Abstract
Recovery following nerve repair can be evaluated based on electrophysiological and morphological assessments of biomechanical properties. This study compared the effects of topical hyaluronic acid (HA), tacrolimus (FK-506) or saline administration on the biomechanical properties of the sciatic nerve at 12 weeks after nerve repair. Materials and Methods: Eighteen male European rabbits (Oryctolagus cuniculus) (weight from 2.5 to 3 kg) were randomly assigned to one of the following experimental groups (six animals per group): Saline, HA, or FK-506. The non-transected left leg was used as a control group (eighteen sciatic nerves). Biomechanical assays were performed and statistically analyzed. Results: The average maximal load, elastic limit load, maximal stress, and elastic limit strain of the control group were significantly different (P<0.001) from those of all three experimental groups. Moreover, the other examined parameters (i.e., maximal displacement, elastic limit stress, and maximal strain) were significantly different between the control group and all three experimental groups (P<0.0001). However, no significant differences in any of the biomechanical parameters were observed between the experimental groups (P>0.05). At 12 weeks after nerve repair, Saline, HA, and FK-506 groups displayed average maximal stress values that were 72.6%, 77.38%, and 73.8% of those in the control group (100%), respectively. Conclusion: The biomechanical properties of the HA and FK-506 groups were similar to those of the saline group at 12 weeks after nerve repair.
Keywords: Biomechanical properties, hyaluronic acid, rabbits, tacrolimus, scar, sciatic nerve healing
Introduction
Peripheral nerve injuries are very common and cause significant clinical problems due to neurological deficits [1]. To reduce the consequences of severe nerve injuries, such as nerve stretching, compression, crushing, and transection (neurotmesis), microsurgical nerve repair is necessary. Different surgical techniques can be utilized depending on the type of nerve injury [2,3]. However, despite the improvements in operational techniques, nerve repair is often followed by the formation of scar tissue surrounding the damaged nerve [4]. Many experimental studies have focused on the application of different pharmacological substances at the site of nerve repair to prevent scar tissue formation and improve nerve regeneration. Hyaluronic acid (HA) and tacrolimus (FK-506) are the compounds most commonly used for this purpose [5,6]. These substances exert anti-inflammatory and immunosuppressive effects and potentially prevent scar formation via several mechanisms [7,8]. Nerve damage due to scar formation alters the functional and morphologic properties of nerves (i.e., the nerve fascicles, the epineurium, the perineurium, the endoneurium, and vessels) [9-11]. The success of nerve regeneration can be evaluated using several functional (e.g., toe-spreading reflex test and electrophysiological recording) [12,13] and morphologic methods (e.g., macroscopic, histomorphometric, and immunohistochemical analyses) [6,14]. In addition to these experimental examinations, the biomechanical properties of nerves that were previously repaired following damage have recently been investigated in several studies [15]. Several biomechanical properties, such as maximal load, elastic limit load, maximal displacement, maximal stress, elastic limit stress, maximal strain, and elastic limit strain, can be assessed. These parameters can be recorded using universal tensile machines [16]. Many factors influence nerve healing and recovery as indicated by these biomechanical parameters. These factors include the type of nerve injury, the method of nerve repair (i.e., direct end-to-end, end-to-site, epineural, perineural, or inter-fascicular, as well as the use of conduits to repair nerve defects) [17], the timing of nerve repair and the use of topical pharmacological agents [4].
This study investigated the biomechanical properties of the sciatic nerve at the site of repair, at which saline, HA, orFK-506 was topically applied.
Materials and methods
Eighteen adult male European rabbits (Oryctolagus cuniculus) ranging in weight from 2.5 kg to 3 kg (average: 2.75 kg) were used for this study. The rabbits were placed in separate cages and were raised under conventional laboratory conditions.
All procedures were performed at the Experimental Animal Breeding and Research Center. Animal care was performed with the prior approval of the Animal Experimental Ethics Committee of the Medical Faculty of the University of Prishtina, Kosovo (No. 1551).
The animals were randomly divided into 3 equal experimental groups (6 per group). The non-transected left leg was used as a control group (eighteen sciatic nerves).
In groups I, II, and III, following transection and repair of the sciatic nerve, the site of nerve repair was wrapped with an absorbable gelatin sponge soaked (AGSS) with 0.5 ml of saline (0.9% NaCl), 0.5 ml of HA (16 mg/2 ml Orthovisc), or 15 μl of FK-506 (Prograf, Astellas Pharma), respectively. FK-506 was prepared from the original 5 mg/ml ampules, and the final concentration (10 ng/ml) was prepared via 2 dilutions [6].
Surgical procedures
All of the rabbits (18) were anesthetized via intravenous injection of 30 g/l sodium pentobarbital (1 mg/kg) into the ear vein. The rabbits were intravenously administered 20 mg/kg cefazolin prior to surgery to prevent postoperative infections.
After the induction of anesthesia, the animals were shaved and placed in the lateral decubitus position. All subsequent procedures were performed aseptically. First, a longitudinal incision was made in the lower 2/3 of the thigh. Next, the right sciatic nerve was exposed via the splitting procedure to separate the semitendinosus and biceps femoris muscles [18]. The sciatic nerve was transversally cut using a surgical blade. After the nerve was transected, it was immediately repaired via end-to-end neurorrhaphy using four equidistantly placed epineural sutures (10-0 nylon suture; Ethicon Inc., Somerville, NJ, USA) that were positioned 1 mm from the site of transection. After neurorrhaphy, the site of nerve repair was wrapped with AGSS (Spongostan®; Ethicon Inc., Somerville, NJ, USA) with 0.5 ml of saline (group I), HA (group II), or FK-506 (group III). Then, the fascia, muscle and skin layers were separately closed. The rabbits were allowed unrestricted movement immediately after anesthesia. All of the surgical procedures were performed using microsurgical instruments under a loupe at 3.5× magnification [5].
Nerve diameter
All of the experimental rabbits were sacrificed via overdose of intracardially administered 100 mg/kg phenobarbital. The sciatic nerves were re-exposed, and the repaired and control nerves were harvested to examine their biomechanical properties. The sciatic nerve diameter (d) was measured using a digital micrometer [19]. In the repaired nerves, the measurements were obtained at the anastomosed sites, and the measurements were obtained at the equivalent sites in the non-transected sciatic nerves (from the left hind leg). Longitudinal representations of the sciatic nerves from the control and experimental groups are presented as schematics in Figure 1, and images of rabbit legs that were exposed (control left sciatic nerve) or re-exposed (the right sciatic nerve in the experimental groups) are presented in Figure 2.
Figure 1.
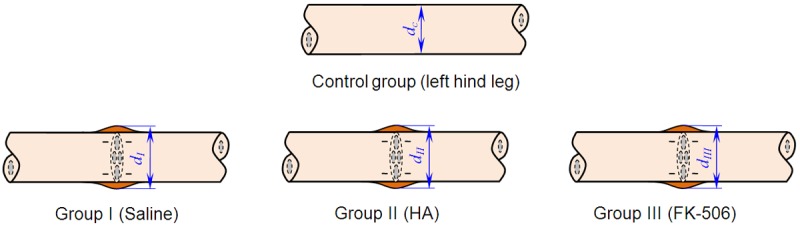
Simplified longitudinal representations of the sciatic nerves and their diameter.
Figure 2.
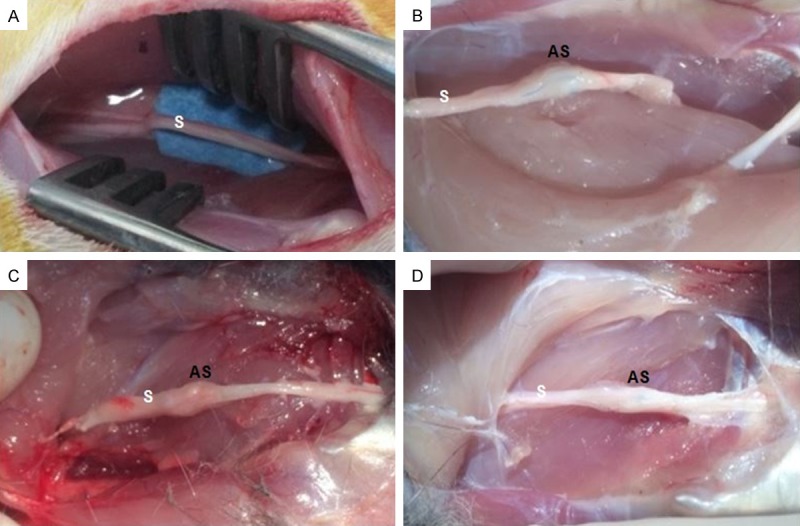
Photographic image of (A) non-transected exposed left sciatic nerve [control group]), and (B-D) right sciatic re-exposed transected nerve from experimental groups (I, II, and III, respectively). (S: sciatic nerve, AS: anastomosed site).
Biomechanical testing
The biomechanical assays were performed at the laboratory of the Faculty of Mechanical Engineering of the University of Prishtina using a universal tensile machine (Figure 3). To perform these experiments, the machine was modified to obtain electronically controlled and suitable measurements of the sciatic nerve load. The universal tensile machine produced adequate forces and jaw adjustment speeds to measure the biomechanical properties of the sciatic nerves. The prepared nerves of 35 mm in length were fixed in the jaws of the tensile machine. The nerves were then loaded under displacement control at 20 mm/min. The repaired site of the sciatic nerve, with an approximate length of 3-4 mm, was also examined via high-resolution video recording during the assay. The video data were analyzed on a computer and were correlated with the data from the tensile machine every 3 seconds, which corresponds to a 1-mm elongation of the sciatic nerve. The sciatic nerves were incrementally loaded until each repaired or control nerve failed.
Figure 3.
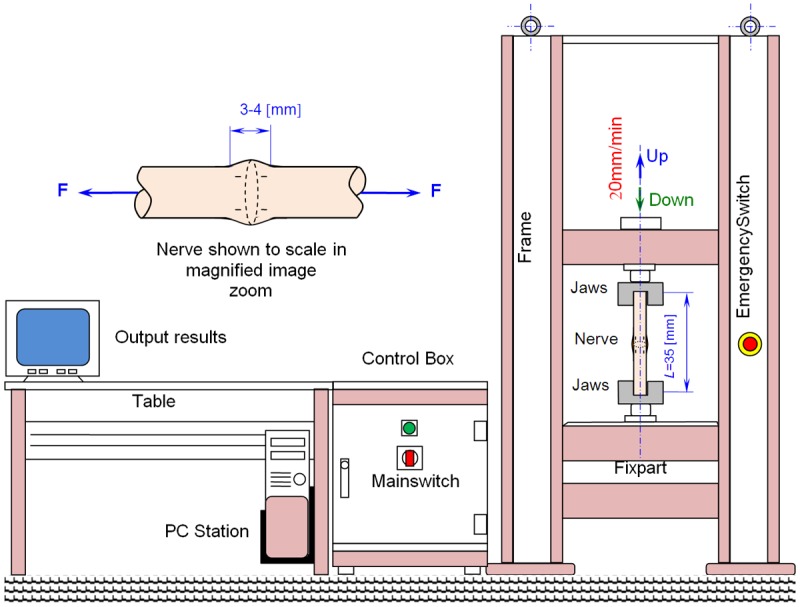
Schematic of the universal tensile machine used for the biomechanical assessment of sciatic nerves.
After experimentation, the stress-strain curves, force-displacement curves, maximum load, maximum displacement, maximum stress, maximum strain, elastic limit load, and elastic limit stress were computed. The schematic approach of the load applied to the sciatic nerve and the functions used to calculate the stress and strain values are presented in Figure 4. Stress represents the applied force over the cross-sectional area of the nerve, whereas strain refers to the amount of elongation in the direction of the applied force divided by the initial length of the nerve.
Figure 4.
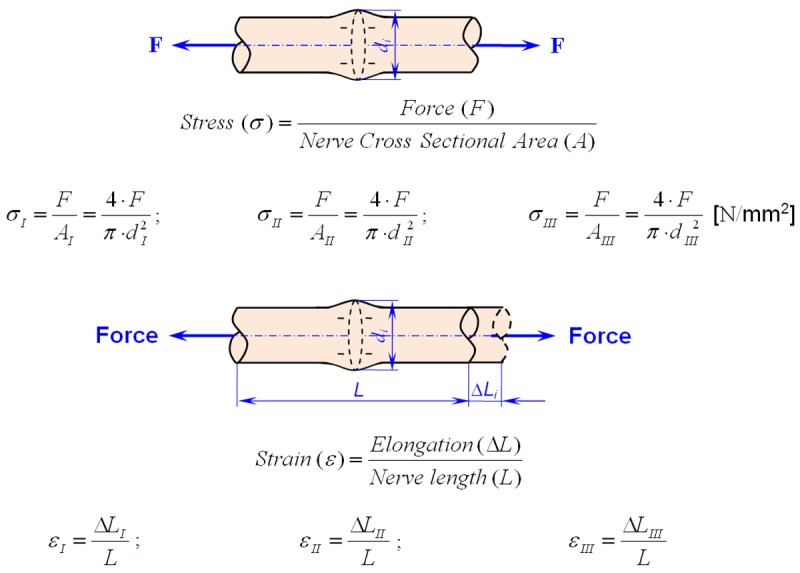
Schematic of the sciatic nerve and definitions of stress and strain.
Statistical analysis
The data were analyzed using SPSS Statistics 21.00 software (IBM, Chicago, IL, USA). Descriptive statistics (means and standard deviations [SDs]) and Student’s t-tests were used to analyze the biomechanical parameters. P<0.05 was considered significant.
Results
In the three experimental groups (6 samples per group), 12 weeks following transection and repair of the right sciatic nerve, the diameter and the biomechanical properties of the nerves were measured. As shown in Table 1, the mean nerve diameter of the control group was smaller than that of all experimental groups. Additionally, significant differences (P<0.05) in the mean nerve diameter were observed between the saline and HA groups and between the saline and FK-506 groups. In contrast, the difference in the mean nerve diameter between the HA and FK-506 groups was not significant (P>0.05).
Table 1.
Nerve diameters of the experimental and control groups
| Experimental groups | Non-operated group | |||
|---|---|---|---|---|
|
| ||||
| Group I-Saline d I [mm] | Group II-HA d II [mm] | Group III-FK-506 d III [mm] | Control dC [mm] | P valuea |
| <0.051 | ||||
| 4.21±0.28 | 3.7±0.43 | 3.66±0.44 | 2.52±0.20 | <0.052 |
| >0.053 | ||||
Nerve diameters:
P value for the control group compared to all three experimental groups;
P value for the saline group compared to both the HA and FK-506 groups;
P value for the HA group compared to the FK-506 group.
A P value <0.05 was considered statistically significant.
The average values and SDs of the biomechanical properties, including maximal load, elastic limit load, maximal displacement, maximal stress, elastic limit stress, maximal strain and elastic limit strain, which were recorded using the universal tensile machine, are presented in Table 2. Differences in the means of pairs of independent samples (i.e., the control group and groups I, II, and III) were assessed based on the t-test. Comparisons of the average maximal load, elastic limit load, maximal stress, and elastic limit strain values revealed highly significant differences between the control group and all three experimental groups (P<0.001). Extremely significant differences (P<0.0001) in the average values of other biomechanical parameters, including the maximal displacement, elastic limit stress, and maximal strain, were observed between the control group and all three experimental groups. However, the mean values of the biomechanical parameters did not significantly differ between the three experimental groups (P>0.05).
Table 2.
Biomechanical nerve properties of each tested group
| Groups | Maximal load [N] | Elastic limit load [N] | Maximal displacement [mm] | Maximal stress [N/mm2] | Elastic limit stress [%] | Maximal strain [%] | Elastic limit strain [%] |
|---|---|---|---|---|---|---|---|
| Control group | 12.09±0.87 | 7.40±0.64 | 19.01±1.40 | 0.84±0.1 | 0.50±0.08 | 60.20±2.48 | 23.20±0.34 |
| Group I | 9.10±1.0* | 5.50±0.87* | 15.53±0.83** | 0.61±0.09* | 0.30±0.05** | 49.10±2.8** | 22.10±0.59* |
| Group II | 9.81±0.9* | 5.62±0.98* | 15.68±0.86** | 0.65±0.01* | 0.33±0.04** | 48.99±2.4** | 21.90±0.33* |
| Group III | 9.22±1.0* | 5.59±0.99* | 15.72±0.97** | 0.62±0.18* | 0.31±0.05** | 49.12±2.36** | 22.03±0.28* |
P value <0.001 vs. Control group;
P value <0.0001 vs. Control group.
A P value <0.05 was considered statistically significant.
The stress-strain curves and the force-displacement curves for experimental groups I, II and III are presented in Figure 5. As shown in this figure, stress and strain initially increased linearly. This linear increase corresponds to the linear-elastic portion of the curve. Within this linear portion, no permanent elongation occurred. In this region, the sciatic nerve returns to its original shape once the stress is removed. However, when stress and strain reach a specific threshold, permanent deformation and failure of the sciatic nerve occur.
Figure 5.
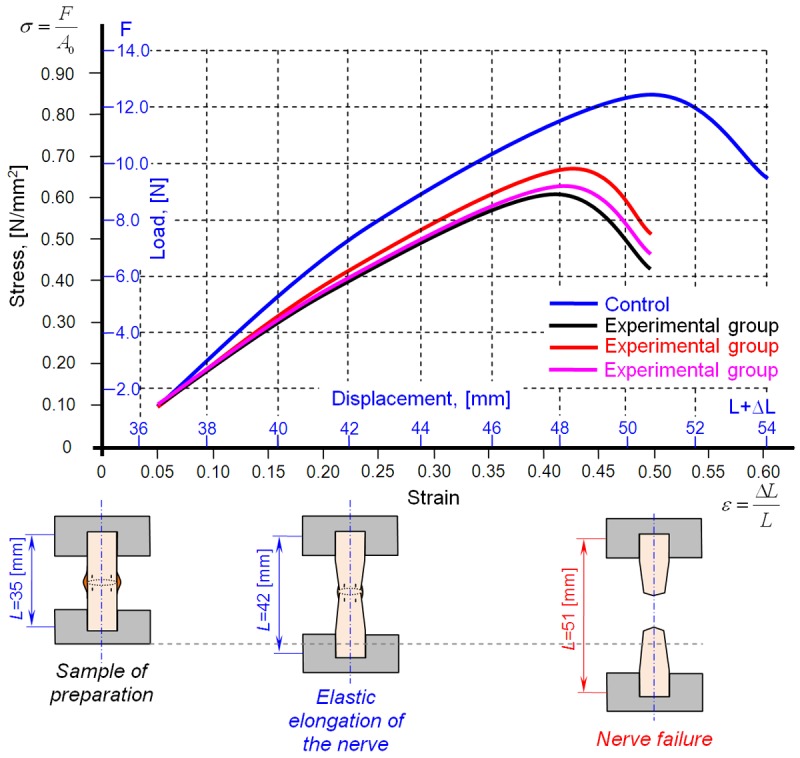
Diagram of the stress-strain and force-displacement relationships in the sciatic nerves of the experimental and control groups.
Discussion
Compared to the central nervous system, which is naturally very well protected, the peripheral nervous system is more vulnerable to different types of trauma [20]. The treatment of peripheral nerve injuries continues to represent a surgical challenge. Despite utilizing the best operating techniques, it is impossible to achieve complete recovery following nerve repair. However, the results of nerve repair depend on the age of the patient, the location and type of nerve injury (i.e., the presence or absence of nerve defects) and the method and timing of nerve repair [17,19]. Consequently, full functional and morphological recovery of the repaired nerve rarely occur due to scar formation, which results in neurological deficits [1]. The topical application of specific pharmacological agents at the site of nerve repair can prevent scar tissue formation and may increase nerve regeneration. HA and FK-506 are the most commonly used agents in experimental studies [5-8]. HA prevents scar formation via several mechanisms. HA exerts anti-inflammatory effects, including the suppression of lymphocyte proliferation, the reduction of granulocyte and macrophage chemotaxis, and the inhibition of granulocyte and macrophage motility and phagocytosis [7]. According to Konofaos et al. [21] and Que et al. [22], FK-506 reduces scar formation via its neurotrophic activities, which mediate neurite elongation and the enhancement of nerve regeneration; both of these activities have been demonstrated in vitro and in vivo. These pharmacological agents have been assessed in research animals undergoing various types of surgery (e.g., ophthalmological, cardiovascular, and dermatological surgeries) and particularly for their ability to prevent failed back surgery syndrome [23]. This study investigated the biomechanical properties of the sciatic nerve at the site of nerve repair following topical application of saline, HA, or FK-506. These properties will be compared to the functional and morphological characteristics of nerve regeneration in these animals (these results are the subject of additional, as-yet unpublished, studies). Immediately after the animals were sacrificed, we measured the nerve diameter at the site of nerve repair in the experimental groups and at similar sites in the control group of non-transected sciatic nerves (Table 1 and Figure 1). The average nerve diameter of the control group was smaller than that of all experimental groups (P<0.05). Moreover, the average nerve diameter of the saline group was significantly different from that of both the HA and FK-506 groups (P<0.05). However, the average nerve diameter was not significantly different between the HA and FK-506 groups (P>0.05). Biomechanical assays were performed using universal tensile machines to record maximal load, elastic limit load, maximal displacement, maximal stress, elastic limit stress, maximal strain and elastic limit strain. These results are presented in Table 2. The stress-strain curves and the force-displacement curves for the control and experimental groups (I, II and III) are presented in Figure 5. Comparisons of the means of selected biomechanical parameters (i.e., maximal load, elastic limit load, maximal stress, and elastic limit strain) revealed highly significant differences between the control group and all three experimental groups (P<0.001). The differences in several other biomechanical parameters (i.e., maximal displacement, elastic limit stress, and maximal strain) were extremely significant between the control group and all three experimental groups (P<0.0001). At 12 weeks after nerve repair, experimental groups I, II, and III displayed average maximal stress values that were 72.6%, 77.38%, and 73.8% of those in the control group, respectively. Additionally, as indicated by these percentages, no significant differences in maximal stress were observed between the experimental groups (P>0.05). These values are greater than the maximal stress values reported by Temple et al. [19] at 8 weeks after neurorrhaphy (63% of the control group). In contrast to our nerve diameter results, in which significant differences were observed between the saline group and both the HA and FK-506 groups, no significant differences in any of the biomechanical parameters were found between the experimental groups (P>0.05). These results can be explained by the delayed measurements of the biomechanical parameters; at 12 weeks, the nerve had almost completely healed. According to Ma et al. [24], biomechanical testing revealed that certain chemical substances disrupted the biomechanical properties of nerves relative to the properties of normal control nerves. However, these authors did not report significant differences (P>0.05). There have been many studies of the biomechanical properties of nerves; however, most of these studies differed from each other regarding the surgical techniques and pharmacological agents utilized.
To our knowledge, the effects of HA and FK-506 on scar formation, nerve regeneration, and the biomechanical properties of nerves have not previously been compared. Therefore, we are unable to compare our results to other published studies. Nevertheless, certain studies in which biomechanical testing was performed should be mentioned, although these studies utilized different materials during nerve repair. Isaacs et al. [25] examined fifty-seven fresh-frozen cadaveric nerve specimens following transection and repair consisting of two epineural sutures. In four of the five intervention groups, nerve repair involved the local application of different glues, i.e., fibrin glue, Tisseel fibrin glue, Evicel fibrin glue or the polyethylene glycol-based hydrogel sealant Duraseal; in the fifth group, no glue was applied. These authors reported no significant differences in the peak loads at failure between the glue-treated groups and the non-glue-treated group. Temple at al. [26] used fibrin glue for nerve repair utilizing five different surgical techniques, and fibrin glue repair was weaker than all other repair techniques. Additionally, similar biomechanical resistance results were reported by Nishimura et al., who studied peripheral nerve repair using glue at different postoperative time points [27]. Peng et al. [16] reported tensile test results, which indicated that maximal load, displacement, stress and strain in the sciatic nerve injury models (in which amniotic membranes were used for neurorrhaphy) were greater than those in autologous nerve anastomosis models. In another study that involved two groups of rabbits, in which immediate or delayed sciatic nerve repair was performed, no significant difference in the biomechanical results between the two groups was observed [28].
Conclusions
Based on our results, we conclude that neither topical HA nor FK-506 application influences the biomechanical properties of the sciatic nerve at 12 weeks after nerve repair. We observed no significant differences in biomechanical properties in the HA and FK-506 groups compared to the saline group. However, several authors have reported improved biomechanical function during the initial weeks following nerve repair, although these authors used different pharmacological agents and distinct microsurgical techniques. Additionally, it should be mentioned that our biomechanical results differ from the unpublished results of our functional and morphological examinations. Future studies are needed to evaluate the biomechanical properties of the sciatic nerve at 12 weeks after nerve repair as well as the effects of topical HA and FK-506 application.
Disclosure of conflict of interest
None.
References
- 1.Grinsell D, Keating CP. Peripheral nerve reconstruction after nerve injury. A review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kucuk L, Gunay H, Erbas O, Kucuk U, Atamaz F, Coskunol E. Effects of platelet-rich plasma on nerve regeneration in a rat model. Acta Orthop Traumatol Turc. 2014;48:449–454. doi: 10.3944/AOTT.2014.13.0029. [DOI] [PubMed] [Google Scholar]
- 3.Fex Svennigsen A, Dahlin LB. Repair of the Peripheral Nerve-Remyelination that Works. Brain Sci. 2013;3:1182–1197. doi: 10.3390/brainsci3031182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekaj AY, Morina AA, Bytyqi CI, Mekaj YH, Duci SB. Application of topical pharmacological agents at the site of peripheral nerve injury and methods used for evaluating the success of the regenerative process. J Orthop Surg Res. 2014;9:94. doi: 10.1186/s13018-014-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozgenel GY. Effects of hyaluronic acid on the peripheral nerve scarring regeneration in rats. Microsurgery. 2003;23:575–581. doi: 10.1002/micr.10209. [DOI] [PubMed] [Google Scholar]
- 6.Azizi S, Mohammadi R, Amini K, Fallah R. Effects of topically administered FK506 on sciatic nerve regeneration and reinnervation after vein graft repair of short nerve gaps. Neurosurg Focus. 2012;32:E5. doi: 10.3171/2012.1.FOCUS11320. [DOI] [PubMed] [Google Scholar]
- 7.Burd DA, Greco RM, Regauer S, Longaker MT, Siebert JW, Garg HG. Hyaluronan and wound healing: a new perspective. Br J Plast Surg. 1991;44:579–584. doi: 10.1016/0007-1226(91)90093-y. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Wang W, Wei G, Wang G, Zhang W, Ma X. ImmunophilinFK506 loaded in chitosan guide promotes peripheralnerve regeneration. Biotechnol Lett. 2010;32:1333–1337. doi: 10.1007/s10529-010-0287-8. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan S, Odaci E, Unal B, Sahin B, Fornaro M. Chapter 2: Development of the peripheral nerve. Int Rev Neurobiol. 2009;87:9–26. doi: 10.1016/S0074-7742(09)87002-5. [DOI] [PubMed] [Google Scholar]
- 10.Smith CE, Atchabahian A, Mackinon SE, Hunter DA. Development of the blood-nerve barrier in neonatal rats. Microsurgery. 2001;21:290–297. doi: 10.1002/micr.1055. [DOI] [PubMed] [Google Scholar]
- 11.Shellswell GB, Restall DJ, Duance VC, Bailey AJ. Identification and differential distribution of collagen types in the central and peripheral nervous system. FEBS Lett. 1979;106:305–308. doi: 10.1016/0014-5793(79)80520-7. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz HC, Beer GM. The toe-spreading reflex of the rabbit revisited-functional evaluation of complete peroneal nerve lesions. Lab Anim. 2001;35:340–345. doi: 10.1258/0023677011911930. [DOI] [PubMed] [Google Scholar]
- 13.Henry FP, Goyal NA, David WS, Wes D, Bujold KE, Randolph MA, Winograd JM, Kochevar IE, Redmound RW. Improving electrophysiologic and histologic outcomes by photochemically sealing amnion to the peripheral nerve repair site. Surgery. 2009;145:313–321. doi: 10.1016/j.surg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Park JS, Lee JH, Han CS, Chung DW, Kim GY. Effect of hyaluronic acid-carboxymethyl cellulose solution on perineural scar formation after sciatic nerve repair in rats. Clinin Orthop Surg. 2011;3:315–324. doi: 10.4055/cios.2011.3.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restaino SM, Abliz E, Wachrathit K, Krauthamer V, Shah SB. Biomechanical and functional variation in rat sciatic nerve following cuff electrode inplantion. J Neuroeng Rehabil. 2014;23:11–73. doi: 10.1186/1743-0003-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng C, Zhang Q, Yang Q, Zhu Q. Strain and stress variations in the human amniotic membrane and fresh corpse autologous sciatic nerve anastomosis in a model of sciatic nerve injury. Neural Regen Res. 2012;7:1179–1185. doi: 10.3969/j.issn.1673-5374.2012.23.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.P M, S H, M D, M S. Advances of peripheral nerve repair techniques to improve hand function: a systematic review of literature. Open Orthop J. 2012;6:60–68. doi: 10.2174/1874325001206010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, Zhou CP, Zhong XM, Guo RM, Griffith JF, Cheng LN, Duan XH, Liang BL. MR neurography: T1 and T2 measurements in acute peripheral nerve traction injury in rabbits. Radiology. 2010;254:729–738. doi: 10.1148/radiol.09091223. [DOI] [PubMed] [Google Scholar]
- 19.Temple CL, Ross DC, Dunning CE, Johnson JA, King GJ. Tensile strength of healing peripheral nerves. J Reconstr Microsurg. 2003;19:483–488. doi: 10.1055/s-2003-44637. [DOI] [PubMed] [Google Scholar]
- 20.Rosso G, Liashkovich I, Gess B, Young P, Kun A, Shahin V. Unravelling crucial biomechanical resilience of myelinated peripheral nerve fibers providedby the Schwann cell basal lamina and PMP22. Sci Rep. 2014;4:7286. doi: 10.1038/srep07286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konofaos P, Terzis JK. FK506 and nerve regeneration: past, present and future. J Reconstr Microsurg. 2013;29:141–148. doi: 10.1055/s-0032-1333314. [DOI] [PubMed] [Google Scholar]
- 22.Que J, Cao Q, Sui T, Du S, Kong D, Cao X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 2013;4:e526. doi: 10.1038/cddis.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mekaj Y, Mekaj A. Prevention of failed back surgery syndrome with applications of different pharmacological agents: A review article. Ther Targets Neurol Dis. 2015;2:e507. [Google Scholar]
- 24.Ma XL, Sun XL, Yang Z, Li XL, Ma JX, Zhang Y, Yuan ZZ. Biomechanical properties of peripheral nerve after acellular treatment. Chin Med J (Engl) 2011;124:3925–3929. [PubMed] [Google Scholar]
- 25.Isaacs JE, McDaniel CO, Owen JR, Wayne JS. Comparative analysis of the biomechanical performance of available “nerve glues”. J Hand Surg Am. 2008;33:893–899. doi: 10.1016/j.jhsa.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Temple CL, Ross DC, Dunning CE, Johnson JA. Resistance to distruption and gapping of peripheral nerve repairs: and in vitro biomechanical assessment of techniques. J Reconstr Microsurg. 2004;20:645–650. doi: 10.1055/s-2004-861525. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura MT, Mazzer N, Barbieri CH, Moro CA. Mechanical resistance of peripheral nerve repair with biological glue and with conventional suture at different postoperative times. J Reconstr Microsurg. 2008;24:327–332. doi: 10.1055/s-2008-1080535. [DOI] [PubMed] [Google Scholar]
- 28.Piskin A, Altunkaynak BZ, Çitlak A, Sezgin H, Yazici O, Kaplan S. Immediate versus delayed primary nerve repair in the rabbit sciatic nerve. Neural Regen Res. 2013;8:3410–3415. doi: 10.3969/j.issn.1673-5374.2013.36.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


