Abstract
Cancer is a global and growing problem. Nodal, which has been showed to be involved in occurrence and development of cancers, is an important embryonic morphogen. The aim of this study was to evaluate the significance of Nodal expression in human cancers based on the published related articles. Online databases were searched to retrieve relevant articles published between 2000 and 2015. The odds ratio (OR) with its 95% confident intervals (CI) were employed to calculate the strength of significance. Finally, a total of 11 articles were screened out, including 801 cancer patients and 372 healthy controls. Nine kinds of cancers were contained, and Nodal was detected in 56.7% of all participants (665/1173). Overall, our result found that Nodal was highly expressed in cancer patients than that in healthy controls, indicating that Nodal expression was significantly associated with cancers progression (OR=21.72, 95% CI=9.94-47.46, P<0.00001). Subgroup analysis showed that Nodal expression was significantly corrected with high WHO grade of human cancers (III+IV versus I+II: OR=2.46, 95% CI=1.63-3.71, P<0.00001). This significant relationship was also found in tumor size, differentiation degree, not observed in gender, age and lymphatic metastasis status of patients with all studied cancers in this meta-analysis. In conclusion, our results demonstrated that Nodal might be implicated in cancer progression, suggesting that it was a potential biomarker and therapeutic target for cancers.
Keywords: Cancer, Nodal, expression, significance, meta-analysis
Introduction
Cancer, the first and the second leading cause of death in economically developed and developing countries respectively, is a major public health problem in many parts of the world [1]. Its occurrence is increasing because of the growth and aging of the population, and the increasing prevalence of established risk factors such as overweight, smoking, and physical inactivity [2-4]. According to GLOBOCAN estimates, about 14.1 million new cancer cases and 8.2 million cancer deaths are estimated to have occurred in 2012 [5]. A population-based study suggests that more than 20 million people will be diagnosed with cancer every year by 2030, with more than 13 million cancer deaths [6]. Although considerable progress and improvement such as immunotherapy [7], radiotherapy [8], and anticancer drugs [9] have been made in cancer treatment strategies, the 5-year relative survival rate still remains low [10], with 68% in the United States between 2004 and 2010 [11]; 66.3% in Korean between 2007 and 2011 [12]; and 30.9% in China between 2003 and 2005 [13] for all sites combined in both sexes. Moreover, most cancers were confirmed at their late-stages, and the recurrence rate after the surgery was high [14]. Thus, finding a more effective biological biomarker which can predict the prognosis, reduce the recurrence and metastasis rates, and play a role in medical treatment of malignant tumor has become an international difficult problem.
Cancer cells can motivate normally dormant embryonic pathways to induce tumorigenesis and metastasis. Nodal, belongs to the transforming growth factor-β (TGF-β) superfamily, is an embryonic morphogen [15]. In humans, Nodal is largely restricted expressed in embryonic tissues and reproductive cell types, and is not detectable in most normal adult tissues [16,17]. However, recent studies have demonstrated that Nodal expression is re-emerged during cancer progression [18], and is corrected with increased tumourigenesis, invasion and metastasis [19]. Evidences from previous researches have shown that Nodal could serve as a biomarker for progression and poor prognosis of cancers, and function as a potential therapeutic target. Chai et al. found that Nodal might be useful for the diagnosis of malignant thyroid tumor, and might be related with the tumorigenesis of thyroid malignancy [20]. Chen et al. demonstrated that Nodal expression was associated with aggressive characteristics of hepatocellular carcinoma, and its aberrant expression might be a predictive factor of unfavorable prognosis for hepatocellular carcinoma after surgery [21]. Kong et al. discovered that high expression of Nodal correlated with reduced patient survival in pancreatic cancer [22]. Strizzi et al. suggested that Nodal was a potential biomarker for disease progression and a promising target for anti-Nodal therapy in breast cancer [23].
Realizing that the results from each single study might show discrepancy because of the different populations, ages, tumor stages of participants present, we conducted this meta-analysis to comprehensive review all the related articles concerning the effect of Nodal expression in human cancers and to obtain a relatively reliable result.
Materials and methods
Search strategy
We performed a systematic literature search in the online databases of Medline, PubMed, Embase, Cochrane Library databases and CNKI (Chinese National Knowledge Infrastructure) to retrieve relevant articles published between January 2000 and July 2015. The following MeSH terms: “cancer or carcinoma or neoplasm or tumor or malignancy”, “Nodal or embryonic morphogen”, “expression” and “clinical significance” as well as their combinations were employed as the searching words. To obtain more articles, we manually searched the references of each included studies. Our study only focused reports that conducted on humans. The languages were limited to Chinese and English.
Inclusion and exclusion criteria
Eligible articles should meet the following criteria: 1) evaluating the significance of Nodal expression in patients with cancers; 2) patients of each cancer were confirmed by at least two pathologists, and must be accorded with the diagnostic standards for a single cancer; 3) the controls should be healthy participants; 4) the Nodal-positive expression was determined by immunohistochemistry (IHC) method; and 5) the related data were available to extract.
The exclusion criteria were as follow: 1) studies were reviews or conference papers; 2) duplicated studies from the same authors or laboratory; 3) without control group; and 4) data couldn’t be extracted.
Data extraction
Two of our authors systematically read all related articles independently, and then assessed the quality of selected articles. They should reach a consensus on each item, and any disagreement was resolved by discussing with a third expert. The first author, published year, country, sources of cases and controls, sample size and number of Nodal-positive expression were extracted from each included study.
Statistical analysis
The pooled odds ratio (OR) with its corresponding 95% confidence interval (CI) were employed to identify the significance of Nodal expression on cancer progression. The Z test was used to estimate the effect with P-value less than 0.05 considering significant. Between-study heterogeneity was calculated by the Q test and the I2 test. When the effect was assumed to be homogeneous (p-value for the Q test more than 0.01 and I2 for the I2 test less than 50%), the fixed-effect model was used; otherwise, the random-effect model was used when it was on its opposite. The RevMan 5.2 program was used to perform all the analysis.
Results
Characteristics of included studies
After applying the inclusion and exclusion criteria, we totally screened out 11 relevant articles, including 1173 participants (801 cancer patients and 372 healthy controls). Figure 1 showed the process of selection. The 11 articles, which were all conducted in Chinese population, contained nine types of cancers (gastric carcinoma [24,25], cervical carcinoma [26], hepatocellular carcinoma [27], bladder urothelial carcinoma [28], colorectal cancer [29,30], lung adenocarcinoma [31], renal cell carcinoma [32], breast cancer [33], prostate carcinoma [34]). The sample size ranged from 23 to 234. Nodal expression was all measured by HIC method, and was detected in 56.7% of all participants (665/1173). The main characteristics of included studies were shown in Table 1.
Figure 1.
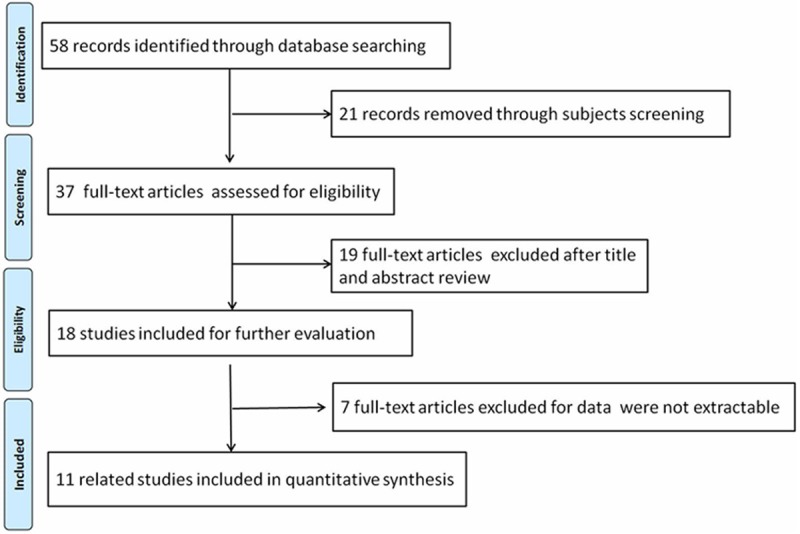
Flow chart for the selection of included articles.
Table 1.
Main characteristics of included studies in this meta-analysis
| First author | Year | Country | Sources of participants | Sample size | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Cases | Controls | Positive | Total | Positive | Total | |||
| Zhou HJ | 2010 | China | Gastric carcinoma | Normal gastric mucosa | 46 | 50 | 1 | 20 |
| Xu RR | 2011 | China | Cervical carcinoma | Normal cervical epithelium | 22 | 26 | 2 | 20 |
| Wang XJ | 2012 | China | Hepatocellular carcinoma | Normal liver tissue | 59 | 95 | 4 | 20 |
| Hu J | 2013 | China | Bladder urothelial carcinoma | Normal bladder mucosa | 17 | 17 | 0 | 6 |
| Ji HY | 2013 | China | Gastric carcinoma | Normal gastric mucosa | 94 | 135 | 22 | 99 |
| Luo HP | 2013 | China | Colorectal cancer | Normal colorectal tissues | 59 | 93 | 17 | 93 |
| Ouyang ZC | 2013 | China | Lung adenocarcinoma | Normal lung tissue | 56 | 80 | 3 | 20 |
| Li YK | 2014 | China | Renal cell carcinoma | Normal renal tissue | 25 | 25 | 0 | 16 |
| Liu P | 2014 | China | Colon adenocarcinoma | Normal colon mucosa | 97 | 100 | 10 | 23 |
| Zeng FC | 2014 | China | Breast cancer | Normal breast epithelium | 83 | 140 | 8 | 35 |
| Zhu W | 2014 | China | Prostate carcinoma | Normal prostatic tissue | 40 | 40 | 0 | 20 |
Significance of Nodal expression in cancer development and progression
We identified the difference of Nodal expression in human cancers and related normal tissues. Between-study heterogeneity was observed, and the random-effect model was used. Overall, we found that Nodal protein was highly expressed in cancer patients than that in healthy controls (74.7% versus 18.0%). This result demonstrated that Nodal expression was significantly associated with the increased risk of patients with cancers (OR=21.72, 95% CI=9.94-47.46, P<0.00001) as shown in Figure 2.
Figure 2.
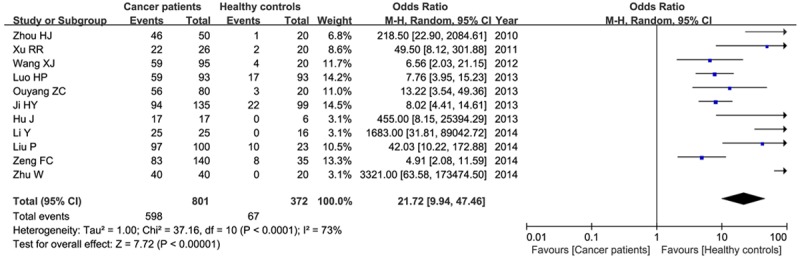
Meta-analysis of Nodal expression and progression of all studied cancers.
Correction of Nodal expression on TNM stage of cancers
According to WHO grade, we divided cancer patients into low- (I+II) and high-grade (III+IV). Six articles contained these data and were available to extract, including 249 high-grade and 281 low-grade patients, respectively. No significant heterogeneity was found between studies (P=0.87, I2=0%), and the fixed-effect model was employed. Our results observed that Nodal was highly expressed in high-grade patients than that in low-grade (79.5% versus 62.9%), indicating Nodal expression was significantly associated with high WHO grade of human cancers (OR=2.46, 95% CI=1.63-3.71, P<0.00001) as shown in Figure 3.
Figure 3.
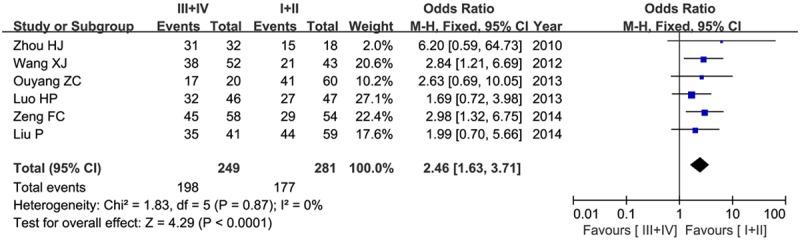
Forest plot of the significance of Nodal expression in tumor grade in a fixed-effect model.
Association between Nodal expression and gender, age, tumor size, lymphatic metastasis status, tumor differentiation degree, and 5-year overall survival of patients with cancers
Four studies concerned the gender issue, including 368 cases (206 male patients and 162 female patients). Our results did not find a significant association between Nodal expression and different gender composition (male versus female: OR=0.83, 95% CI=052-1.84, P=0.44) in a fixed-effect model as shown in Figure 4A.
Figure 4.
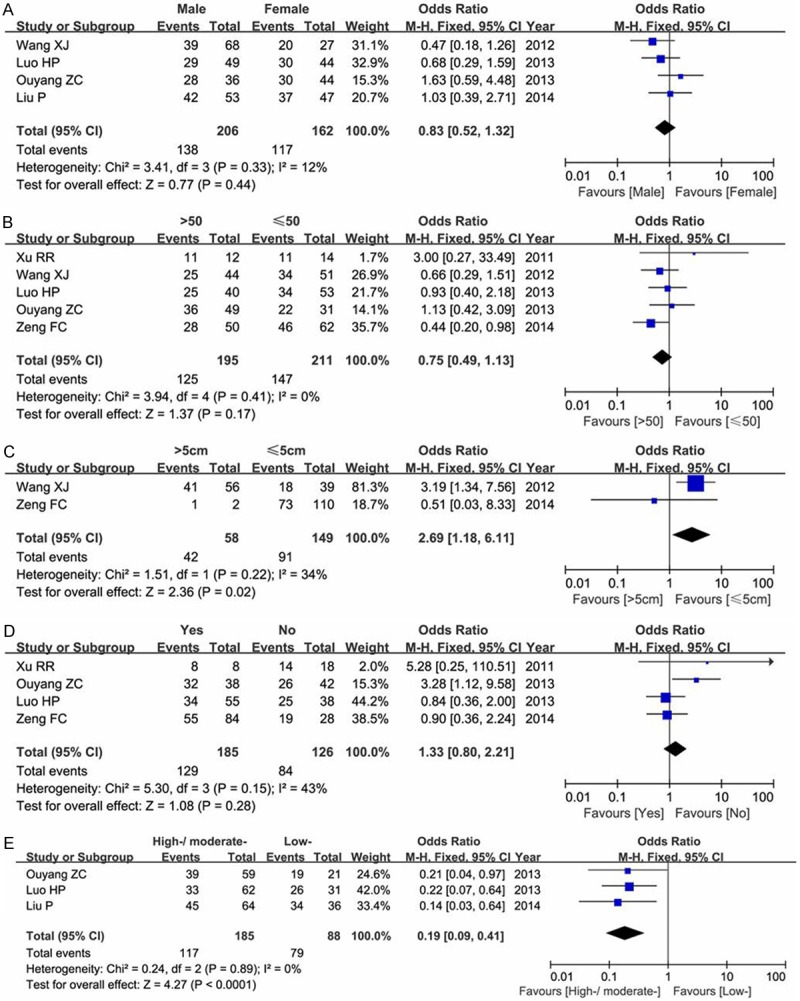
Meta-analysis of Nodal expression in gender (A), age (B), tumor size (C), lymphatic metastasis status (D) and differentiation degree (E) of patients with human cancers.
Five studies focused on the age issue, containing 407 cancer patients. We divided ages into two comparable groups (>50-year-old group and ≤50-year-old group). As shown in Figure 4B, our results showed no difference between Nodal expression and age groups (OR=0.75, 95% CI=0.49-1.13, P=0.17) in a fixed-effect model.
Two articles concerned the tumor size (207 cancer patients). Our results indicated that Nodal expression was significant associated with the difference of tumor size (>5 cm versus ≤5 cm OR=2.69, 95% CI=1.18-6.11, P=0.02) in a fixed-effect model as shown in Figure 4C.
Four studies concerned the lymphatic metastasis status (311 patients). Our results demonstrated that Nodal expression was not associated with whether the lymphaden metastasizes or not (Yes versus No: OR=1.33, 95% CI=0.80-2.21, P=0.28) in a fixed-effect model as shown in Figure 4D.
Three studies focused on the tumor differentiation degree. Our analysis found a significant relationship between Nodal expression and differentiation of cancers (high-/moderate-versus low-: OR=0.19, 95% CI=0.09-0.41, P<0.00001) in a fixed-effect model as shown in Figure 4E.
Only one study identified the 5-year overall survival (OS) and disease-free survival (DFS). The results showed that Nodal expression was significant associated with 5-year OS and 5-year DFS (P<0.01).
Sensitivity analysis and publication bias
Each study was deleted to reveal any single study on the overall effect. Our results indicated that the pooled OR was not significantly influenced by omitting any single study at a given time. The funnel plot also demonstrated no publication bias in this meta-analysis as shown in Figure 5.
Figure 5.
Publication bias of the funnel plot of Nodal expression in cancer progression.
Discussion
Nodal is an oncofetal protein. It plays a key role not only in regulating the growth and differentiation of embryonic stem cells [35], but also in the plasticity and invasion of malignant tumor cells [36]. Nodal expression has been shown to be correlated with tumor grade. In this present meta-analysis, we retrieved 11 related articles containing nine tumors. Our results showed that Nodal expression was significant associated with progression, grade, size, and differentiation degree of tumors. No relationship was found between Nodal expression and gender, age, lymphatic metastasis status of cancer patients. This is the first systematic meta-analysis evaluating the significance of Nodal expression in human cancers.
Nodal protein plays a critical role in normal embryonic development, and regulates the proliferation and numerous developmental processes in various normal physiological systems. Harrison et al. have found that Nodal was highly expressed in the proliferative and early secretory phases of human endometrium during remodelling, and was abruptly downregulated by the mid-secretory phase [37]. Kenney et al. have found that members of the Nodal signaling pathway were cyclically expressed during mammary gland remodeling [38]. These results indicated that Nodal might promote the proliferative phenotypes in dynamic epithelial cell types.
Recent findings have revealed that Nodal is an important regulator of cancer growth, plasticity, and tumorigenicity. Understanding the impact of Nodal signaling pathways and the regulatory programs holds significant potential for new cancer therapies. Previous studies have showed that TGF-β1 promoted the expression of Nodal via activating Smads and MAPK pathways, and thereby promoted the growth of glioma cells [39]. Nodal also promotes the self-renewal of human colon cancer stem cells via an autocrine manner through Smad2/3 signaling pathway [40]. Duan et al. discovered that Nodal overexpression enhanced pancreatic cancer cells migration and invasion via the Smad2/3 pathway, and inhibition of Nodal signaling reduced distant metastasis and reversed the invasive, indicating that targeting Nodal signaling might be a promising therapeutic strategy for pancreatic cancer [41]. Moreover, Nodal is involved in promoting invasion in multiple cellular contexts via a mitogen-activated protein kinase-dependent pathway, indicating Nodal inhibition may be useful as a therapeutic target for patients with progressive disease [42]. Fang et al. revealed that Nodal promoted the aggressive phenotype of B16 murine melanoma cells by inducing EMT via up-regulation of Snail [43]. In human breast cancer, Nodal signaling promoted a tumorigenic phenotype [44], AND supported tumor growth at primary and secondary sites by increasing the ratio of proliferation and apoptosis in breast cancer cells [45].
Nodal might be involved in tumor angiogenesis, thus promoting the occurrence and development of malignant tumors. The extent of tumor neovasclarzation can reflect the degree and invasion of malignancy, and the prognosis of patients. Hueng et al. demonstrated that overexpression of Nodal promoted glioma growth and angiogenesis in vivo, and inhibition of Nodal suppressed U87MG glioma growth and angiogenesis in the brain [46]. Chen et al. found that Nodal was highly expressed in hepatocellular carcinoma tissues which was was positively correlated with high microvascular density and low accumulated survival rate, indicating that Nodal could promote angiogenesis, and be used as a predictor of poor prognosis [47]. Quail et al. showed that Nodal promoted vascular recruitment in vivo, suggesting Nodal as a potential target for the treatment of breast cancer angiogenesis and progression [48].
There were several limitations in our study. Firstly, all the included studies were conducted in Chinese population, while other ethnicities should be considered. Secondly, total nine kinds of tumors were included, more articles concerned the same tumor are needed due to the difference of tumor biology. Thirdly, other genes, such as VEGF, Cripto-1 which may interact with Nodal should be focused on. Lastly, high quality studies with complete reports, including TNM stage and survival data, were limited, which may affect our conclusions.
In conclusion, our results showed that Nodal expression was associated with progression, stage, size, and differentiation of cancers. Future large-scale, well-designed studies with more nations are still needed to further exploring the significance of Nodal expression in human cancers.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81272311, 81273538 and 81472643) and Postdoctoral Startup Foundation of Guangzhou City (No. Fen Ning).
Disclosure of conflict of interest
None.
References
- 1.Mathers C, Fat DM, Boerma JT. The global burden of disease: 2004 update. World Health Organization. 2008 [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Banavali A, Pyle D, Fincham K, Miller C, Nadler B. Global Cancer Epidemiology and the Cancer Divide. Global Perspectives on Cancer: Incidence, Care, and Experience. 2015:5. [Google Scholar]
- 4.Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014;383:549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 6.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 7.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaromina A, Krause M, Baumann M. Individualization of cancer treatment from radiotherapy perspective. Mol Oncol. 2012;6:211–221. doi: 10.1016/j.molonc.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homer RJ. Cancer Treatment Drugs. 2013 [Google Scholar]
- 10.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 12.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46:109–23. doi: 10.4143/crt.2014.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, Zhang L, Tang J, Chen J, Wei K. Cancer survival in China, 2003-2005: A populationbased study. Int J Cancer. 2015;136:1921–1930. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- 14.Seinen JM, Styring E, Verstappen V, von Steyern FV, Rydholm A, Suurmeijer AJ, Hoekstra HJ. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700–2706. doi: 10.1245/s10434-012-2310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strizzi L, Hardy KM, Kirschmann DA, Ahrlund-Richter L, Hendrix MJ. Nodal expression and detection in cancer: experience and challenges. Cancer Res. 2012;72:1915–1920. doi: 10.1158/0008-5472.CAN-11-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 17.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U S A. 2008;105:4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence MG, Margaryan NV, Loessner D, Collins A, Kerr KM, Turner M, Seftor EA, Stephens CR, Lai J, Postovit LM. Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Prostate. 2011;71:1198–1209. doi: 10.1002/pros.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quail DF, Siegers GM, Jewer M, Postovit LM. Nodal signalling in embryogenesis and tumourigenesis. Int J Biochem Cell Biol. 2013;45:885–898. doi: 10.1016/j.biocel.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Chai YJ, Kim YA, Jee HG, Yi JW, Jang BG, Lee KE, Park YJ, Youn YK. Expression of the embryonic morphogen Nodal in differentiated thyroid carcinomas: Immunohistochemistry assay in tissue microarray and The Cancer Genome Atlas data analysis. Surgery. 2014;156:1559–1568. doi: 10.1016/j.surg.2014.08.050. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Liu WB, Jia WD, Xu GL, Ma JL, Ren Y, Chen H, Sun SN, Huang M, Li JS. Embryonic morphogen nodal is associated with progression and poor prognosis of hepatocellular carcinoma. PLoS One. 2014;9:e85840. doi: 10.1371/journal.pone.0085840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong B, Wang W, Esposito I, Friess H, Michalski CW, Kleeff J. Increased expression of Nodal correlates with reduced patient survival in pancreatic cancer. Pancreatology. 2015;15:156–161. doi: 10.1016/j.pan.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Strizzi L, Hardy KM, Margaryan NV, Hillman DW, Seftor EA, Chen B, Geiger XJ, Thompson EA, Lingle WL, Andorfer CA. Potential for the embryonic morphogen Nodal as a prognostic and predictive biomarker in breast cancer. Breast Cancer Res. 2012;14:R75. doi: 10.1186/bcr3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H. The expression of Nodal and Survivin proteins and their association with gastric cancer. Central South University. 2010 [Google Scholar]
- 25.Ji H, Xin Y, Zhao J, Xiao Y. Expression and significance of Nodal protein in human gastric carcinoma. Chin J Pract Int Med. 2013;33:561–562. [Google Scholar]
- 26.Xu R. Expression and clinical significance of Cripto-1, Nodal in cervical carcinoma. Anhui Medical University. 2011 [Google Scholar]
- 27.Xiujun W, Weidong J, Geliang X. Expression and significance of Nodal protein in hepatocellular carcinoma. J Clin Hepatol. 2012;1:15. [Google Scholar]
- 28.Hu J. Expression and significance of Nodal protein in bladder urothelial carcinoma. Yangtze University. 2013 [Google Scholar]
- 29.Luo H, Tong S, Zheng Y, Shi Q. Expression and clinical significance of Dpr2 and Nodal in colorectal cancer. Chin J Clinicians (Electronic Edition) 2013;7:10439–10443. [Google Scholar]
- 30.Liu P. The clinicopathological significance of the embryonic morphogen Nodal expression in colon adenocarcinoma. Luzhou Medical College. 2014 [Google Scholar]
- 31.Ouyang Z. Expression and significance of Nodal protein in lung adenocarcinoma. Central South University. 2013 [Google Scholar]
- 32.Li Y, Zhou J, Zhang X, Zhong W. Expression and significance of Nodal in renal cell carcinoma. Guizhou Medical Journal. 2014;38:295–299. [Google Scholar]
- 33.Zeng F. Expression, function and clinical significance of Nodal and its receptor ALK47 and ALK4 in breast cancer. University of Electronic Science and Technology of China. 2014 [Google Scholar]
- 34.Zhu W. Expression and significance of Nodal protein in prostate carcinoma. Yangtze University. 2014 [Google Scholar]
- 35.Mfopou JK, Chen B, Sui L, Sermon K, Bouwens L. Recent advances and prospects in the differentiation of pancreatic cells from human embryonic stem cells. Diabetes. 2010;59:2094–2101. doi: 10.2337/db10-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaminska B, Wesolowska A, Danilkiewicz M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol. 2005;52:329. [PubMed] [Google Scholar]
- 37.Papageorgiou I, Nicholls PK, Wang F, Lackmann M, Makanji Y, Salamonsen LA, Robertson DM, Harrison CA. Expression of nodal signalling components in cycling human endometrium and in endometrial cancer. Reprod Biol Endocrinol. 2009;7:b2. doi: 10.1186/1477-7827-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenney NJ, Adkins HB, Sanicola M. Nodal and Cripto-1: embryonic pattern formation genes involved in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2004;9:133–144. doi: 10.1023/B:JOMG.0000037158.91940.1c. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Liu SZ, Lin Y, Cao XP, Liu JM. TGF-β promotes glioma cell growth via activating Nodal expression through Smad and ERK1/2 pathways. Biochem Biophys Res Commun. 2014;443:1066–1072. doi: 10.1016/j.bbrc.2013.12.097. [DOI] [PubMed] [Google Scholar]
- 40.Gong Y, Guo Y, Hai Y, Yang H, Liu Y, Yang S, Zhang Z, Ma M, Liu L, Li Z. Nodal promotes the self-renewal of human colon cancer stem cells via an autocrine manner through Smad2/3 signaling pathway. Biomed Res Int. 2014;2014:364134. doi: 10.1155/2014/364134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li R, Duan W, Ma J, Lei J, Xu Q, Jiang Z, Nan L, Li X, Wang Z, Huo X. Overexpression of Nodal induces a metastatic phenotype in pancreatic cancer cells via the Smad2/3 pathway. Oncotarget. 2014;5:1490–506. doi: 10.18632/oncotarget.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quail D, Zhang G, Findlay S, Hess D, Postovit L. Nodal promotes invasive phenotypes via a mitogen-activated protein kinasedependent pathway. Oncogene. 2014;33:461–473. doi: 10.1038/onc.2012.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang R, Zhang G, Guo Q, Ning F, Wang H, Cai S, Du J. Nodal promotes aggressive phenotype via Snail-mediated epithelial-mesenchymal transition in murine melanoma. Cancer Lett. 2013;333:66–75. doi: 10.1016/j.canlet.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Kirsammer G, Strizzi L, Margaryan NV, Gilgur A, Hyser M, Atkinson J, Kirschmann DA, Seftor EA, Hendrix MJ. Nodal signaling promotes a tumorigenic phenotype in human breast cancer. Semin Cancer Biol. 2014;29:40–50. doi: 10.1016/j.semcancer.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quail DF, Zhang G, Walsh LA, Siegers GM, Dieters-Castator DZ, Findlay SD, Broughton H, Putman DM, Hess DA, Postovit LM. Embryonic morphogen nodal promotes breast cancer growth and progression. PLoS One. 2012;7:e48237. doi: 10.1371/journal.pone.0048237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hueng DY, Lin GJ, Huang SH, Liu LW, Ju DT, Chen YW, Sytwu HK, Chang C, Huang SM, Yeh YS. Inhibition of Nodal suppresses angiogenesis and growth of human gliomas. J Neurooncol. 2011;104:21–31. doi: 10.1007/s11060-010-0467-3. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Li J, Liu W, Jia W, Xu G, Ma J. [Expression of Nodal in hepatocellular carcinoma and its relationship with angiogenesis and epithelial-mesenchymal transition] . Zhonghua Wai Ke Za Zhi. 2014;52:188–192. [PubMed] [Google Scholar]
- 48.Quail DF, Walsh LA, Zhang G, Findlay SD, Moreno J, Fung L, Ablack A, Lewis JD, Done SJ, Hess DA. Embryonic protein nodal promotes breast cancer vascularization. Cancer Res. 2012;72:3851–3863. doi: 10.1158/0008-5472.CAN-11-3951. [DOI] [PubMed] [Google Scholar]


