Abstract
TNFα played a dominant role in the development and progression of rheumatoid arthritis (RA). Clinical trials proved the efficacies of anti-TNFα agents for curing RA. However, most researchers were concentrating on their abilities of neutralizing TNFα, the potencies of different anti-TNFα agents varied a lot due to the antibody-dependent cell-mediated cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC). For better understanding and differentiating the potentiality of various candidate anti-TNF reagents at the stage of new drug research and development, present study established a cell model expressing the transmembrane TNFα for usage in in vitro ADCC or CDC assay, meanwhile, the assay protocol described here could provide guidelines for screening macromolecular antibody drugs. A stable cell subline bearing transmembrane TNFα was first established by conventional transfection method, the expression of transmembrane TNFα was approved by flow cytometer, and the performance of the stable subline in ADCC and CDC assay was evaluated, using human peripheral blood mononuclear cells as effector cells, and Adalimumab as the anti-TNFα reagent. The stable cell subline demonstrated high level of surface expression of transmembrane TNFα, and Adalimumab exerted both ADCC and CDC effects on this cell model. In conclusion, the stable cell line we established in present research could be used in ADCC or CDC assay for screening antibody drugs, which would provide in-depth understanding of the potencies of candidate antibody drugs in addition to the traditional TNFα neutralizing assay.
Keywords: Transmembrane TNFα, antibody-dependent cell-mediated cytotoxicity, complement dependent cytotoxicity, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory disease, the self-immune system of the patient who is suffering from this disease destroys synovial joints and accessory structures. Wrists, fingers, knees, feet, and ankles are the most commonly affected body sites. If this disease progresses further, the autoimmune condition can bring additional articular complications within several other body organs [1]. Patients can be diagnosed with RA at any age, but more is commonly seen in their middle age. Approximately 1% adult around the world is affected by RA. Regarding the gender, the frequency of RA happening to women is 2 or 3 times higher than man [2]. Although RA has been characterized long ago, the etiology is still not fully revealed. Some environmental and genetic elements are presumed to be potential precipitating factors [3]. However, recent biomedical research and clinical trials proved that tumor necrosis factor α (TNFα) played dominant roles in RA [4].
Human TNFα consists of the following three domains, a 35 amino acid (aa) cytoplasmic domain, a 21 aa transmembrane segment, and a 177 aa extracellular domain (ECD). TNFα is a pleiotropic molecule playing central roles in inflammation, apoptosis, and immune system development. A broad array of immune and epithelial cells can produce TNFα, like monocytes, macrophages, T and B cells, even fibroblasts. It’s the key cytokine that triggers inflammatory response in RA patients [5]. It acts as a paracrine inducer of other inflammation-associated cytokines, including interleukin-1, interleukin-6, as well as granulocyte monocyte-colony stimulating factor (GM-CSF) [2]. In the cultured synovial cells derived from patients with RA, blockade of TNFα significantly inhibited the secretion of interleukin 1, interleukin 6, as well as GM-CSF [6]. Moreover, clinical study also found RA patients with high level of TNFα in the synovial fluid, and anti-TNFα therapy shifted the cytokine equilibrium resulting in more anti-inflammatory cytokines [7]. All these existing reports support the irreplaceable role of TNFα during the progression of RA. And pharmaceutical companies across the world are dedicating their efforts to design and develop new drugs targeting TNFα. Some macromolecular drugs have been clinically used and could effectively remit the pains caused by RA, like Etanercept [8], Infliximab [9], and Adalimumab [10]. All these macromolecular drugs can inactivate TNFα through a physically binding mechanism, preventing it from activating TNF receptors, which is a common feature shared by these three antibody drugs. Therapeutic antibody drugs have been successfully used in clinical trials and their potentiality is influenced by multiple factors [11], as the clinical outcome using the three anti-TNFα agents is not comparable, which may cause by antibody-dependent cell-mediated cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC) to the transmembrane TNFα-bearing cells [12]. Therefore, it becomes necessary to develop a simple cell model to systematically evaluate the biologic effects of candidate anti-TNFα agents via ADCC or CDC. In present research, we developed a stable CHO suspension cells with transmembrane TNFα expression, and further validated the performance of the stable cell line with Adalimumab in ADCC and CDC assay.
Materials and methods
Cell line and reagents
The CHO suspension cells were purchased from Gbico, Invitrogen, and were routinely maintained in Gibco® FreeStyle™ CHO Expression Medium. The L929 cell line was purchased from ATCC (Cat No. CCL-1). Adalimumab was a product of Abbott. PE-labeled anti-transmembrane TNFα (CatNo. FAB210P) and the mouse IgG1 isotype control (CatNo. 1C002P) were both purchased from R & D, as well as the recombinant TNFα (CatNo. 210-TA-100). The CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) was a product of Promega (CatNo. G3581). LDH detection kit was produced by Roche (CatNo. 11644793001). Lymphoprep™ for isolating peripheral blood mononuclear cell (PBMC) was from Axis-Shield (CatNo. 1114547). The usage of the human blood samples from healthy donors has been approved by the ethic committee of the second affiliated hospital of Shenyang Medical College.
Development of stable cell line expressing transmembrane TNFα
The sequence corresponding to the transmembrane TNFα was synthesized by GenScript, USA, and subcloned into pcDNA3.1+ vector. And after linearization, the recombinant expression vector was transfected into CHO suspension cells with Lipofectamine 2000. At 24 hourspost transfection, 500 µg/ml G418 was supplemented into the culture medium to screen the stable clones. After continuously cultured for 14 days, the stable cell pool was obtained. Then, the single-cell-derived stable clones were sorted by BD FACSJazz™ after stained with the PE-labeled anti-transmembrane TNF alpha antibody.
Determination of the surface expression of transmembrane TNFα
Approximately 1×106 cells were harvested by centrifugation at 800× g for 5 min at 4°C. Prior to staining the cells with PE-labeled primary antibody, the cells were washed with pre-cold PBS for three times, and then incubated with primary antibody at 4°C for 1 h in dark. After being washed for three times, the surface expression of transmembrane TNFα was analyzed with BD FACSCalibur.
TNFα neutralization assay
Approximately, 1.5×104 cells/well were pre-seeded into a 96-well-plate, and incubated overnight in a 37°C, 5% CO2 incubator. Adalimumab was prepared at 3× stock solution by 5-fold serial dilution starting from 2666.7 ng/ml. TNFα was prepared at 3× stock solution with final concentration of 3 ng/ml. Actinomycin D was also prepared at 3× stock solution of 3 µg/ml and was protected from light. The L929 cells were then incubated for extra 16 hours in the culture medium containing 1 µg/ml actinomycin D, 1 ng/ml TNFα, and Adalimumab at indicated concentration. Before assay of the samples, culture medium was carefully removed from each well and 50 µl Cell Titer solution was added into each well. After incubation for 10 minutes, the luminescence was read out on a PHERStar. The value from blank well (assay medium only) was subtracted before analysis.
Isolation of PBMC
PBMC was isolated according to the manual of Lymphoprep™. Briefly, peripheral blood samples from the healthy donors were collected into a tube containing anticoagulant (EDTA, heparin). Before isolation of PBMC, the blood samples were diluted by addition of an equal volume of 0.9% NaCl. 6 ml diluted blood samples were carefully loaded over 3 ml Lymphoprep reagent in a 15 ml centrifuge tube. After centrifugation at 800× g for 20 minutes at 20°C in a swing-out rotor, a distinct band was visualized at the sample/medium interface, and removed into a new tube with Pasteur pipettes without disturbing the upper layer. The harvested fraction was diluted further with 0.9% NaCl to reduce the density of the solution and centrifuged for 10 minutes at 250× g to pellet the lymphocytes.
ADCC assay
The transmembrane TNFα-bearing CHO-S cell line was used as target cells; while the PBMC isolated from human peripheral blood was used as effector cells. All cells were routinely maintained in their corresponding culture medium. At the day of conducting the ADCC assay, both target cells and effector cells were washed three times with the assay buffer consisting of 99% Freestyle CHO Expression Medium and 2% FBS. 100 µl of PBMCs (effector cells), with effector cells: target cells (E:T) ratios of 25:1, were added into the 96-well-plate containing target cells and Adalimumab at indicated serial concentrations. 1% Triton X-100 was added to the control wells without effector cells and Adalimumab to completely lysate the target cells, reading value from these wells represented the maximal lysis capacity; assay buffer was added into other control wells without effector cells and Adalimumab and reading value served as minimal lysis control. Target cells incubated with effector cells without Adalimumab were taken asnoisy background to exclude the non-specific LDH release. The plate was incubated in a 37°C, 5% CO2 incubator for additional 6 hours. After that, the cell viability was calculated.
CDC assay
For CDC assay, the transmembrane TNFα-bearing CHO-S cell line served as target cells. 5,000 cells/well in assay buffer were seeded into a 96-well-plate. 10 µl diluted Adalimumab was added into each well at the indicated final concentration, as well as the human IgG control. After incubation at room temperature for 30 minutes, 10 µl normal human serum or heat-inactivated human serum was supplemented into each well to initiate the CDC cascade. At 2 hours post addition of normal human serum, the cell viability was determined using Cell Titer kit.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). p value of <0.05 was accepted as statistically significant.
Result
Development of stable CHO suspension cells bearing transmembrane TNFα expression
Transmembrane TNFα is a precursor of the soluble form of TNFα, consisting of 233 amino acid residues. After being cleaved by the TNFα-converting enzyme (TACE) between the residue alanine and valine, the mature and soluble form of TNFα with 157 amino acids will be released and secreted to exert its biological function. In present research, the sites that TACE recognized were completely removed, and the transmembrane part of TNFα supposed to be retained on the surface of the host cell line. For better proving and understanding this assumption, the surface expression of transmembrane TNFα was probed by flow cytometer. And our data demonstrated that transmembrane TNFα was successfully halted on the cell surface, being protected from cleavage by TACE (Figure 1). Meanwhile, the efficiency of stable TNFα expression CHO cell model has also been examined. The results indicated that the efficiency of stable expression CHO cell line achieved to 83.6%, compared to the normal CHO cell (4.3%) (Figure 2).
Figure 1.
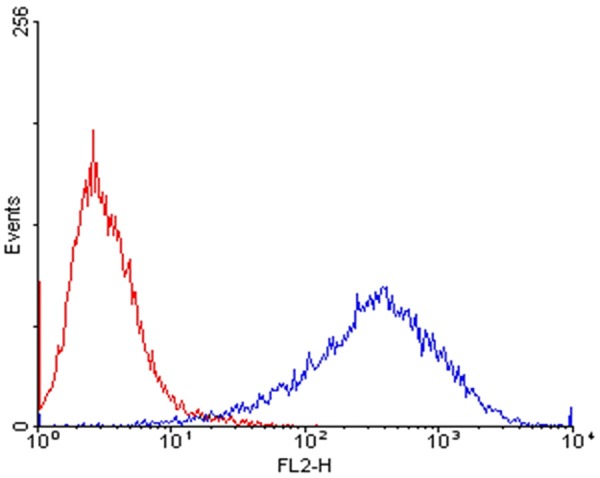
FACS analysis of surface expression of transmembrane TNFα. Red line represented untransduced parental cells, and the blue line represented the stable cell clones.
Figure 2.
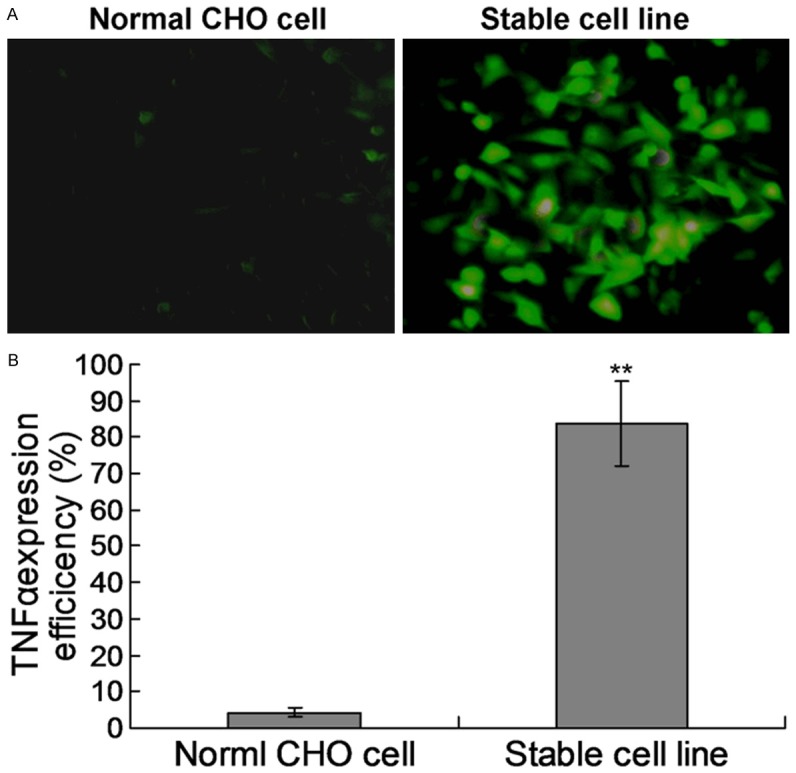
Examination of the stable expression CHO cell line. A. The immunofluorescence examination for TNFα. The TNFα on the CHO cell surface was detected by the FITC-labled mouse anti-TNFα antibody. B. Statistical analysis for the stable CHO cell line expression.
Adalimumab could neutralize the bioactivity of TNFα
Adalimumab has been designed to inhibit TNFα activity [13]. In present study, before it was used for ADCC or CDC assay, its biological activity was first probed by the neutralization assay. Our data showed that TNFα could induce L929 cells into apoptosis in a dose-dependent manner (Figure 3). After being applied at indicated concentration into the TNFα containing medium, Adalimumab could efficiently counteract the cytotoxicity effects of TNFα with maximal 100% neutralization.
Figure 3.
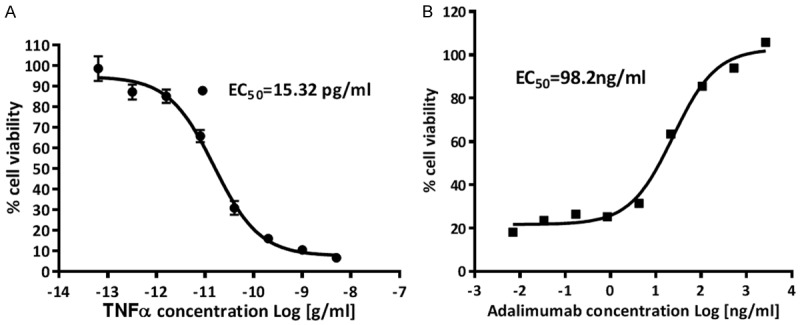
Cell viability assay A: TNFα demonstrated cytotoxicity to L929 cells in a dose-dependent manner; B: Adalimumab could neutralize the apoptotic effects of TNFα.
As a control, the direct effects of Adalimumab to L929 cellular viability were also investigated. As shown in Figure 4, Adalimumab could not cause cell death significantly, except the case of highest concentration. Our data indicated Adalimumab could function properly.
Figure 4.
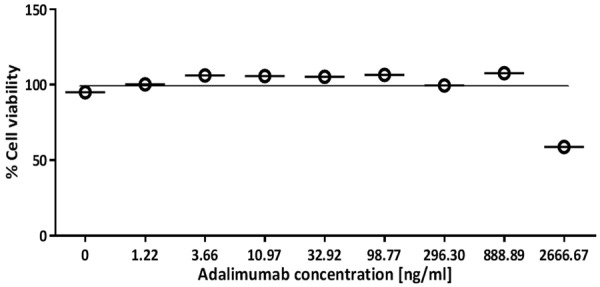
Effects of Adalimumab to L929 cellular viability.
ADCC by Adalimumab
PBMCs were used as effector cells and incubated with transmembrane TNFα-expressing CHO suspension cells (Target cells) at an fixed E:T ratio (25:1) in the presence of Adalimumab at indicated concentration. As shown in Figure 5, 0.001 µg/ml Adalimumab could produce a detectable ADCC at E:T ratio of 25:1; when the concentration reached up to 10 µg/ml, Adalimumab generated a specific cell lysis of up to 76.01%, while the control IgG almost did not cause any target cell lysis (less than 5%), indicating the transmembrane-bearing CHO suspension cells demonstrated specific ADCC effects mediated by those antibody drugs targeting TNFα.
Figure 5.
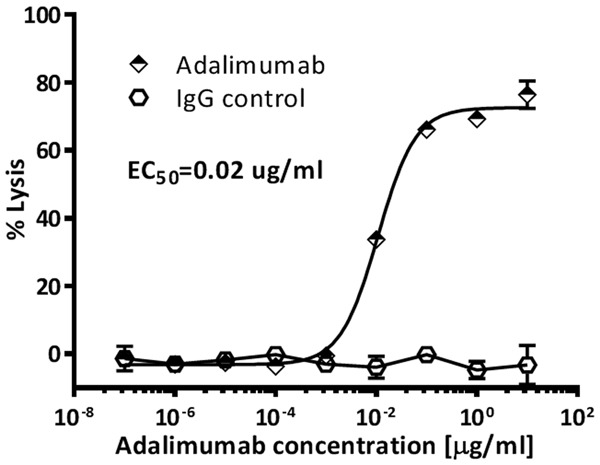
Antibody-dependent cell-mediated cytotoxicity of anti-TNFα agents against transmembrane TNFα-expressing cells.
CDC by Adalimumab
Adalimumab was incubated with the transmembrane TNFα-expressing CHO suspension cells for 2 hours in the presence of freshly prepared human normal serum to investigate the CDC activities. Adalimumab showed significant cytotoxicity to this transmembrane TNFα-expressing CHO suspension cells. As shown in Figure 6, the percentages of lysed cells induced by Adalimumab kept increasing in a dose-dependent manner. 10 µg/ml Adalimumab induced 90.6% cell death, and the cytotoxicity effects of Adalimumab was almost completely abolished by heat-inactivated human serum, suggesting that the cytotoxic effects were specifically induced by activation of complement.
Figure 6.
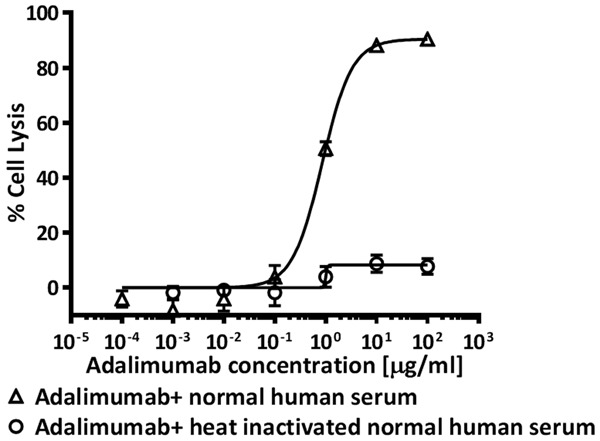
Complement-dependent cytotoxicity of anti-TNF agents against transmembrane TNF-expressing cells.
Discussion
Therapeutic antibody drugs have been successfully used in clinical trials,and up to nowabout five anti-TNF drugs that have been approved by U.S. Food and Drug Administration to treat moderate to severe RA that has not responded to one or more of the traditional disease-modifying anti-rheumatic drugs. However, data from clinical application demonstrated that the potency of these agents was not identical. One of the possible reasons that bring such difference is the antibody-dependent cell-mediated cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC) to the transmembrane TNFα-bearing cells [12]. However, most drug developers are focusing on their abilities of neutralizing TNF alpha, overlooking the ADCC or CDC effects trigged by these antibody drugs. Hereby we developed a transmembrane-bearing CHO suspension cells as the target cells in ADCC or CDC assay, and fully described the assay protocols using human peripheral blood monocyte cells as effector cells. In present research, we involved Adalimumab as the anti-TNF alpha reagent, and our data demonstrated that the transmembrane TNF alpha-bearing CHO cells could be specifically lysated by the Adalimumab-mediated ADCC or CDC, indicating the assay system we established here could be used to differentiate the potentiality of candidate antibody drugs. However, we did not have a chance to compare the potentiality of commercially available antibody dugs targeting TNF alpha, due to our limited resources. Nevertheless, we could refer to associated references. For example, Infliximab and Adalimumab (both of which are anti-TNF monoclonal antibodies targeting TNFα) showed similar antibody-dependent cell-mediated cytotoxicity (ADCC), which were much more potent ADCC when compared with Etanercept, while Certolizumab pegol did not show any ADCC response [14]. Structurally Infliximab, Etanercept and Adalimumab carried the similar CH2 and CH3 domains of the Fc domain of IgG1, while Certolizumab pegol did not. These domains were crucial for the binding of antibodies with the Fc receptors of NK cells [15]. It was reported that all these antibody drugs weakly bound to the Fcγ receptors in the absence of soluble TNFα [16], this point was also taken into consideration in present research, and the noisy background readings was subtracted from the corresponding control group, making the system produce repeatable conclusions.
Complement-dependent cytotoxicity was also involved in the process of these anti-TNFα drugs exerting their biological functions. From our data we knew that Adalimumab could trigger CDC and targeted the TNF alpha-bearing cells. In combination with other reports, the potency of Infliximab and Adalimumab inducing CDC activity was much higher than Etanercept, while Certolizumab pegol did not demonstrate any CDC effects [14], which were caused by the absence of Fc domain of IgG1.
Considering the point that Infliximab and Adalimumab induced ADCC or CDC through the transmembrane TNFα, besides neutralization of its soluble form, both of these two anti-TNF agents showed better potency than Etanercept in the elimination of transmembrane-expressing cells, and the clinical efficacies could partially be explained by the differences of these anti-TNF agents on transmembrane TNFα. to the assay system we established in present research is a simple and practical system that canaccurately reflect the potency of candidate agents. The CHO suspension cell baring transmembrane TNFα expression is an ideal cell model for evaluating the performance of anti-TNFα agents. Our ADCC and CDC results proved that the new model could be used for screening new anti-TNFα agents for in vitro pharmacology.
Disclosure of conflict of interest
None.
References
- 1.Tobon GJ, Youinou P, Saraux A. The envionment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J Autoimmun. 2010;35:10–14. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Mclnnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Zhou Y, Chen H, Wang Z, Tang Z, Liu J. The efficacy and safty of certolizumab pegol (CZP) in the treatment of active rheumatoid arthritis (RA): a meta-analysis from nine randomized controlled trials. Int J Clin Exp Med. 2014;7:3870–3880. [PMC free article] [PubMed] [Google Scholar]
- 4.Callejas-Rubio JL, Lopez-Perez L, Ortego-Centeno N. Tumor necrosis factor alpha inhibitor treatment for sarcoidosis. Ther Clin Risk Manag. 2008;4:1305–1313. doi: 10.2147/tcrm.s967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 6.Daien CI, Duny Y, Bametche T, Daures JP, Combe B, Morel J. Effect of TNF inhibitor on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862–868. doi: 10.1136/annrheumdis-2011-201148. [DOI] [PubMed] [Google Scholar]
- 7.Deon D, Ahmed S, Tai K, Scaletta N, Herrero C, Lee IH, Krause A, Ivashkiv LV. Cross-talk between IL-1 and IL-6 signaling in rheumatoid arthritis synovial fibroblasts. J Immunol. 2001;167:5395–5403. doi: 10.4049/jimmunol.167.9.5395. [DOI] [PubMed] [Google Scholar]
- 8.Russell AS, Brunkhorst R. Sensitivity and specificity of tests for autoantibodies: comment on the article by Tan et al. Arthritis Rheum. 1999;42:2020. doi: 10.1002/1529-0131(199909)42:9<2020::AID-ANR37>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Ye XQ, Zhu YZ, Liu Z, Zou Y, Deng Y, Guo CC, Garg SK, Feng JS. Short-term effect of adverse events of adalimumab versus placebo in inducing remission for moderate-to-severe ulcerative colitis: a meta-analysis. Int J Clin Exp Med. 2015;8:86–93. [PMC free article] [PubMed] [Google Scholar]
- 10.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 11.Internati MC, Mirandola L, Bot A. Editorial: international reviews of immunology. Int Rev Immunol. 2014;33:365–366. doi: 10.3109/08830185.2014.941239. [DOI] [PubMed] [Google Scholar]
- 12.Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immjne effector functions. Cytokine. 1995;7:251–259. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- 13.van Schouwenburg PA, van de Stadt LA, de Jong RN, van Buren EE, Kruithof S, de Groot E, Hart M, van Ham SM, Rispens T, Aarden L, Wolbink GJ, Wouters D. Adalimumab elicits a restricted anti-idiotypic antibody response to autoimmune patients resulting in functional neutralisation. Ann Rheum Dis. 2013;72:104–109. doi: 10.1136/annrheumdis-2012-201445. [DOI] [PubMed] [Google Scholar]
- 14.Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, Brown D, Robinson M, Bourne T. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13:1323–1332. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 15.Gessner JE, Heiken H, Tamm A, Schnidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76:231–48. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 16.Arora T, Padaki R, Liu L, Hamburger AE, Ellison AR, Stevens SR, Louie JS, Kohno T. Differences in binding and effector functions between classes of TNF antagonists. Cytokine. 2009;45:124–131. doi: 10.1016/j.cyto.2008.11.008. [DOI] [PubMed] [Google Scholar]


