Abstract
Objective: This study is to investigate effects of bisphenol A (BPA) on the blastocyst implantation in endometrium. Methods: Pregnant mice were orally administered with BPA. Implantation sites were examined, and serum estrogen level was assayed with ELISA. Protein expression levels were detected with immunohistochemistry and immunofluorescence staining. Results: High doses (400 and 600 mg/kg/day) of BPA remarkably reduced the implantation sites in the pregnant mice. No significant differences were observed in the serum estrogen level across the groups. Moreover, high doses (400 and 600 mg/kg/day) of BPA significantly declined the expression level of endometrial estrogen receptor α (ERα) in the pregnant mice. In addition, high doses (400 and 600 mg/kg/day) of BPA significantly declined the expression levels of integrin β3 and trophinin in the endometrium and blastocysts. Conclusion: BPA declines ERα expression in endometrium, and inhibits adhesion protein expression in endometrium and blastocysts, causing the adhesion failure of blastocyst implantation.
Keywords: Blastocyst implantation, bisphenol A (BPA), integrin β3, trophinin, estrogen receptor
Introduction
Bisphenol A (BPA), an estrogenic chemical, is one of the most widely used compounds in the production of high-polymer materials, such as epoxy resin, polysulfone resin, polyphenylene ether resin, and unsaturated polyester resin. BPA can act on the reproductive system and affect human health [1-4]. It has been demonstrated in male animals that, BPA reduces sperm motility and disturbs spermatogenesis, thus influencing fertility, libido levels, and sexual behavior [5-7]. Moreover, BPA promotes follicle atresia [8] and oocyte apoptosis [9], inhibits the expression of steroidogenic enzyme in granulosa cells [10], and interrupts the production and secretion of steroid hormones [11]. Furthermore, BPA has been shown to influence the endometrial receptivity at the implantation stage [12].
The endometrium development and endometrial receptivity, and the blastocyst implantation in the endometrium, are precisely regulated by estrogen. Integrin and trophinin are markers for the endometrial receptivity during the blastocyst implantation process, which mediate the adhesion between the blastocyst and endometrium [13,14]. It has been shown that, estrogen could activate the expression of integrin to improve the blastula adhesion in the endometrium [15]. Moreover, estrogen regulates the expression of trophinin in mouse endometrium, and no expression of trophinin could be detected in the uterus in ovariectomized mice [16]. On the other hand, the injection of BPA into the newborn female mice could significantly decrease the expression levels of estrogen receptor α (ERα) in the endometrium, which alters the Hoxa10 signaling pathway, and influences the endometrial decidualization, leading to the habitual abortion and infertility [17]. In addition, BPA could also affect the role of estrogen in the uterine development in rats, resulting in uterine abnormalities [18]. However, the effects of BPA on the expression levels of adhesion proteins have not yet been fully elucidated.
In this study, the fertilized mice were administered with BPA. The expression levels of estrogen, integrin β3, and trophinin in the uterine tissues and blastocysts were detected. The mechanisms for the effects of BPA on the blastocyst implantation were investigated.
Materials and methods
Chemicals and animals
All reagents were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA), unless otherwise stated. Kunming mice were purchased from the Changchun Hi-Tech Laboratory Animal Research Center (Changchun, Jilin, China). These mice were housed in with a 14 h/10 h day/night cycle. All the animal experiments were carried out following the protocol approved by the Jilin Medical University Institutional Animal Care and Use Committee.
Animal treatment and drug administration
The 2-month-old female mice were mated naturally with healthy fertile young males. In the next morning, the presence of a vaginal plug in the vagina was considered as day 0.5 of pregnancy. Not every female mouse with a plug would become pregnant. Mice with the presence of blastocysts in the reproductive tract were considered pregnant.
Pregnant females were randomly divided into four groups (n=30 per group). From day 0.5 to 4.5 of pregnancy, the pregnant females were daily gavaged with 0 (control), 200, 400, and 600 mg/kg/day BPA in the sesame oil, respectively. The estrogenicity of 100 mg/kg/day BPA was assumed to be equivalent to that of 0.01 mg/kg/day estrogen [19,20].
Implantation site staining and blastocyst collection
On day 5 of pregnancy, the mice were anesthetized by the intraperitoneal injection of chloral hydrate. The implantation sites were stained with Chicago sky blue, and the number of the implantation sites was counted. Uteri of the pregnant mice treated with or without BPA were flushed with PBS to collect the blastocysts. Uterine tissues were collected and fixed in 10% formalin at room temperature for 24 h before further processing.
Enzyme-linked immunosorbent assay (ELISA)
On day 5 of pregnancy, the mice were anesthetized with chloral hydrate. The trunk blood was collected, and serum was separated and stored at -20°C. The serum content of estrogen was determined with an ELISA kit (Shanghai Elisa Biotech Co., Ltd., Shanghai, China), according to the manufacturer’s instructions. The optical density (OD) was read with a Model 680 microplate reader (Bio-Rad, Hercules, CA, USA), and the estrogen level was determined based on the standard curve.
Immunohistochemistry
The uterine tissue was embedded with paraffin and cut into 5-μm serial sections. After the antigen retrieval, these sections were incubated with rabbit anti-mouse anti-ERα antibody (1:100 dilution; Santa Cruz Biotechnology, Dallas, TX, USA), or rabbit anti-mouse anti-integrin β3 antibody (1:100 dilution; Santa Cruz Biotechnology), at 4°C overnight. Then the sections were developed using the streptavidin-biotin peroxidase method. The staining was observed with a microscope (Leica, Heidelberg, Germany). Image analysis was performed using the Image Pro-Plus 6.0 software.
Immunofluorescence staining
For the uterine tissue staining, the sections were blocked with bovine serum albumin (BSA; Sigma-Aldrich, Munich, Germany) at room temperature for 1 h. They were incubated with rabbit anti-mouse anti-trophinin antibody (1:50 dilution; Santa Cruz Biotechnology) at 4°C overnight, and then incubated with FITC-conjugated secondary antibody (1:400 dilution; Life Technologies, Grand Island, NY, USA) at room temperature for 1 h. After staining with 1 μM Hoechst33342, the sections were detected with a laser-scanning confocal microscopy (FV-500; Olympus, Tokyo, Japan). Image analysis was performed with the Image J software (http://rsb.info.nih.gov/ij/).
For the blastocyst staining, the blastocysts were fixed with 4% paraformaldehyde at room temperature for 40 min, and permeabilized with 0.2% Triton X-100 for 10 min. The procedures were the same as above mentioned, and the primary antibody was replaced with the rabbit anti-mouse anti-integrin β3 polyclonal antibody (1:100 dilution; Santa Cruz Biotechnology) and rabbit anti-mouse anti-trophinin polyclonal antibody (1:100 dilution; Santa Cruz Biotechnology), respectively.
Statistical analysis
Data were expressed as mean ± SD. SPSS 13.0 software was used for statistical analysis. One-way ANOVA and LSD method were performed for group comparison. P < 0.05 was considered statistically significant.
Results
Effects of BPA on implantation site numbers in pregnant mice
To determine the effects of BPA on the blastocyst implantation, the implantation site was stained and the implantation site number was counted on day 5 of pregnancy. As shown in Figure 1, compared with the control group (treated with 0 mg/kg/day BPA), no significant difference was observed in the implantation site number for the mice treated with 200 mg/kg/day BPA (P > 0.05). However, in mice treated with 400 mg/kg/day BPA, the implantation site number was remarkably reduced compared with the control group (P < 0.05). Furthermore, no implantation sites were found in the mice treated with 600 mg/kg/day BPA (P < 0.05). These results suggest that BPA at higher concentrations (greater than 400 mg/kg/day) could significantly reduce the number of implantation sites in the pregnant mice.
Figure 1.
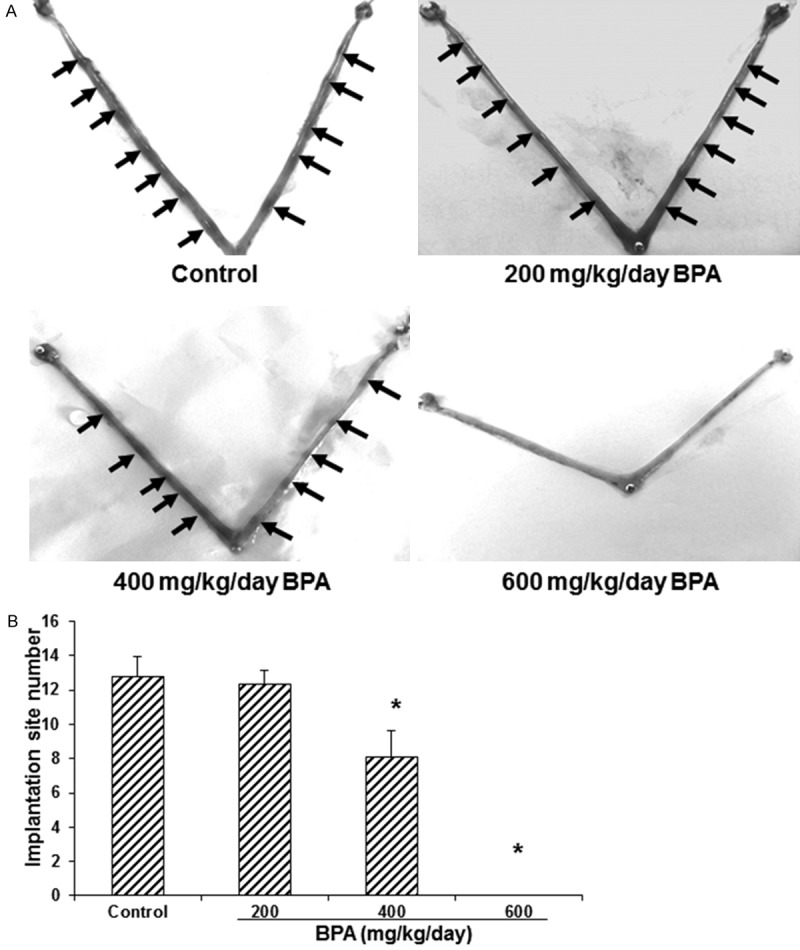
Effects of BPA on the implantation site numbers in pregnant mice. A. The implantation site staining was performed in mice treated with BPA on day 5 of pregnancy. Arrow indicated the implantation sites. B. Statistical analysis of the implantation site numbers in the pregnant mice (N=10-14). Compared with the control group, *P < 0.05.
Effects of BPA on serum estrogen level and endometrial ERα expression in pregnant mice
To investigate the effects of BPA on the secretion of ovarian steroid hormones and the expression of steroid hormone receptor, the serum estrogen level and the expression of ERα in the endometrium were detected with ELISA and immunohistochemistry, respectively. Our results from ELISA showed that, no significant differences were observed in the serum estrogen concentration across all the groups (P > 0.05) (Figure 2A). On the other hand, our results from the immunohistochemistry showed that, compared with the control group, the expression level of endometrial ERα was not significantly altered in the mice treated with 200 mg/kg/day BPA (P > 0.05). However, the expression levels of endometrial ERα were significantly declined in the mice treated with 400 and 600 mg/kg/day BPA, compared with the control group (both P < 0.05), in a dose dependent manner (Figure 2B, 2C). Taken together, these results suggest that, BPA could not significantly influence the secretion of ovarian estrogen, however high concentrations of BPA could significantly reduce the expression levels of ERα in the endometrium in pregnant mice.
Figure 2.
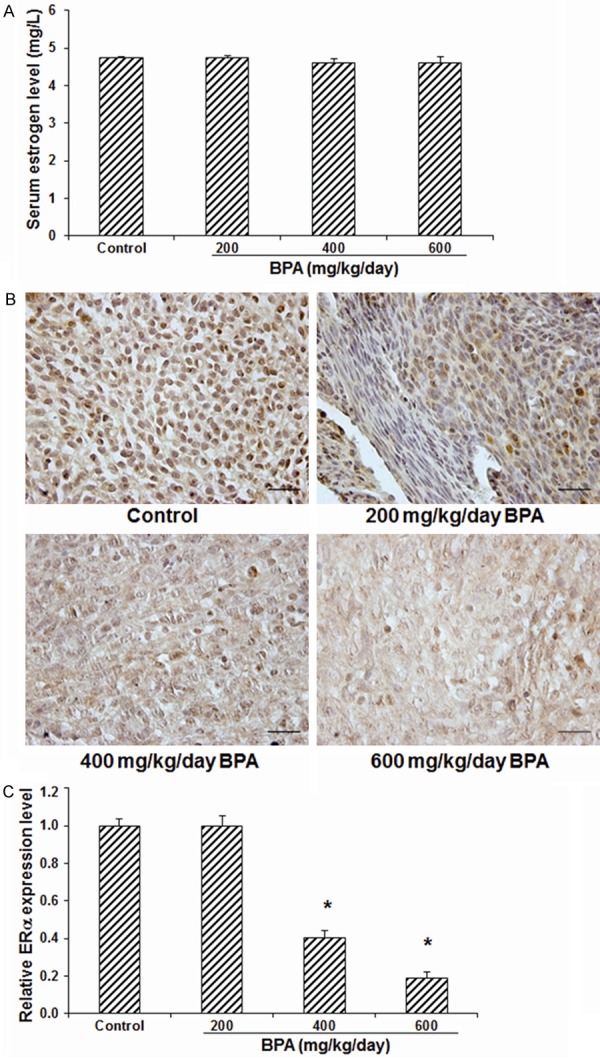
Effects of BPA on serum estrogen level and endometrial ERα expression in pregnant mice. A. The serum estrogen levels in mice treated with BPA on day 5 of pregnancy were determined with ELISA (N=7-9). B. The expression levels of estrogen in the uterine tissues were detected with immunohistochemistry. Scale bar, 50 μm. C. Statistical analysis of the estrogen expression levels. Compared with the control group, *P < 0.05.
Effects of BPA on integrin β3 and trophinin expression in uterine tissues
To investigate the effects of BPA on the blastocyst implantation-related adhesion proteins, the expression levels of integrin β3 and trophinin in the endometrial epithelium and glands were detected with immunohistochemistry or immunofluorescence staining. Our results showed that, compared with the control group, the treatment of 200 mg/kg/day BPA did not significantly change the expression level of integrin β3 (Figure 3) or trophinin (Figure 4) in either endometrial epithelium or glands. However, in the mice treated with 400 or 600 mg/kg/day BPA, both the expression levels of integrin β3 (Figure 3) and trophinin (Figure 4) in the endometrial epithelium and glands were significantly declined (all P < 0.05). These results suggest that, the BPA treatment at high concentrations could significantly decrease the expression levels of blastocyst implantation-related adhesion proteins in endometrial epithelium and glands, which might affect the adhesion between blastocyst and endometrium.
Figure 3.
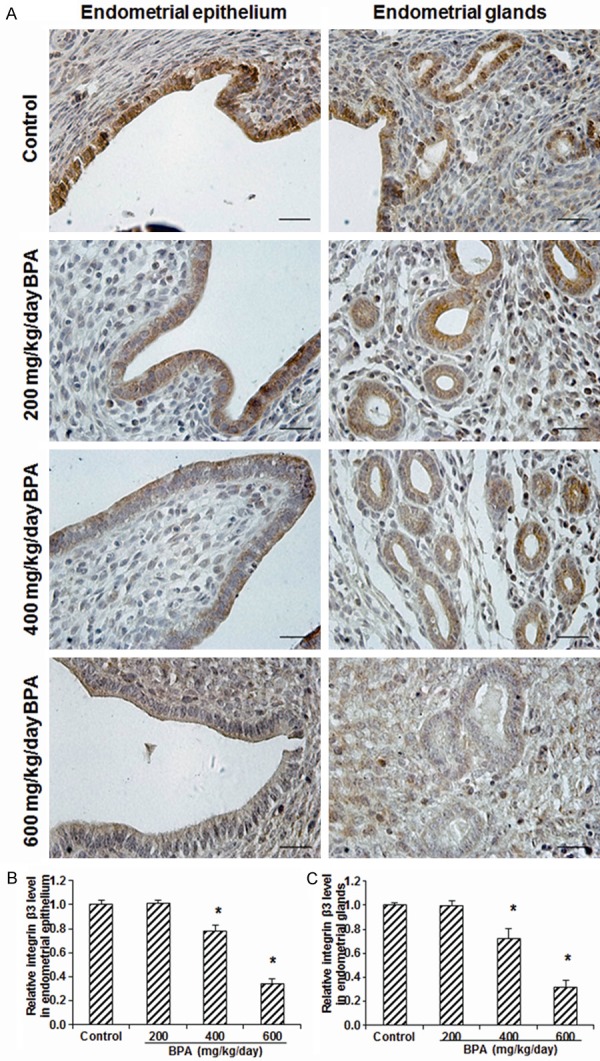
Effects of BPA on the integrin β3 expression levels in the uterine tissues. (A) The expression levels of integrin β3 in the endometrial epithelium and glands in mice treated with 0 (control), 200, 400, and 600 mg/kg/day BPA on day 5 of pregnancy were detected with immunohistochemistry. Scale bar, 50 μm. (B, C) Statistical analysis of the integrin β3 expression levels in the endometrial epithelium (B) and glands (C), respectively. Compared with the control group, *P < 0.05.
Figure 4.
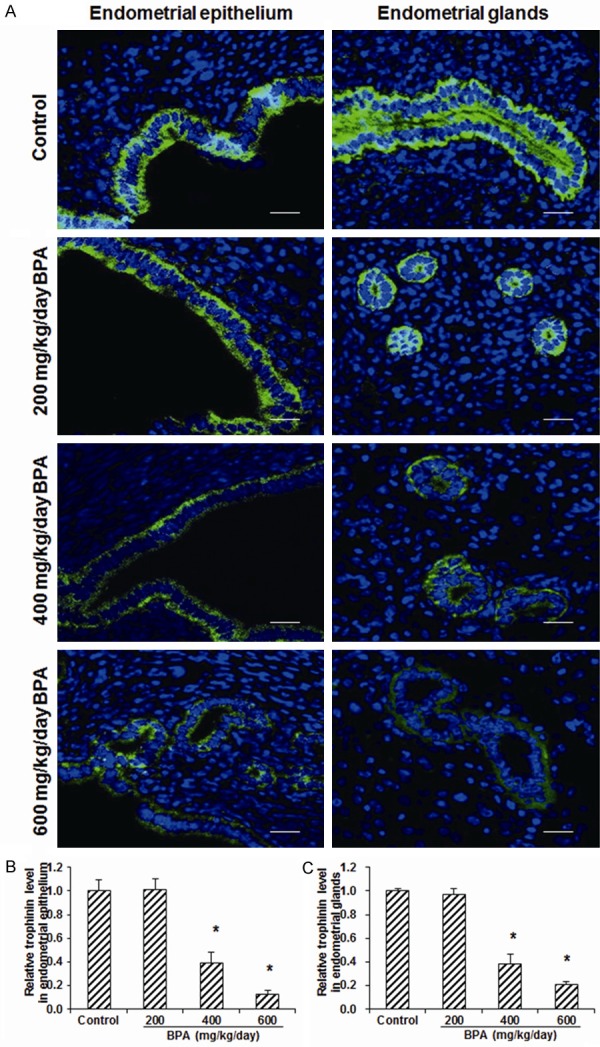
Effects of BPA on the trophinin expression levels in the uterine tissues. (A) The expression levels of trophinin in the endometrial epithelium and glands in mice treated with 0 (control), 200, 400, and 600 mg/kg/day BPA on day 5 of pregnancy were detected with immunofluorescence staining (green for trophinin). DNA was stained with Hoechst33342 (blue). Scale bar, 50 μm. (B, C) Statistical analysis of the trophinin expression levels in the endometrial epithelium (B) and glands (C), respectively. Compared with the control group, *P < 0.05.
Effects of BPA on integrin β3 and trophinin expression in blastocysts
The effects of BPA on the expression levels of integrin β3 and trophinin in D5 blastocysts were then detected with the immunofluorescence staining. Our results showed that, the polar expression pattern was observed for integrin β3 (Figure 5) and trophinin (Figure 6) in the blastocysts, which might develop into one pole of the blastocyst. No significant difference was observed in the expression level of integrin β3 (Figure 5) or trophinin (Figure 6) between blastocysts from the control group and the mice treated with 200 mg/kg/day BPA. However, compared with the control group, the treatments of 400 and 600 mg/kg/day BPA significantly decline the expression levels of integrin β3 (Figure 5) and trophinin (Figure 6) in the blastocysts (all P < 0.05). These results suggest that, in addition to the endometrium, the treatment of BPA at high concentrations could significantly decrease the expression levels of blastocyst implantation-related adhesion proteins in the blastocysts.
Figure 5.
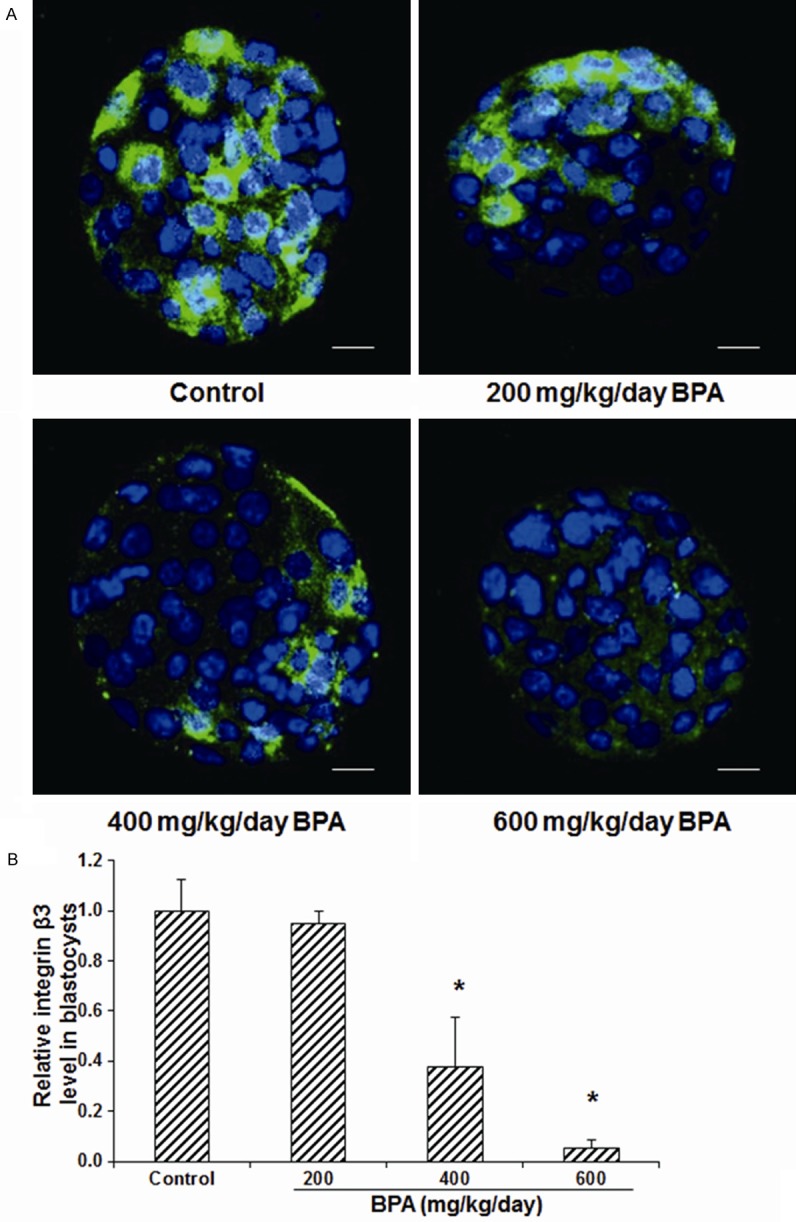
Effects of BPA on the integrin β3 expression levels in the blastocysts. A. The expression levels of integrin β3 in the blastocysts in mice treated with 0 (control), 200, 400, and 600 mg/kg/day BPA on day 5 of pregnancy were detected with immunofluorescence staining (green for integrin β3). DNA was stained with Hoechst33342 (blue). Scale bar, 50 μm. B. Statistical analysis of the expression levels of integrin β3 in the blastocysts. Compared with the control group, *P < 0.05.
Figure 6.
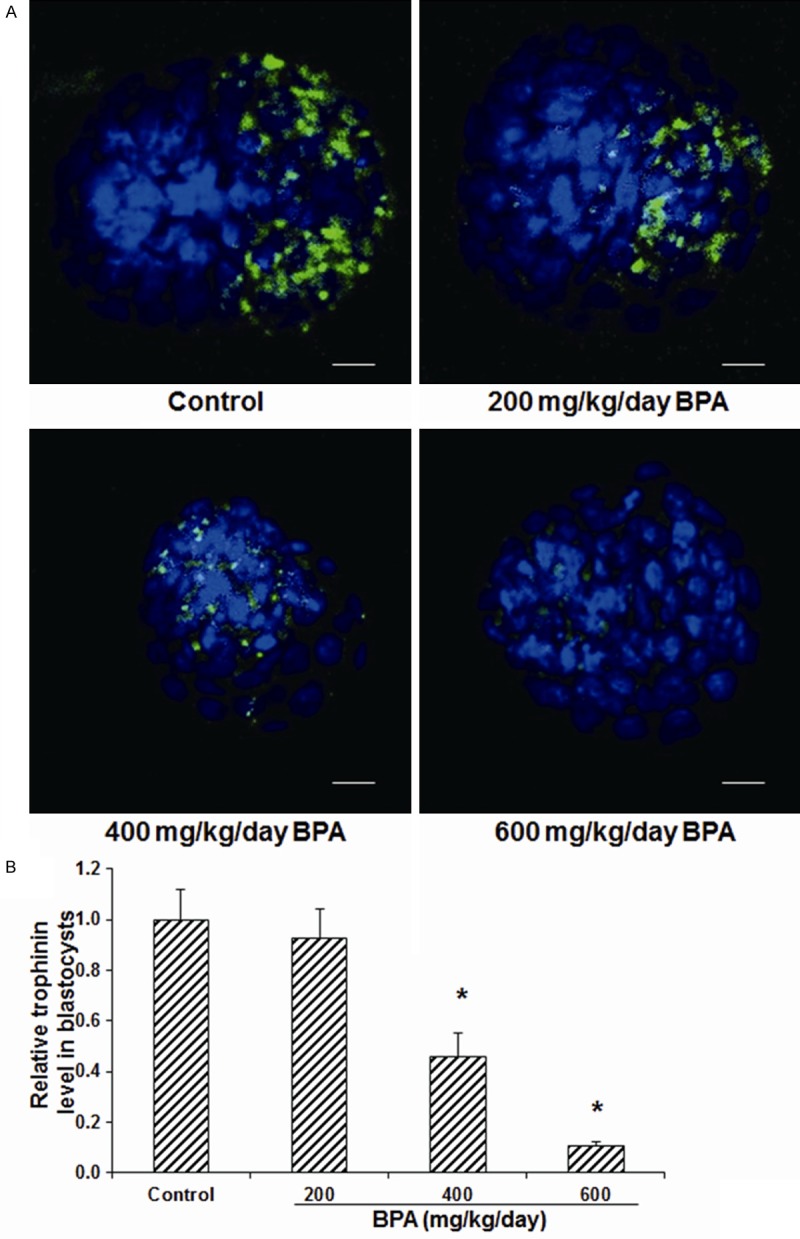
Effects of BPA on the trophinin expression levels in the blastocysts. A. The expression levels of trophinin in the blastocysts in mice treated with 0 (control), 200, 400, and 600 mg/kg/day BPA on day 5 of pregnancy were detected with immunofluorescence staining (green for trophinin). DNA was stained with Hoechst33342 (blue). Scale bar, 50 μm. B, C. Statistical analysis of the expression levels of trophinin in the blastocysts. Compared with the control group, *P < 0.05.
Discussion
Studies have shown that BPA can affect blastocyst implantation in endometrium, however the underlying mechanisms are still unclear. In this study, to investigate the effects of BPA on the blastocyst implantation, the fertilized mice were subjected to the BPA treatment with different concentrations. Our results showed that, the BPA treatment could significantly reduce the implantation site number, and decline the expression levels of implantation-related adhesion proteins in the endometrium and blastocysts. Moreover, BPA did not influence the secretion of estrogen in pregnant mice, however the treatment significantly declined the expression levels of ERα in the endometrium, therefore reducing the endometrial sensitivity to estrogen. In line with this, a previous study has shown that, BPA disturbs the expression levels of Hoxa10 and Hoxa11, influences the normal development of endometrium, and reduces the endometrial sensitivity to sex hormones, resulting in the blastocyst implantation failure in endometrium [17]. Taken together, these findings suggest that the endocrine disruptor BPA would disrupt the normal uterine function through interfering with the hormonal action.
The effects of BPA on the reproductive system depend on the animal models, developmental stage, and dosage. Previous studies have confirmed the effects of BPA on the adolescent initiation, sexual cycle, and ovary structure [21-23]. Based on these results, BPA might act on the reproductive capacity via its toxic effects on the multiple sites along the hypothalamus-pituitary gland-gonad axis. Moreover, the BPA treatment at high concentrations could significantly reduce the expression levels of ERα in the endometrium, without affecting the serum estrogen level. La et al. [24] have shown that, the treatment of BPA could significantly decline the mRNA and protein expression levels of ERα in the MCF-7 cells, therefore reducing the stimulating effects of estrogen to the target cells. In our study, BPA reduced the expression level of ERα in the endometrium, which would influence the expression levels of the implantation-related proteins regulated by estrogen.
During the blastocyst implantation, integrin β3 is one of the key molecules involved in the recognition and adhesion between the blastocyst and endometrium, which is considered to be the connection molecule between the blastocyst and endometrium [25]. It has been shown that the expression levels of integrin β3 in the endometrium and blastocyst depend on estrogen, which could stimulate the endometrial gland to secrete osteopontin (OPN). OPN could subsequently activate integrin in the blastocysts, and form the adhesion complex, thereby activating the blastocyst adhesion [15]. Integrin β3 has also been recognized as a marker for the endometrial receptivity, whose expression change is the main cause for the unexplained infertility in females [26]. In this study, the BPA treatment at high concentrations significantly declined the expression levels of integrin β3 in the endometrium and blastocysts. This might be due to the fact that BPA reduced the expression level of ERα in the endometrium, which disturbed the expression levels of estrogen-dependent integrin β3 and its activators.
Trophinin is one of the recently discovered cell adhesion molecules, which is specifically expressed during the implantation window in endometrial cells and blastocyst trophoblast cells [27]. Immunofluorescence staining has indicated the polar distribution pattern of trophinin in the blastocysts in monkeys and apes, which might contribute to the recognition between the blastocyst and endometrium. Suzuki et al. [16] have found that, in the uterus of pregnant mice, the timing of trophinin expression coincides with the blastocyst implantation process, and the trophinin expression is regulated by estrogen secreted by ovary, which could not be induced by the progesterone or the implanting blastocyst. In this study, our results showed that, the BPA treatment at high concentrations could decline the expression levels of trophinin in the endometrium and blastocysts.
Different drug administration approaches and dosages might influence the effects of BPA on the blastocyst implantation. Our results showed that 400 mg/kg/day BPA by gavage could affect the blastocyst implantation. Moreover, no implantation sites were observed in the pregnant mice treated with 600 mg/kg/day BPA, indicating the fully inhibited blastocyst implantation. Xiao et al. [12] have shown that the subcutaneous injection of BPA at 100 mg/kg/day could inhibit the blastocyst implantation, indicating that the subcutaneous injection of BPA was more efficient compared with the oral administration. However, the most common administration approach of BPA for humans is the oral administration with food packaging [28]. Therefore, the oral administration was used in this study to better simulate the adverse effects in humans. On the other hand, different administration timing of BPA might also lead to differential effects on the blastocyst implantation. Berger et al. [29] have found that on day 0 or 1 of pregnancy, the subcutaneous injection of 10.125 mg/mouse (approximately 400 mg/kg/day) of BPA would significantly reduce the number of implantation sites in pregnant mice. However, the injection of BPA at the same dosage on day 2 of pregnancy did not dramatically influence the blastocyst implantation site number. These findings indicate that the mice are more sensitive to the endocrine disrupter treatment on day 0.5 to 1.5 of pregnancy than on day 2.5 of pregnancy. Further in-depth studies are still needed to investigate the influence of different administration timing of BPA with oral administration on the drug efficiency.
In conclusion, our results showed that the BPA treatment at high concentrations could significantly reduce the blastocyst implantation in the endometrium. BPA could reduce the expression levels of ERα in the endometrium, and inhibit the expression levels of estrogen-dependent adhesion proteins in the endometrium and blastocysts, therefore inducing the blastocyst implantation adhesion failure in the endometrium. These findings might contribute to the understanding of the molecular mechanism for the endometrial receptivity, and the risks of adverse effects of the BPA exposure for the reproductive system.
Acknowledgements
This study was supported by the Scientific and Technological Research Project of Jilin Province (20140204033YY), the Natural Science Foundation Project of Shandong Province (ZR2011HL005), and the Open Foundation Projects from the State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (KF2014-12).
Disclosure of conflict of interest
None.
References
- 1.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 2.Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30:2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varayoud J, Ramos JG, Bosquiazzo VL, Muñozde-Toro M, Luque EH. Developmental exposure to Bisphenol A impairs the uterine response to ovarian steroids in the adult. Endocrinology. 2008;149:5848–5860. doi: 10.1210/en.2008-0651. [DOI] [PubMed] [Google Scholar]
- 5.Aikawa H, Koyama S, Matsuda M, Nakahashi K, Akazome Y, Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315:119–24. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- 6.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85:742–752. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Toyama Y, Yuasa S. Effects of neonatal administration of 17beta-estradiol, beta-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reprod Toxicol. 2004;19:181–188. doi: 10.1016/j.reprotox.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Rivera OE, Varayoud J, Rodríguez HA, Muñozde-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32:304–312. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 9.Brieño-Enríquez MA, Reig-Viader R, Cabero L, Toran N, Martínez F, Roig I, Garcia Caldés M. Gene expression is altered after bisphenol A exposure in human fetal oocytes in vitro. Mol Hum Reprod. 2012;18:171–183. doi: 10.1093/molehr/gar074. [DOI] [PubMed] [Google Scholar]
- 10.Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSHstimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect. 2010;118:400–406. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli F, Baratta L, Baioni L, Bussolati S, Ramoni R, Grolli S, Basini G. Bisphenol A disrupts granulosa cell function. Domest Anim Endocrinol. 2010;39:34–39. doi: 10.1016/j.domaniend.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and utetine receptivity in mice. Reprod Toxicol. 2011;32:434–441. doi: 10.1016/j.reprotox.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang YJ, Forbes K, Carver J, Aplin JD. The role of the osteopontin-integrin αvβ3 interaction at implantation: functional analysis using three different in vitro models. Hum Reprod. 2014;29:739–749. doi: 10.1093/humrep/det433. [DOI] [PubMed] [Google Scholar]
- 14.Tamura N, Sugihara K, Akama TO, Fukuda MN. Trophinin-mediated cell adhesion induces apoptosis of human endometrial epithelial cells through PKC-δ. Cell Cycle. 2011;10:135–143. doi: 10.4161/cc.10.1.14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaen T, Konno T, Egashira M, Bai R, Nomura N, Nomura S, Hirota Y, Sakurai T, Imakawa K. Estrogen-dependent uterine secretion of osteopontin activates blastocyst adhesion competence. PLoS One. 2012;7:e48933. doi: 10.1371/journal.pone.0048933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N, Nadano D, Paria BC, Kupriyanov S, Sugihara K, Fukuda MN. Trophinin expression in the mouse uterus coincides with implantation and is hormonally regulated but not induced by implanting blastocysts. Endocrinology. 2000;141:4247–4254. doi: 10.1210/endo.141.11.7738. [DOI] [PubMed] [Google Scholar]
- 17.Varayoud J, Ramos JG, Bosquiazzo VL, Lower M, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology. 2011;152:1101–1011. doi: 10.1210/en.2009-1037. [DOI] [PubMed] [Google Scholar]
- 18.Vigezzi L, Bosquiazzo VL, Kass L, Ramos JG, Muñoz-de-Toro M, Luque EH. Developmental exposure to bisphenol A alters the differentiation and functional response of the adult rat uterus to estrogen treatment. Reprod Toxicol. 2015;52:83–92. doi: 10.1016/j.reprotox.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Milligan SR, Balasubramanian AV, Kalita JC. Relative potency of xenobiotic estrogens in an acute in vivo mammalian assay. Environ Health Perspect. 1998;106:23–26. doi: 10.1289/ehp.9810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings AM, Laws SC. Assessment of estrogenicity by using the delayed implanting rat model and examples. Reprod Toxicol. 2000;14:111–117. doi: 10.1016/s0890-6238(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Thakur MK. Perinatal exposure to bisphenol-A impairs spatial memory through upregulation of neurexin1 and neuroligin 3 expression in male mouse brain. PLoS One. 2014;9:e110482. doi: 10.1371/journal.pone.0110482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Peluso ME, Munnia A, Ceppi M. Bisphenol-A exposures and behavioural aberrations: median and linear spline and meta-regression analyses of 12 toxicity studies in rodents. Toxicology. 2014;325:200–208. doi: 10.1016/j.tox.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Sadowski RN, Wise LM, Park PY, Schantz SL, Juraska JM. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience. 2014;279:122–131. doi: 10.1016/j.neuroscience.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Rosa P, Pellegrini M, Totta P, Acconcia F, Marino M. Xenoestrogens alter estrogen receptor (ER) α intracellular levels. PLoS One. 2014;9:e88961. doi: 10.1371/journal.pone.0088961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu LH, Sun LH, Hu YL, Jiang Y, Liu HY, Shen XY, Jin XY, Zhen X, Sun HX, Yan GJ. PCAF impairs endometrial receptivity and embryo implantation by down-regulating β3-integrin expression via HOXA10 acetylation. J Clin Endocrinol Metab. 2013;98:4417–4428. doi: 10.1210/jc.2013-1429. [DOI] [PubMed] [Google Scholar]
- 26.Franasiak JM, Holoch KJ, Yuan L, Schammel DP, Young SL, Lessey BA. Prospective assessment of midsecretory endometrial leukemia inhibitor factor expression versus ανβ3 testing in women with unexplained infertility. Fertil Steril. 2014;101:1724–1731. doi: 10.1016/j.fertnstert.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda MN, Sugihara K. Cell adhesion molecules in human embryo implantation. Sheng Li Xue Bao. 2012;64:247–258. [PubMed] [Google Scholar]
- 28.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(Suppl 6):S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 29.Berger RG, Shaw J, de Catanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17beta-estradiol. Reprod Toxicol. 2008;26:94–99. doi: 10.1016/j.reprotox.2008.06.007. [DOI] [PubMed] [Google Scholar]


