J Surg Oncol. 2014;40:311–317. doi: 10.1016/j.ejso.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi S, Katada N, Sakuramoto S, Kobayashi N, Shimao H, Watanabe M, Hiki Y. Survival after surgical treatment of early gastric cancer: surgical techniques and long-term survival. Langenbecks Arch Surg. 2004;389:69–74. doi: 10.1007/s00423-004-0462-2. [DOI] [PubMed] [Google Scholar]
- 38.Zu H, Wang F, Ma Y, Xue Y. Stage-stratified analysis of prognostic significance of tumor size in patients with gastric cancer. PLoS One. 2013;8:e54502. doi: 10.1371/journal.pone.0054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao G, Chen J, Ren C, Li R, Du S, Xie G, Deng H, Yang K, Yuan Y. Robotic versus open gastrectomy for gastric cancer: A meta-analysis. PLoS One. 2013;8:e81946. doi: 10.1371/journal.pone.0081946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patru CL, Surlin V, Georgescu I, Patru E. Current issues in gastric cancer epidemiology. Rev Med Chir Soc Med Nat Iasi. 2013;117:199–204. [PubMed] [Google Scholar]
- 41.Wang SJ, Emery R, Fuller CD, Kim JS, Sittig DF, Thomas CR. Conditional survival in gastric cancer: a SEER database analysis. Gastric Cancer. 2007;10:153–158. doi: 10.1007/s10120-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 42.Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bharadwaj U, Eckols TK, Kolosov M, Kasembeli MM, Adam A, Torres D, Zhang X, Dobrolecki LE, Wei W, Lewis MT, Dave B, Chang JC, Landis MD, Creighton CJ, Mancini MA, Tweardy DJ. Drug-repositioning screening identified piperlongumine as a direct STAT3 inhibitor with potent activity against breast cancer. Oncogene. 2015;34:1341–1353. doi: 10.1038/onc.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palagani V, Bozko P, El KM, Belahmer H, Giese N, Sipos B, Malek NP, Plentz RR. Combined inhibition of Notch and JAK/STAT is superior to monotherapies and impairs pancreatic cancer progression. Carcinogenesis. 2014;35:859–866. doi: 10.1093/carcin/bgt394. [DOI] [PubMed] [Google Scholar]
- 45.Yoon J, Cho SJ, Ko YS, Park J, Shin DH, Hwang IC, Han SY, Nam SY, Kim MA, Chang MS, Lee HS, Kim WH, Lee BL. A synergistic interaction between transcription factors nuclear factor-kappaB and signal transducers and activators of transcription 3 promotes gastric cancer cell migration and invasion. BMC Gastroenterol. 2013;13:29. doi: 10.1186/1471-230X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, Rave-Frank M, Kramer F, Beissbarth T, Kitz J, Wienands J, Ghadimi BM, Ebner R, Ried T, Grade M. STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer. 2014;134:997–1007. doi: 10.1002/ijc.28429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


