Abstract
The PI3K/Akt signal pathway was suggested to be associated with apoptosis. However, it was still unclear whether activated PI3K/Akt signaling pathway could inhibit hypoxic cardiomyocytes apoptosis. In this research, the recombinant PI3KCG lentiviral vector plasmid (PLV-PI3KCG) was constructed and transfected into neonatal rat hypoxia/reoxygenation (H/R) injury cardiomyocytes models which were randomly divided into five groups as the normal control group, H/R group, HR empty plasmid group (HRE group), HR PLV-PI3KCG transfection preconditioning group (HRP group), and HR PLV-PI3KCG transfection + LY294002 group (HRPL group). Compared with the H/R, HRE and HRPL groups, the cardiomyocytes beat frequency and survival rate in the HRP group were significantly increased (P<0.05) and the released LDH were significantly decreased (P<0.05). The Bcl-2/Bax ratio was significantly lower in H/R, HRE and HRPL groups than that in HRP group (P<0.05). Activated PI3K/Akt signaling pathway could play a protection role in the cardiomyocytes H/R injury process which could be inhibited by LY294002.
Keywords: Lentiviral vector, PI3KCG, virus packaging, cardiomyocytes, hypoxia/reoxygenation
Introduction
Cardiomyocyte apoptosis participates in various cardiac diseases including acute myocardial infarction, hypoxia/reperfusion injury, cardiac allograft, and heart failure. The studies on the cardiomyocyte apoptosis mechanism and inhibition approach have become the research focus [1]. Researches have shown that as one of the important cellular signal pathways, phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt) signal pathway plays a key role in the regulating cell cycle course, differentiation and survival [2,3]. However, the concrete mechanism of PI3K/Akt signal pathway activation preventing the cardiomyocytes hypoxia/reoxygenation (H/R) injury was still unclear. In the current research, the lentiviral vector containing human phosphatidylinositol 3-kinase, catalytic subunit gamma (PI3KCG) gene has been constructed and identified by using lentiviral package technology. Moreover, the recombinant PI3KCG lentiviral vector plasmid pLenti6/V5-PI3KCG (PLV-PI3KCG) was transfected into neonatal rat cardiomyocytes and intervened by H/R procedure to analyze the PI3KCG gene function. It will lay the foundation for the following in vivo study on the protection mechanism of PI3KCG gene in AMI progress.
Material and methods
Construction of recombinant PLV-PI3KCG lentiviral vector
The PLV-PI3KCG was constructed by gateway LR homologous recombination method in vitro. The LR Clonase™ II enzyme was unfrozen and mixed with vortex on ice. The pEntry-PI3KCG plasmid (Inventrogen Inc, 150 ng, 1 µL), pLenti6/V5-DEST (Invitrogen Inc, 150 ng, 1 µL), TE buffer (30 mM Tris·Cl, 1 mM EDTA, pH 8.0, 8 μL) were added into the 1.5 mL centrifuge tube successively and mixed at room temperature which was joined by 2 μL LR Clonase™ II enzyme mixture soon afterwards, mixed together again and incubated for 1 hour at room temperature. 1 μL Proteinase K solution was then added into the above mixture and the reaction was terminated at 37°C for 10 minutes. The above termination reaction solution was taken out for 2 μL which was put into 100 μL competence XL-BLUE Escherichia coli together and absorbed for 30 minutes on ice. Then 2 μL termination reaction solution and Escherichia coli mixture was swashed for 50 seconds at 42°C which were rapidly cooled for 2 minutes on ice, added by 1 mL LB culture medium, oscillated for 1 hour at 37°C by 180 rpm and then centrifuged for 3 minutes by 6000 rpm. The supernatant were abandoned and the remaining mixture was mixed with 200 mL LB culture medium. The 100 mL solution were taken out of the above mixture, coated onto the LB culture plating with 100 μg/mL ampicillin and cultured overnight at 37°C. Ten clones were picked out on the second day, inoculated into 3 mL LB culture medium with 100 μg/mL ampicillin, shaken and cultured overnight at 37°C by 250 rpm.
Positive clones identification by colony PCR and bi-directional sequencing verification of positive clones
The recombinant PLV-PI3KCG lentiviral vector genomic DNA was extracted. The PCR reaction was performed to identify the PLV-PI3KCG lentiviral vector by using PI3KCG gene primers. The upstream and downstream primers were PI3KCG-F1, PI3KCG-R1, PI3KCG-F2 and PI3KCG-R2. The reaction system were 20 μL including 10× Buffer 2 μL, Mg2+ (25 mmol/L) 2 μL, dNTPs 0.4 μL, upstream and downstream primers (5 μmol/L) 1 μL respectively, Taq enzyme (5 U/μL) 0.1 μL, ddH2O 12.5 μL, and template 1 μL. The PCR amplification procedure is pre-denaturation for 3 minutes at 94°C, denaturation for 30 seconds at 94°C, annealing for 30 seconds at 55°C, extension for 30 seconds at 72°C for 35 cycles and extension for 7 minutes at 72°C at last. The positive clones were bi-directionally sequenced and verified by using CMV-F and V5 reverse primers (Table 1).
Table 1.
The sequence table of the PCR primers
| Primers name | Primers sequence 5’ to 3’ |
|---|---|
| PI3KCG-F1 | CATCCCCATCGAGTTCGTG |
| PI3KCG-R1 | CAGTTG TTGGCAATCTTCTTCC |
| PI3KCG-F2 | AACCTATTTCATATTGACTTCGGG |
| PI3KCG-R2 | AATTAAACTGCACAG TCCATCCTT |
| CMV-F | CGCAAATGGGCGGTAGGCGTG |
| V5 reverse | ACCGAGGAGAGGGTTAGGGAT |
Recombinant PLV-PI3KCG lentiviral vector package in HEK 293T cells and identification
The 10 cm culture dish was coated by gelatin (Sigma Inc, USA) for 24 hours before transfection. The well-grown HEK 293T cells were inoculated into the culture dish at 37°C and 5% CO2. When the 293T cells were grown to about 85% confluence, the recombinant PLV-PI3KCG lentiviral vector or empty lentiviral vector was transfected into the 293T cells by using LipofectaminTM 2000 reagent (Invitrogen Inc, USA) and the null plasmid with GFP was also transfected at the same time as the control to observe the transfection effect. The opti-MEM (without serum and antibiotics) were added into two Eppendorf tubes, of which the DNA (conjugative plasmid: recombinant PLV-PI3KCG plasmid, package diolame pLP1, pLP2 and VSVG, Addgene Inc) were added into one Eppendorf tube and LipofectaminTM 2000 was added into another Eppendorf tube. The solutions of the two Eppendorf tubes were mixed for 20 minutes at room temperature, added into the 10 cm culture dish and shaken up. The viral solution was collected after incubation for 48 hours and filtrated by 0.45 μm filter membrane. The 5 μL viral solution was picked out and used to perform PCR amplification and sequence to identify the target gene expression. The PCR amplification primers and condition were the same to that in the above mentioned text. The remained viral solution was stored for standby application at -80°C.
The cardiomyocytes preparation and hypoxia model establishment in vitro [4]
The present research was approved by the ethnic committee of the First Affiliated Hospital of Nanjing Medical University. Cardiomyocytes were prepared from 1- to 3-day-old neonatal Sprague-Dawley rats (150-180 g, Nanjing Qinglongshan Multiplying Farm, China). The chest was opened after the rat was washed by alcohol and the heart was taken out and put into phosphate buffer solution (PBS) at 4°C. The blood stasis was removed by washing repeatedly and the atrial tissue was cut off. The remained ventricular tissue was digested repeatedly by adding 0.1% trypsin solution and the cardiomyocytes were isolated by differential attachment method. The cardiomyocytes were inoculated into 24-well plates at a density of 1×105 cells/mL in Dulbecco modified Eagle medium (DMEM) supplemented with 15% fetal bovine serum. After 72 hours incubation at 37°C and 5% CO2, cardiomyocytes were grown to about 80% confluence, followed by random allocation experiments. Cardiomyocytes were then transfected at 50 MOI with empty lentiviral vector, PLV-PI3KCG, or PLV-PI3KCG + LY294002 (10 mM) at 105 pfu/mL by adding 10 mg/L polybrene transfection reagent (Merk Inc, German). After 12 hours transfection, the viral transfection solution was replaced by DMEM with 10% calf serum. The isolated cardiomyocytes were subsequently random divided into five groups after being cultured for 3 days: the normal control group, H/R group, HRE group, HRP group, and HRPL group. The same experiments were repeated for five times.
The H/R injury model of neonatal rat myocardial cells was established. After the cardiomyocytes were co-cultured with recombinant PLV-PI3KCG or empty plasmid for 72-96 hours, except the control group, all of the other four groups were treated with hypoxia for 180 minutes by using hypoxia bag (Becton Dickinsonand Inc, USA) (95% N2 and 5% CO2) and they were then dealt with reoxygenation for 120 minutes (70% N2, 25% O2, and 5% CO2) to simulate the H/R process.
Identification of PI3KCG gene in the transfected cardiomyocytes
The total RNA was extracted from the transfected cardiomyocytes. The RT-PCR reaction was performed to identify the PLV-PI3KCG lentiviral vector by using CMV-F and V5 reverse primers which were the same to the above mentioned primers (Table 1). The RT-PCR products were sequenced subsequently.
Analysis of LDH in cardiomyocytes
The LDH detection kit (Nanjing Jiancheng Bioengineering Institute) was used to analyze LDH level. Measurements of LDH from cell supernatants were analyzed by full-automatic biochemical analyzer (Beckman, USA).
Cardiomyocytes survival rate assay by cell count kit-8 method (CCK-8, WST-8) [5]
The cardiomyocytes were inoculated into 96-well plates with 100 μL suspension per cell. Additionally, 100 μL DMEM per well was acted as the blank control group. The five group experiment was performed as the above mentioned. After the H/R procedure, CCK-8 was added by 10 μL/well and incubated with cardiomyocytes for another 0.5 hour at 37°C. The 450 nm wave length of microplate reader was selected and absorbance value A was measured. The cardiomyocytes survival rate was calculated by the following formula: viable cell percent (%)=(value A of experiment group/value A of control group) ×100%.
Cardiomyocyte apoptosis assay by western blot
The cardiomyocytes were inoculated into 6-well plates with 2 ml suspension per cell. After the H/R procedure, DMEM was discarded, and the cardiomyocytes were twice rinsed and digested by trypsin. The cardiomyocytes were collected by 1200 g centrifuging for 3 minutes and added by 0.1 mL RIPA Lysis buffer, 1 μL protease inhibitor, and 1 μL PMSF. The cardiomyocytes and lysate were transferred into 1.5 mL Eppendorf tubes, placed on ice for 5 minutes and centrifuged by 13000 g for 5 minutes at 4°C. The supernatant was transferred into new Eppendorf tubes. The protein concentration of the supernatant was measured by BCA protein measure kit and BSA standard substance. Then the protein (50 µg) was separated by 10% SDS-PAGE gel, transferred to PVDF membrane and sealed by 5% skim milk powder for 2 hours. The 1:1000 diluted rabbit anti-rat antibodies as β-Actin, PI3KCG, Bax and Bcl-2 (Cell Signaling Technology Inc, USA) were incubated with the transferred PVDF membrane overnight at 4°C. β-Actin antibody acted as internal reference antibody. After PVDF membrane was washed by Tris-Buffered-Saline with Tween (TBST), 1:2000 diluted goat anti rabbit IgG-HRP (Santa Cruz Biotechnology Inc, USA) were added and incubated with the transferred PVDF for 40 minutes at 37°C. After the PVDF membrane was washed by TBST for three times, it was exposed by ECL for 1 minute to perform the band density analysis. Image Lab software was used to analyze the band and perform the protein quantification.
Statistical analysis
The SPSS software system (SPSS for Windows, version 10.0) was used to perform the statistical analysis. Measurement data were expressed as mean ± standard deviation (mean ± SD). One-way analysis of variance (ANOVA) followed by Dunnett’s test. A statistically significant difference was defined as P<0.05.
Results
The PLV-PI3KCG plasmid construction and polymerase chain reaction (PCR) identification
The objective bands were distinctly observed in the 540 bp and 382 bp after the agarose gel electrophoresis for PCR products of the transfected recombinant PLV-PI3KCG lentiviral plasmid positive clones. The band size was in agreement with the expected results. The sequencing results were completely in agreement with the given human PI3KCG gene sequence which suggested the recombinant PLV-PI3KCG lentiviral plasmid was successfully constructed (Figure 1A, 1B). Figure 2A depicted the original pLenti6/V5-DEST vector map and Figure 2B displayed the recombinant PLV-PI3KCG lentiviral plasmid map.
Figure 1.
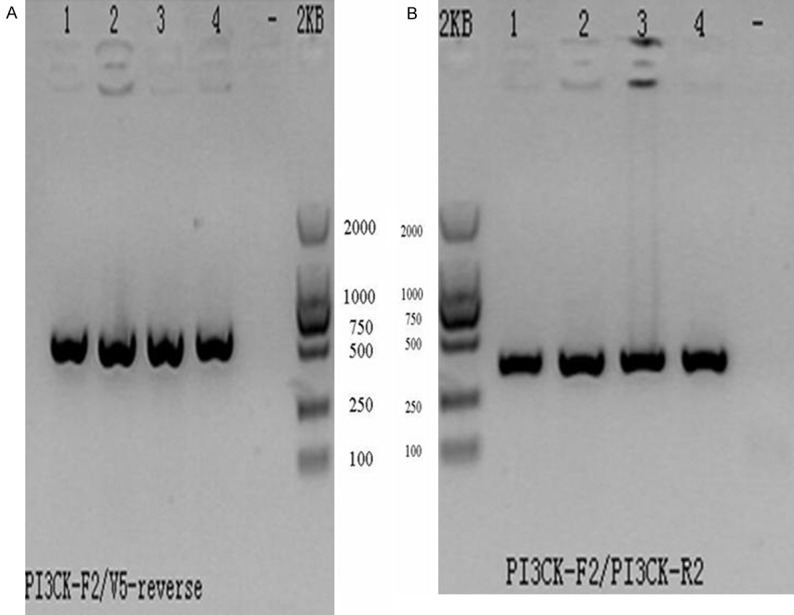
The recombinant PLV-PI3KCG plasmid was identified by PCR reaction. A. The PCR amplification agarose gel electrophoretogram of the positive clone of the transfected HEK293 cells by using PI3KCG-F2/V5 primers. Lane 1-4: The PCR amplification products by using PI3KCG-F2/V5 primers (540 bp); Lane-: The blank control by using H2O. B. The PCR amplification agarose gel electrophoretogram of the positive clone of the transfected HEK293 cells by using PI3KCG-F2/R2 primers. Lane 1-4: The PCR amplification products by using PI3KCG-F2/R2 primers (382 bp); Lane-: The blank control by using H2O.
Figure 2.
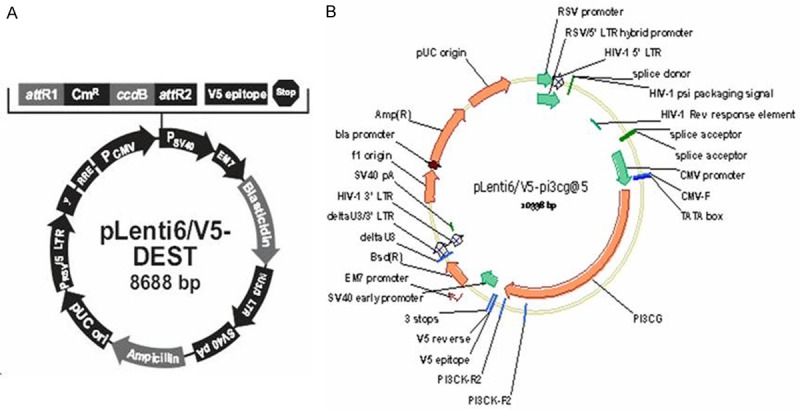
The vector map used in the current research. A. Map of pLenti6/V5-DEST; B. Map of recombinant PLV-PI3KCG vector.
Recombinant PLV-PI3KCG plasmid package and identification
The lipofection transfection method was used to co-transfect the conjugative plasmid PLV-PI3KCG or empty plasmid and packaging diolame plasmid into human embryonic kidney (HEK) 293T cells. The GFP protein expression could be observed in the empty lentiviral plasmid group after transfection for 24 hours by fluorescence microscopy. The GFP protein fluorescence intensity was further strengthened after transfection for 48 hours. The positive package ratio of GFP protein expression was up to 100% after transfection for 72 hours which implied that the PLV-PI3KCG was successfully packaged. It was verified that the PI3KCG gene was contained in the recombinant lentiviral genome by PCR and sequencing which suggested that the recombinant PLV-PI3KCG or empty plasmid was successfully transfected into HEK 293T cells. The virus titer of the recombinant PLV-PI3KCG or empty plasmid was up to 106 IU/mL after the transfected HEK 293T cells were multi-round amplified.
Identification results of PI3KCG gene in the transfected cardiomyocytes
The transfected cardiomyocytes total RNA was sucessfully extracted and the reverse transcription PCR products were also observed in the 540 bp and 382 bp by agarose gel electrophoresis. The bi-directional sequencing results were completely in accordance with the PI3KCG gene which suggested the PI3KCG gene was successfully transfected into cardiomyocytes (Figure 3).
Figure 3.
The RT-PCR products sequence identification report of PI3KCG gene in transfected cardiomyocytes.
The PI3KCG gene attenuated cardiomyocytes viability loss in the H/R procedure
The cardiomyocyte did not adhere originally and presented as roundness which soon adhered and gradually presented as fusiformis and polygon. The cardiomyocyte size was uniform and grew up quickly. The single cardiomyocyte wriggled by itself and when many cardiomyocytes grew up to flakiness and beaten rhythmically like an island. After the H/R intervention, the cardiomyocytes survival rate and rhythm of the heart were significantly reduced than the control group (P<0.05). However, the cardiomyocytes survival rate and beat frequency in the PLV-PI3KCG transfection preconditioning group (HRP group) were significantly increased than that in the H/R, HR empty plasmid group (HRE) and HR PLV-PI3KCG transfection + LY294002 (HRPL) groups (P<0.05) (Figure 4).
Figure 4.
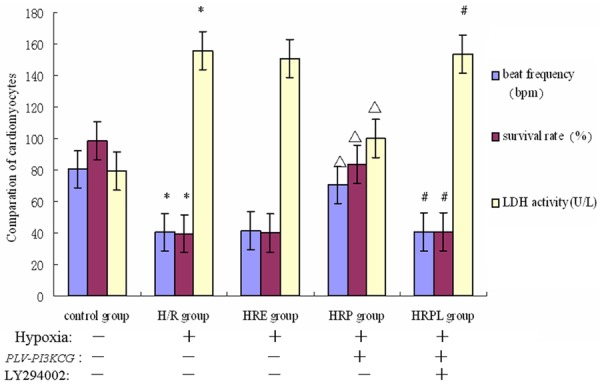
Comparation results of cardiomyocytes beat frequency, survival rate and supernatant LDH level between the five groups (n=5). *P<0.05 vs. control group; ΔP<0.05 vs. H/R group; #P<0.05 vs. HRP group.
The PI3KCG gene attenuated lactate dehydrogenase (LDH) release amount
The LDH release amount in the medium was measured as the cardiomyocytes apoptosis index. As shown in Figure 4, the LDH level of the cardiomyocytes medium supernatant was significantly higher in the H/R group than that in the control group (P<0.05). However, the LDH level was significantly lower in the HRP group than that in the H/R group (P<0.05). No significant difference was detected in LDH level among HRE, H/R or HRPL groups (P>0.05).
The intracellular mechanisms of PI3KCG gene protecting cardiomyocytes against apoptosis in the H/R injury
The western blot results of the transfected cardiomyocytes with recombinant PLV-PI3KCG plasmid in the H/R procedure were shown in Figure 5. The apoptosis-promoting protein (Bax) was highly expressed in H/R, HRE and HRPL groups which were significantly decreased in HRP group. By contrast, the apoptosis-inhibiting proteins (Bcl-2) were lowly expressed in H/R, HRE and HRPL groups which were significantly increased in HRP group. The PI3KCG protein expression was also decreased in H/R and HRE groups which were significantly increased in HRP and HRPL groups. No significant difference was detected in the β-Actin protein expression among the five groups (Figure 5A).
Figure 5.
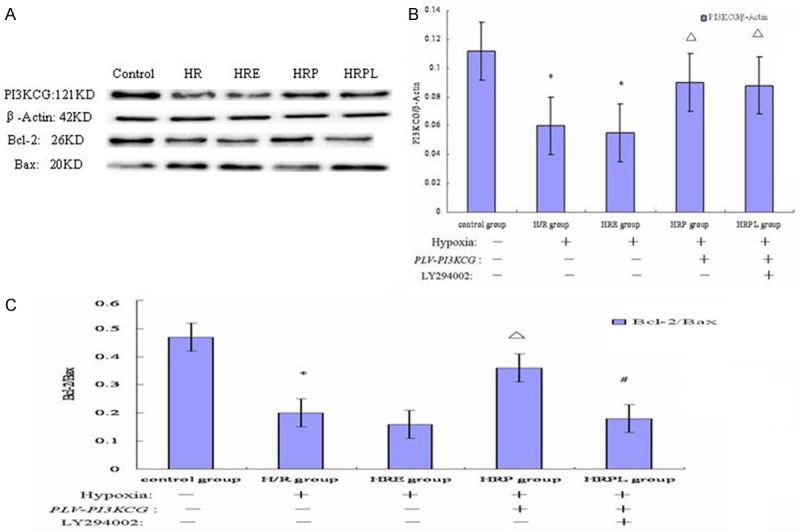
The intracellular mechanisms of PI3KCG gene protecting cardiomyocytes against apoptosis in the H/R injury. A. The western blot results of the transfected cardiomyocytes with recombinant PLV-PI3KCG plasmid in the H/R procedure. The effect of recombinant PLV-PI3KCG plasmid on the protein levels of PI3KCG, Bcl-2, and Bax. The recombinant PLV-PI3KCG plasmid mediated PI3KCG, Bcl-2 protein increase, and Bax protein decrease. The Bcl-2 protein increase and Bax protein decrease were abrogated by the PI3K inhibitor LY294002 treatment. Data are presented as the mean ± SD (n=5). The β-Actin was analyzed as loading control. B. The effect of recombinant PLV-PI3KCG plasmid on the PI3KCG/β-Actin protein ratio in hypoxia-induced cardiomyocytes apoptosis. The recombinant PLV-PI3KCG plasmid mediated PI3KCG protein increase. Data are presented as the mean ± SD. (n=5). *P<0.05 vs. control group; ΔP<0.05 vs. H/R group. C. The effect of recombinant PLV-PI3KCG plasmid on the Bcl-2/Bax protein ratio in hypoxia-induced cardiomyocyte apoptosis. The recombinant PLV-PI3KCG plasmid-mediated Bcl-2 protein increase and Bax protein decrease were abrogated by the PI3K inhibitor LY294002 treatment. Data are presented as the mean ± S.D. (n=5). *P<0.05 vs. control group; ΔP<0.05 vs. H/R group; #P<0.05 vs. HRP group.
Figure 5B has shown the PI3KCG/β-Actin ratio was decreased both in the H/R and HRE groups (0.060±0.004, 0.055±0.005, respectively, P<0.05, compared to control: 0.112±0.003) and this decrease was largely inhibited by recombinant PLV-PI3KCG plasmid administration in HRP and HRPL groups (0.090±0.002, 0.088±0.003, respectively, P<0.05, compared to H/R group). Compared with H/R group, no significant difference was detected in the HRE group (0.055±0.004, P>0.05, compared to H/R group). There was also no significant difference in PI3KCG/β-Actin ratio between HRP and HRPL groups (P>0.05).
Figure 5C depicted that the Bcl-2/Bax ratio was decreased in the HR group (0.20±0.02, P<0.05, compared to control: 0.47±0.03) and this decrease was greatly blocked by administration of recombinant PLV-PI3KCG plasmid in HRP group (0.36±0.04, P<0.05, compared to H/R group). In addition, the recombinant PLV-PI3KCG plasmid-mediated Bcl-2 protein increase and Bax protein decrease were abrogated by the PI3K inhibitor LY294002 treatment in HRPL group (0.18±0.02, P<0.05, compared to HRP group). Compared with H/R and HRPL group, no significant difference was detected in the HRE group (0.16±0.03, P<0.05, compared to HRP group).
Discussion
In the current research, the recombinant PLV-PI3KCG plasmid was successfully constructed and packaged. The transfected PI3KCG gene function was also confirmed to have the protection effect on the hypoxia cardiomyocytes. The human PI3KCG gene spans 3.3 kb which is located in 7q22.3. Given that the PI3KCG gene is relatively large, the green fluorescent protein (GFP) gene of the pLenti6/V5-DEST lentiviral vector was removed by enzyme digestion to avoid influencing the PI3KCG gene function in the recombinant PLV-PI3KCG plasmid. Hence, in the host HEK 293T cells transfected with PLV-PI3KCG plasmid, the GFP protein expression could not be observed which could only be observed in the HEK 293T cells transfected with empty plasmid to see the transfection efficiency. The following PCR and sequence methods were used to determine whether the transfection process was successfully performed or not. In the PLV-PI3KCG plasmid recombination process, the traditional repeat digestion and link technologies were not adopted and the gateway homologous recombination method was used. Thus, the PLV-PI3KCG plasmid was constructed simply and efficiently [6].
The current research is the first study to explore the protection effect of PI3KCG gene on hypoxia cardiomyocytes by using lentiviral vector. As well known, there are many gene transfection methods as physical, chemical, retrovirus vector, adenovirus vector methods and so on. The transfection efficiency was low by using physical or chemical method. The retrovirus vector has the bad capacity to accommodate the exogenous gene and can only infect the division stage cells. Additionally, it is difficult for the adenovirus vector to realize the stable expression of the exogenous gene. The lentiviral vector, constructed on the basis of human immunodeficiency virus-1, has the large capacity to accommodate the exogenous gene, can realize the stable expression of the transferred gene and infect the non-division stage cells. The lentivirus titer demanding for transfection is high up to 1×107-1×108 IU/mL with little host immunoreaction. The aforementioned merits of the lentiviral vector overcome the deficiency of the retrovirus and adenovirus vectors.
The treatment of acute myocardial infarction (AMI) with thrombolysis and percutaneous transluminal angioplasty methods always lead to the ischemia reperfusion injury, especially cell apoptosis and necrosis which caused the patients condition worsened. Hence, inhibition of cardiomyocytes apoptosis becomes the main way for the treatment of AMI and congestive heart failure sequela [7]. PI3K/Akt is the important celluar signal transduction pathway. The activated PI3K/Akt signal pathway could further active or inhibit the downstream target protein by phosphorylation, thus play the role of regulating cell proliferation, differentiation, glucose metabolism and migration [8-20]. In 2007, Arab et al found that in the reversible myocardial ischemia-reperfusion during cardiac surgery, the ischemia preconditioning could cause the Akt phosphorylation level increase. The PI3K inhibitors such as Wortmannin and LY294002 could enlarge the myocardial infarction zone, inhibit the ischemic cardiac function improvement and reduce the phosphorylation level [21]. Murphy et al reported that lack of PI3K could not help the cardiac function recover during the ischemia reperfusion procedure, increased the dead cells number, and weakened the adenosine protection effect [22]. Matsui et al found that hypoxia induced cell apoptosis could be reduced by transfecting the PI3K or Akt gene into cardiomyocytes using adenovirus vector [23]. In 2010, Li et al reported that Asperosaponin VI had a protective effect against hypoxia-induced cardiomyocytes apoptosis probably by activating the PI3K/Akt and CREB pathways [24].
The current research results have shown that the cardiomyocytes survival rate in the H/R group was significantly reduced and LDH release quantity was significantly increased compared with control group (P<0.05) which suggested that the cardiomyocytes H/R injury model was successfully constructed. After the recombinant PLV-PI3KCG plasmid was transfected into cardiomyocytes, PI3KCG protein overexpression was detected in HRP and HRPL groups which suggested that the PLV-PI3KCG plasmid was successfully constructed, transfected and expressed. In the following function test, it was observed that the cardiomyocytes beat frequency, survival rate, and Bcl-2 protein expression in the HRP group was significantly increased than that in the H/R, HRE and HRPL groups. The LDH release amount and Bax protein expression in the HRP group were significantly decreased than that in the H/R, HRE and HRPL groups. It was verified that in the present study that PI3KCG gene has the significant protection effect on the cardiomyocytes hypoxia reoxygenation injury by activating the PI3K/Akt signal pathway. In addition, the protection effect was lost after the PI3KCG inhibitor LY294002 was added.
There are still some limitations in the present research. The current study was just an experiment in vitro. The in vivo research on the PI3KCG gene protection effect on the cardiomyocytes hypoxia reoxygenation injury still needed to be carried out in the near future. Furthermore, the protection effect was possibly mediated by other cytokines as transforming growth factor-beta 1 which needed to be verified by more studies.
Taken together, PLV-PI3KCG plasmid was expected to become an available vector to investigate PI3K/Akt pathway in the cardiomyocytes H/R injury process. PI3KCG gene was markedly associated with the protection effect on the cardiomyocytes H/R injury. The current conclusion might help us to formulate novel ischemia coronary disease therapy strategies. Given the limitations, more studies in vivo are needed to clarify the significance of the conclusion.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to Dr Yan-yan Li), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for Dr Yan-Yan Li (2013-2015, JX2161015034) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thank all our colleagues working in the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University.
Disclosure of conflict of interest
None.
References
- 1.Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, Lau WB, Koch W, Ma XL, He B. Cardiomyocyteexpressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. 2013;34:1834–45. doi: 10.1093/eurheartj/ehs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease--a novel therapeutic target? FASEB J. 2002;16:135–46. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- 3.Sunkomat JN, Gaballa MA. Stem cell therapy in ischemic heart disease. Cardiovasc Drug Rev. 2003;21:327–42. doi: 10.1111/j.1527-3466.2003.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 4.Spector DL, Goldam RD, Leinwand LA. Cells: a Laboratory Mannul. New York: Cold Spring Harbor Laboratory Press; 1998. p. 129. [Google Scholar]
- 5.Yu C, Xing F, Tang Z, Bronner C, Lu X, Di J, Zeng S, Liu J. Anisomycin suppresses Jurkat T cell growth by the cell cycle-regulating proteins. Pharmacol Rep. 2013;65:435–44. doi: 10.1016/s1734-1140(13)71019-3. [DOI] [PubMed] [Google Scholar]
- 6.Que WZ, Chen JM. Gateway technical supported construction of a recombinant adenovirus vector pAd-NK4. Chinese Pharmacological Bulletin. 2011;27:467–72. [Google Scholar]
- 7.Cai ZL, Yin Jk, Han TL, et al. Construction of Grb2-SH2 genetic restructuring-slow virus carrier with Gateway system. Progress in Modern Biomedicine. 2012;12:616–8. [Google Scholar]
- 8.Paul A, Binsalamah ZM, Khan AA, Abbasia S, Elias CB, Shum-Tim D, Prakash S. A nanobiohybrid complex of recombinant baculovirus and Tat/DNA nanoparticles for delivery of Ang-1 transgene in myocardial infarction therapy. Biomaterials. 2011;32:8304–18. doi: 10.1016/j.biomaterials.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Yoon K, Jung EJ, Lee SY. TRAF6-mediated regulation of the PI3 kinase (PI3K)-Akt-GSK3beta cascade is required for TNF-induced cell survival. Biochem Biophys Res Commun. 2008;371:118–21. doi: 10.1016/j.bbrc.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch E, Ciraolo E, Ghigo A, Costa C. Taming the PI3K team to hold inflammation and cancer at bay. Pharmacol Ther. 2008;118:192–205. doi: 10.1016/j.pharmthera.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Salphati L, Heffron TP, Alicke B, Nishimura M, Barck K, Carano RA, Cheong J, Edgar KA, Greve J, Kharbanda S, Koeppen H, Lau S, Lee LB, Pang J, Plise EG, Pokorny JL, Reslan HB, Sarkaria JN, Wallin JJ, Zhang X, Gould SE, Olivero AG, Phillips HS. Targeting the PI3K pathway in the brain--efficacy of a PI3K inhibitor optimized to cross the blood-brain barrier. Clin Cancer Res. 2012;18:6239–48. doi: 10.1158/1078-0432.CCR-12-0720. [DOI] [PubMed] [Google Scholar]
- 12.Wallin JJ, Guan J, Prior WW, Lee LB, Berry L, Belmont LD, Koeppen H, Belvin M, Friedman LS, Sampath D. GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin Cancer Res. 2012;18:3901–11. doi: 10.1158/1078-0432.CCR-11-2088. [DOI] [PubMed] [Google Scholar]
- 13.Wallin JJ, Guan J, Edgar KA, Zhou W, Francis R, Torres AC, Haverty PM, Eastham-Anderson J, Arena S, Bardelli A, Griffin S, Goodall JE, Grimshaw KM, Hoeflich KP, Torrance C, Belvin M, Friedman LS. Active PI3K pathway causes an invasive phenotype which can be reversed or promoted by blocking the pathway at divergent nodes. PLoS One. 2012;7:e36402. doi: 10.1371/journal.pone.0036402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan W, Stawiski E, Janakiraman V, Chan E, Durinck S, Edgar KA, Kljavin NM, Rivers CS, Gnad F, Roose-Girma M, Haverty PM, Fedorowicz G, Heldens S, Soriano RH, Zhang Z, Wallin JJ, Johnson L, Merchant M, Modrusan Z, Stern HM, Seshagiri S. Conditional activation of Pik3ca (H1047R) in a knock-in mouse model promotes mammary tumorigenesis and emergence of mutations. Oncogene. 2013;32:318–26. doi: 10.1038/onc.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, Lesnick JD, Lewis C, Nonomiya J, Pang J, Salphati L, Olivero AG, Sutherlin DP, O‘Brien C, Spoerke JM, Patel S, Lensun L, Kassees R, Ross L, Lackner MR, Sampath D, Belvin M, Friedman LS. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–36. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 16.Wallin JJ, Guan J, Prior WW, Edgar KA, Kassees R, Sampath D, Belvin M, Friedman LS. Nuclear phospho-Akt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci Transl Med. 2010;2:48ra66. doi: 10.1126/scitranslmed.3000630. [DOI] [PubMed] [Google Scholar]
- 17.Salphati L, Wong H, Belvin M, Bradford D, Edgar KA, Prior WW, Sampath D, Wallin JJ. Pharmacokinetic-pharmacodynamic modeling of tumor growth inhibition and biomarker modulation by the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab Dispos. 2010;38:1436–42. doi: 10.1124/dmd.110.032912. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA, Guan J, Berry L, Prior WW, Amler LC, Belvin M, Friedman LS, Lackner MR. Predictive biomarkers of sensitivity to the phosphatidylinositol 3’ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010;16:3670–83. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- 19.Edgar KA, Wallin JJ, Berry M, Lee LB, Prior WW, Sampath D, Friedman LS, Belvin M. Isoformspecific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors. Cancer Res. 2010;70:1164–72. doi: 10.1158/0008-5472.CAN-09-2525. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Chunlei W, Pei W, Zhen L, Xiangzhen L. Simvastatin activates Akt/glycogen synthase kinase-3beta signal and inhibits caspase-3 activation after experimental subarachnoid hemorrhage. Vascul Pharmacol. 2010;52:77–83. doi: 10.1016/j.vph.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Arab S, Konstantinov IE, Boscarino C, Cukerman E, Mori A, Li J, Liu PP, Redington AN, Coles JG. Early gene expression profiles during intraoperative myocardial ischemia-reperfusion in cardiac surgery. J Thorac Cardiovasc Surg. 2007;134:74–81. 81. doi: 10.1016/j.jtcvs.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Murphy E, Tong H, Steenbergen C. Preconditioning: is the Akt-ion in the PI3K pathway? J Mol Cell Cardiol. 2003;35:1021–5. doi: 10.1016/s0022-2828(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 23.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated hosphatidylinositol 3’-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–9. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Tian J, Li G, Jiang W, Xing Y, Hou J, Zhu H, Xu H, Zhang G, Liu Z, Ye Z. Asperosaponin VI protects cardiac myocytes from hypoxia-induced apoptosis via activation of the PI3K/Akt and CREB pathways. Eur J Pharmacol. 2010;649:100–7. doi: 10.1016/j.ejphar.2010.08.060. [DOI] [PubMed] [Google Scholar]


