Abstract
The Heart failure (HF) is considered as the end-stage of various heart disease and associated with high mortality globally. Progressive loss of cardiac myocytes via apoptosis is considered as the most important factor for HF pathology. In this study, we demonstrated that Safflower extract was able to inhibitthe apoptosis inducted by Angiotensin II (AngII) in a ratmyocardium derived cell line H9C2. Further examination of LC-3II conversion and autophagosome formation suggested Safflower extract induced autophagy in treated cell. Inhibition of Safflower extract induced autophagy by 3-methyladenine (3MA) abolished anti-apoptotic function of Safflower extract, while application of autophagy stimulator Rapamycin in H9C2 inhibited apoptosis as well. Moreover, treatment of H9C2 cell with Safflower extract also inhibited expression of pro-apoptotic genes BAD and Bax. In conclusion, our data indicated that Safflower extract inhibit apoptosis via inducing autophagy in myocardium cell and demonstrated the potential as novel therapeutic drug for Heart failure.
Keywords: Heart failure, apoptosis, safflower extract, autophagy, pro-apoptotic genes
Introduction
Heart failure (HF), often referred as chronic heart failure (CHF), has been considered as the end-stage of various heart disease and is generally associated with high mortality all over the world [1]. The progression and the cause of heart failure has been shown to involve multiple factors and processes, such as coronary artery disease, high blood pressure, atrial fibrillation, smoking, alcoholic, infection, and cardiomyopathy as well as some other unknown causes [2-5]. As many clinical and basic studies have been conducted to clarify the complex pathophysiology of HF, several mechanisms that affect cardiac function were identified and the β-adrenergic receptor (β-AR)-mediatedsignaling pathway has beenconsidered as the most important one which regulates cardiac function [2]. As a result, modulating this pathway via β blocker is the most commonly used therapy for HF [2]. Moreover, except β blocker, RAS inhibitors, antiplatelet agents and statins has been used to improve the survival rate of HF patients as well [2]. However, with such understanding of HF pathogenesis and development of therapy, the prognosis for HF is stillpoor, suggested the needing of novel strategy to treating HF.
On the other hand, as the progressive loss of cardiac myocytes is considered as the most important factor for HF pathology, accumulating evidence from both human and animal models had suggested that undesired apoptosis playing indispensable role for the progressive loss of cardiac myocytes [6]. This speculation is supported by numerous studies from animal HF models as well [6]. In HF patients, the tissue samples from individuals undergoing heart transplantation demonstrated a high level of apoptosis in the failed hearts (the one been replaced) [7-9]. Moreover, recent reports also revealed the pathways leading to apoptosis in HF, such as p53, the famous tumor suppressor, is considered as the major player in the development of HF [10-12]. Additionally, during β-AR blocking therapy, several mechanisms of β-AR stimulation-induced apoptosis had been reported as well, such as Inducible cAMP Early Repressor (ICER) mediated apoptosis [13], as well as Ca2+/Calmodulin Kinase (CaMK) and Calcineurin depended apoptosis [14]. Therefore, preventing the undesired apoptosis of failing heart provided a promising target for development of future HF therapy.
In recent years, application of complementary and alternative medicines (CAMS) for chronic HF has been exclusively investigated. In China, Traditional Chinese Medicine (TCM) as well as TCM based Herbologyhas been widely investigatedas the treatment for HF [15-21]. A randomized controlled trial was ongoing to evaluate the efficiency of TCM [22]. However, the mechanism behind TCM and Herbology is still largely unknown. In this study, we investigated the exact from Safflower (Carthamustinctorius L.) for its role in the treatment of heart failure. Our data suggested Safflower extract could inhibit Angiotensin II mediates apoptosis in H9C2 cell in an autophagy dependent manner. Moreover, H9C2 cell treated with Safflower extract also demonstrated down-regulated expression of pro-apoptotic genes. In sum, our data suggested Safflower extract could be a novel treatment for heart failure.
Materials and methods
Cells and chemicals
H9C2 cell (ATCC® CRL-1446™) were purchased from ATCC and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). Angiotensin II (AngII, A9525, Sigma, St. Louis, MO, USA), Rapamycin (Sigma) and 3-methyladenine (3MA, Sigma) was used for apoptosis induction, autophagy induction and autophagy inhibition in H9C2 cells, respectively. Rapamycin and 3MA were used to treat the cell at the concentration of 50 nM and 5 mM, respectively. Safflower extract (Safflower Yellow) was purchased from Yunnan Rainbow Bio-Tech Co. Ltd (Kunming, Yunnan, China) and dissolved in ddH2O for further experiment.
Western blot analysis
H9C2 cells with indicated treatment were lysed by the Laemmli Sample Buffer as previously described [23,24]. Cell lysate then was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to Western blot as previously described [24]. Briefly, separated proteins during SDS-PAGE were then transferred onto PVDF membrane and probed with rabbit anti-LC-3 antibody (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-BAD antibody (Sigma) and rabbit anti-Bax antibody (Santa Cruz, Santa Cruz, CA, USA). Specific reactions were detected by using goat anti-rabbit IgG conjugated with horseradish peroxidase (Sigma) and revealed by a chemiluminescence substrate. The membrane was also blotted with Tubulin antibody (Santa Cruz) for normalization. The chemiluminescence signal was recorded by the ChemiDoc XRS imaging system (Bio-Rad Laboratories, Hercules, CA, USA). Data analysis and quantification were conducted by the Quantity One Program (Version 4.6).
Cell proliferation assay (MTT)
The trypsinized H9C2 cells were stained by trypan blue for counting of viable cells before further seeding. Then the single cell suspensionwas added in 96 well plates with 2 × 104 cells per well. After overnight incubation, the Safflower extract and AngII were added to each well accordingly. Cell added with PBS was included as the control group. Then proliferation of indicated groups was determined at the indicated time points by using MTS Cell Proliferation Colorimetric Assay kit (Biovision, Milpitas, CA, USA) following manufacturer’s instruction.
Immunofluorescenceassay (IFA) and fluorescence microscopy
IFA and fluorescence microscopy was conducted as previously described [25,26]. Briefly, after treating the cell seeded in cover slides with Safflower extract for 12 hours, the cell were fixed with 2% paraformaldehyde (Sigma) and penetrated with 1% Triton-X 100 (Sigma). The LC-3 antibody was used as the primary antibody and incubated for 30 minutes with PBS washed for 3 times. Then the specificreactions between antibody and LC-3 were detected by a FITC conjugated goat anti-rabbit IgG antibody (Sigma). Then the cover glass was mountedon to slides using SlowFadeGoldanti-fadereagent containing 406-diamidino-2-phenylindole (DAPI) (Life Technologies Corporation, Carlsbad, CA) and observedunderfluorescent microscopy.
Flow cytometry based cell apoptosis assay
A totally 1 × 106 H9C2 cells of each group were treated accordingly as indicated. Then the cells were trypsinized and fixed with 70% ethanol and permeabilized by PBS containing 1% Triton-X100 (Sigma). After single cell suspension was stained with FITC labeled Annexin V and Propidium iodide, the stained cells were analyzed via flow cytometry machine (FACSCalibur, BD Biosciences, San Jose, CA, USA) for apoptosis.
Statistical analysis
The significant differences of apoptosis and cell proliferation status between the control group sand groups of the cell in the presence or absence of Safflower extract, AngII, Rapamycin and 3MA were assessed by two tailed Student’s tests and represent analysis of at least 3 replicates per condition. A P-value of less than 0.05 was considered as statistically significant.
Results
The safflower extract inhibit Angiotensin IIinduced apoptosis in H9C2 cell
The Safflower (Carthamustinctorius L.) has been used extensively for the treatment of acute ischemic stroke in China according to TCM [27]. Chemical analysis for the constituents of safflower indicated it includes pigments, flavonoids, and phenolic acid with safflower yellow considered as the major active component of safflower extract [27]. Previous report demonstrated that Safflower extract could inhibit the myocardial apoptosis after acute myocardial infarction (AMI) in rats model [28]. Therefore, we first investigated if Safflower extract could inhibit apoptosis inducted by Angiotens in II (AngII) in a ratmyocardium derived cell line H9C2. Based on our data, after treated the cell with 0.2 μM AngII, these cells undergoing early apoptosis increased to 23.8% from 4.92% of control (Figure 1A). On the other hand, cells undergoing late apoptosis in AngII treated group reached 11.4% compared with 0.059% of control (Figure 1A). However, with adding of Safflower extract, the H9C2 cells undergoing early and late apoptosis significantly decreased to 13.4% and 0.196%, respectively, suggesting a protective role by Safflower extract. Moreover, by repeating the flow cytometry (FCM) based apoptosis analysis for at least 3 times, we confirmed that Safflower extract deed inhibit the apoptosis inducted by AngII (Figure 1B).
Figure 1.
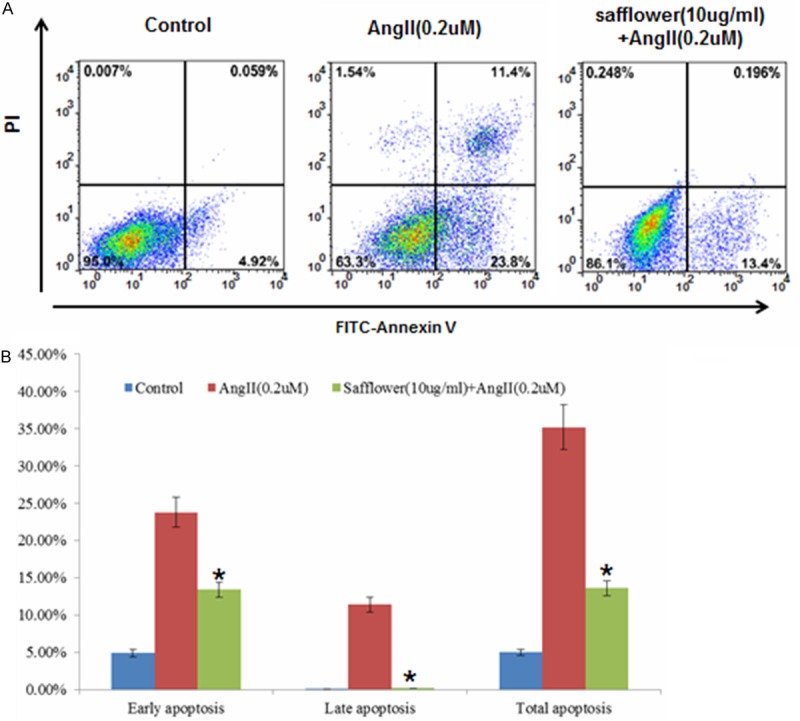
The Safflower extract inhibit AngII induced apoptosis in H9C2 cell. A. Flow cytometry based apoptosis analysis for H9C2 cell. Cellswere treated with AngII, Safflower extract, or left untreated, then stained with Annexin V and PI for FCM based apoptosis analysis. B. The quantification of cell apoptosis, three repeated experiments had been conducted for each group. Significant differences between different groups are shown by “*” to indicate significant difference (P<0.01).
The safflower extracttreatment prevent Angiotensin IIinducted cell proliferation inhibition
To further confirmed Safflower extract deed protect the cell from apoptosis, we examine the cell proliferation via MTT assay. MTT assay was conducted for cells neither treated by Safflower extract or AngII, as well as a combination of both. Compared to control cells without any treatment, Safflower treatment or combined Safflower and AngII together showed similar cell proliferation from day 1 until day 3 (Figure 2B). However, AngII treated cells demonstrated a significantly low proliferation (Figure 2B). Moreover, we also examined if Safflower extract wastoxic to H9C2 cell. In a dose-dependentassay, increased dose (ranging from 10 μg/L to 100 μg/L) of Safflower extract correlated with less proliferation of H9C2 cells. Therefore, 10 μg/L dose would be used in future experiments.
Figure 2.
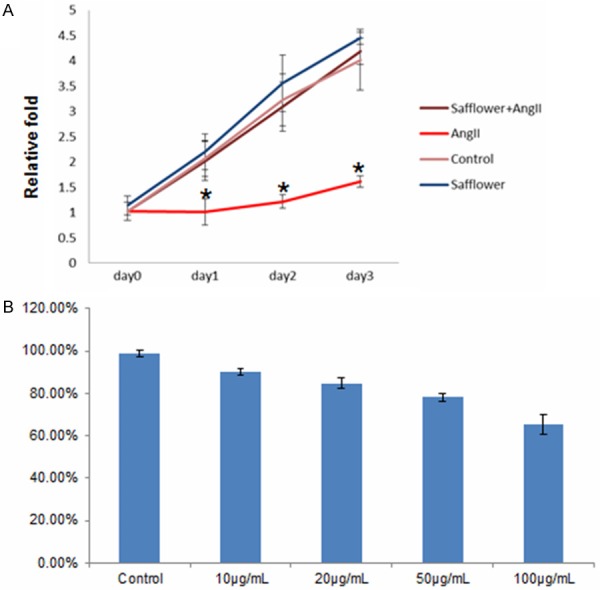
The Safflower extract treatment prevent Angiotensin II inducted cell proliferation inhibition. A. The cell proliferation assay for H9C2 cell treated with Safflower extract and AngII. The MTT cell proliferation kit was used to determine the cell proliferation in indicated time points, error bars represent standard errors of three repeated experiments. Significant differences between different groups are shown by “*”. B. Dose-dependent cell proliferation assay for Safflower extract. Cells were treated with Safflower extracted at indicated dose, then MTT assay was conducted to measure cell proliferation. Three repeating experiments had been conducted for each group for statistical analysis.
Safflower extract induced autophagy in H9C2 cell
As our data confirmed Safflower extract inhibited AngII induced cell apoptosis, it was still unclear for the mechanism of Safflower extract conducting its anti-apoptotic role. Recently, autophagy, an intracellular degradation system that plays an important role for homeostasis, has been suggested to play a protective role for many cell types during apoptosis [29,30]. Therefore, it is interesting to know if Safflower extract treatment could induceautophagy to antagonize apoptosis. To verify our speculation, we firstly examined the converting status of Microtubule-associated protein 1A/1B-light chain 3 (LC-3), which is a hall marker for autophagy in cell [31]. During the formation of autophagosome, which is a necessary step for macro autophagy, a cytosolic form of LC-3 (LC-3I) is conjugated to phosphatidylethanolamine to form LC-3-phosphatidylethanolamine conjugate (LC-3II), which is finally recruited to autophagosomal membranes [31]. Our data indicated that AngII treatment did not change the level of LC-3II (Figure 3A). However, LC-3II ratio was significantly increased to 1.9 folds in Safflower extract treated cells and was at the similar level (2.1 folds) in Safflower extract and AngII treated cells (Figure 3A). Moreover, we observed autophagosomes formation in Safflower extract treated cell as well. During the IFA, there were more autophagosomes in Safflower extract treated cells than control cells, which further confirmed the induction of autophagy (Figure 3B).
Figure 3.
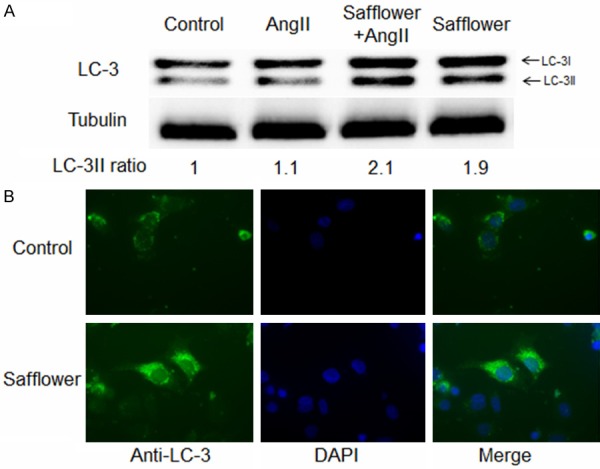
Safflower extract induced autophagy in H9C2 cell. A. Western Blot analysis for LC-3II conversion. Differently treated cells was lysed and subjected to SDS-PAGE, then the separated proteins were transferred to PVDF membrane with anti-LC-3 antibody to detect the conversion of LC3-I to LC3-II. Quantification of image was conducted in QuantityOne program. B. Immunofluorescence assay for formation of autophagosome. Differently treated cells was fixed and penetrated by 2% paraformaldehydeand 1% Triton-X 100, respectively. The LC-3 antibody and FITC conjugated goat anti-rabbit IgG antibody were used to detection autophagosome formation.
Safflower extract induced autophagydepended inhibition of AngII induced apoptosis
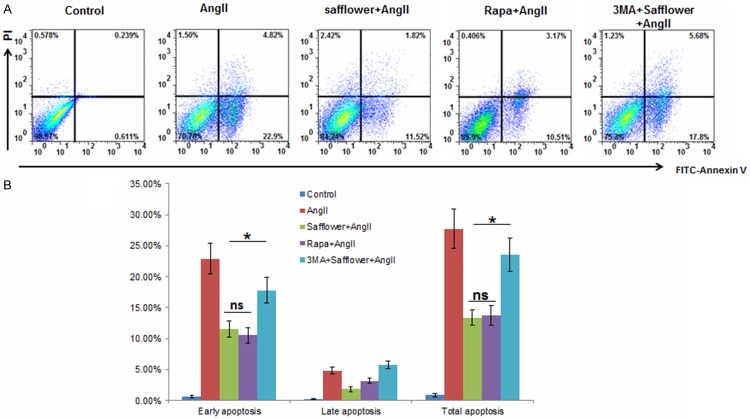
As safflower extract induced autophagy in H92C cell, it is unclear if autophagy induced by safflower extract was the main reason for apoptosis inhibition. As a result, we used Rapamycin (an autophagy inducer) and 3MA (an autophagy inhibitor) to confirm our expectation. In FCM assay, apoptosis status in Safflower treated cell with or without AngII still demonstrated the similar results as we previously observed (Figure 4A and 4B). However, if 3MA was added to antagonize the autophagy induced by Safflower extract, the cell undergoing apoptosis reached similar level as AngII treated cells (Figure 4A and 4B), which suggested the protective role of Safflower extract during apoptosis was autophagy depended. Moreover, if we replaced the Safflower extract with Rapamycin to induced autophagy in H9C2 cell, there were also less cells undergoing apoptosis as well as Safflower extract treated cells (Figure 4A and 4B). Taken together, these data suggested that Safflower extract induced autophagy was the major factor to antagonize apoptosis induction.
Figure 4.
Safflower extract induced autophagy depended inhibition of apoptosis. A. Flow cytometry based apoptosis assay for H9C2 cell. The H9C2 cellswere treated with AngII, Safflower extract, Rapamycin (Rapa), 3MA or combination of indicated agents. Then cells from different groups were stained with Annexin V and PI for apoptosis analysis. B. The statistical quantification of cell apoptosis, three repeated experiments had been conducted for each group. Significant differences between different groups are shown by “*” to indicate significant difference (P<0.01).
Safflower extract inhibiting expression of pro-apoptosis genes BAD and Bax
As our data had shown that Safflower extract induced autophagy to inhibit apoptosis in H9C2, it is interesting to know if the expression of pro-apoptosis genes were also inhibited by Safflower extract treatment. Two pro-apoptotic genes: Bcl-2-associated death promoter (BAD) and Bcl-2-associated X protein (Bax) were examined in here. The function of BAD is to promote apoptosisby forming heterodimers with anti-apoptotic protein BCL-2 [32]. Bax also served as an apoptotic activator by forms a heterodimer with BCL2, which is similar as BAD [33]. When H9C2 cell treated with AngII for apoptosis, expression of BAD and Bax were significantly increased (Figure 5). However, in the Safflower extract and AngII co-treated cells, there only a slide increasing of BAD and Bax expression, suggesting Safflower extract inhibited expression of pro-apoptotic genes as well. There may be another reason why Safflower extract could inhibit apoptosis.
Figure 5.
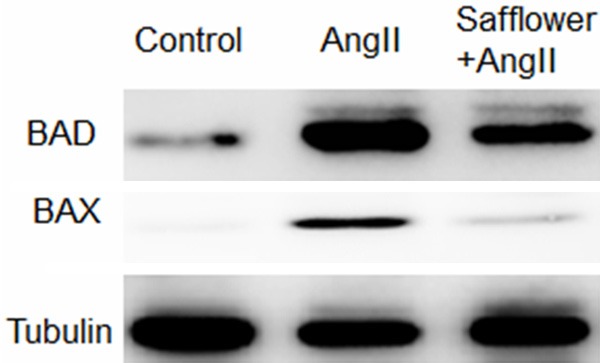
Safflower extract inhibit expression of pro-apoptosis genes BAD and Bax. H9C2 cells were treated as indicated and then lysed for SDS-PAGE. After the proteins were transferred to PVDF membrane, anti-BAD and anti-Bax antibodies were used to probe the expression of indicated genes. Tubulin was probed in the same membrane as reference control.
Discussion
Heart failure refers to a physiological state in which cardiac output is insufficient to meet the needs of the body [5]. Heart failure could be caused by any condition reducing the working efficiency of heart muscle, such as damage to heart muscle due to any reason or overloading to heart muscle. Many factors had been shown to contribution the development and progression of HF, including myocardial infarction (in which the heart muscle is starved of oxygen and dies), hypertension (which increases the force of contraction needed to pump blood) and amyloidosis (in which misfolded proteins are deposited in the heart muscle, causing it to stiffen) [16]. Current therapy for HF management is still limit, which mainly focus on reliving the symptoms, or reversingthe causes of the heart failureas well as cardiac transplantation.
In this study, we examined the protective effect of Safflower extract in in vitro cell culture system as progressive loss of heart muscle cell due to apoptosis was implied to play important role in heart failure. The active components in safflower exact are Safflower Yellow or hydroxysafflor yellow A [34]. Our data indicated treatment of H9C2 cell with Safflower extract could induce autophagy to inhibit AngII induced apoptosis. Further experiment also showed that inhibition of autophagy via 3MA abolished the protective effect of Safflower extract, suggesting the anti-apoptotic role of Safflower extract was autophagy depended. This function of Safflower extract appears to be new. However, the mechanism of how Safflower extract evoking autophagy in vitro cultured cell still needs further investigation.
On the other hand, Safflower Yellow Injection was certificated in China by the State Pharmaceutical Administration of China (SPAC) in 2002 (number 2002ZL0176) and was indicated to have protective effect of in ischemic stroke [27]. Another study also indicated the Safflower Yellow Injection could inhibit myocardial apoptosis after acute myocardial infarction (AMI) in rats and also observedsignificantly lower percentage of Bax-positive cells in drug treated rats [28]. As our data demonstrated the similar result for apoptosis inhibition and down-regulated Bax expression in H9C2 cell, it is interesting to know if Safflower Yellow Injection also induced autophagy in vivo to protect the neuron from apoptosis during the stroke. Moreover, as Safflower Yellow Injection has already been approved in China, it is worth to find out if this injection could benefit for patients with heart failure as well. In conclusion, our resulted demonstrated autophagy depended anti-apoptotic role of Safflower extract, suggested the potential therapeutic value for heart failure of Safflower extract as well as the potential new application for its drug form Safflower Yellow Injection.
Disclosure of conflict of interest
None.
References
- 1.Cleland JG, Khand A, Clark A. The heart failure epidemic: exactly how big is it? Eur Heart J. 2001;22:623–626. doi: 10.1053/euhj.2000.2493. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T, Ishikawa Y. Apoptosis in heart failure. -The role of the beta-adrenergic receptor-mediated signaling pathway and p53-mediated signaling pathway in the apoptosis of cardiomyocytes- Circ J. 2011;75:1811–1818. doi: 10.1253/circj.cj-11-0025. [DOI] [PubMed] [Google Scholar]
- 3.Grazette LP, Rosenzweig A. Role of apoptosis in heart failure. Heart Fail Clin. 2005;1:251–261. doi: 10.1016/j.hfc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update. London: 2010. [PubMed] [Google Scholar]
- 5.McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–1889. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 6.Kang PM, Izumo S. Apoptosis and heart failure: A critical review of the literature. Circ Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 7.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 8.Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med. 1996;335:1190–1196. doi: 10.1056/NEJM199610173351604. [DOI] [PubMed] [Google Scholar]
- 9.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 10.Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovasc Res. 2006;70:457–465. doi: 10.1016/j.cardiores.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Naito AT, Okada S, Minamino T, Iwanaga K, Liu ML, Sumida T, Nomura S, Sahara N, Mizoroki T, Takashima A, Akazawa H, Nagai T, Shiojima I, Komuro I. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ Res. 2010;106:1692–1702. doi: 10.1161/CIRCRESAHA.109.214346. [DOI] [PubMed] [Google Scholar]
- 12.Das B, Young D, Vasanji A, Gupta S, Sarkar S, Sen S. Influence of p53 in the transition of myotrophin-induced cardiac hypertrophy to heart failure. Cardiovasc Res. 2010;87:524–534. doi: 10.1093/cvr/cvq068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Tomita H, Nazmy M, Kajimoto K, Yehia G, Molina CA, Sadoshima J. Inducible cAMP early repressor (ICER) is a negative-feedback regulator of cardiac hypertrophy and an important mediator of cardiac myocyte apoptosis in response to beta-adrenergic receptor stimulation. Circ Res. 2003;93:12–22. doi: 10.1161/01.RES.0000079794.57578.F1. [DOI] [PubMed] [Google Scholar]
- 14.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YL, Ju JQ, Yang CH, Jiang HQ, Xu JW, Zhang SJ. Oral Chinese herbal medicine for improvement of quality of life in patients with chronic heart failure: a systematic review and meta-analysis. Qual Life Res. 2014;23:1177–1192. doi: 10.1007/s11136-013-0582-7. [DOI] [PubMed] [Google Scholar]
- 16.Tang WH, Huang Y. Cardiotonic modulation in heart failure: insights from traditional Chinese medicine. J Am Coll Cardiol. 2013;62:1073–1074. doi: 10.1016/j.jacc.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai H, Li Y, Han K, Gong M, Ma A. Effectiveness of Chinese herbal medicine as an adjunctive treatment for dilated cardiomyopathy in patients with heart failure. J Altern Complement Med. 2013;19:811–819. doi: 10.1089/acm.2012.0361. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Yao Y, Chen H, Kwong JS. Shengmai (a traditional Chinese herbal medicine) for heart failure. Cochrane Database Syst Rev. 2012;11:CD005052. doi: 10.1002/14651858.CD005052.pub4. [DOI] [PubMed] [Google Scholar]
- 19.Wen-Ting S, Fa-Feng C, Li X, Cheng-Ren L, Jian-Xun L. Chinese medicine shenfu injection for heart failure: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012:713149. doi: 10.1155/2012/713149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu S, Zhang J, Menniti-Ippolito F, Gao X, Galeotti F, Massari M, Hu L, Zhang B, Ferrelli R, Fauci A, Firenzuoli F, Shang H, Guerra R, Raschetti R. Huangqi injection (a traditional Chinese patent medicine) for chronic heart failure: a systematic review. PLoS One. 2011;6:e19604. doi: 10.1371/journal.pone.0019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng H, Chen Y, Chen J, Kwong J, Xiong W. Shengmai (a traditional Chinese herbal medicine) for heart failure. Cochrane Database Syst Rev. 2011:CD005052. doi: 10.1002/14651858.CD005052.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Luo L, Chen J, Guo S, Wang J, Gao K, Zhang P, Chen C, Zhao H, Wang W. Chinese Herbal Medicine in the Treatment of Chronic Heart Failure: Three-Stage Study Protocol for a Randomized Controlled Trial. Evid Based Complement Alternat Med. 2015;2015:927160. doi: 10.1155/2015/927160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel D, Nan Y, Shen M, Ritthipichai K, Zhu X, Zhang YJ. Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation. J Virol. 2010;84:11045–11055. doi: 10.1128/JVI.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nan Y, Wang R, Shen M, Faaberg KS, Samal SK, Zhang YJ. Induction of type I interferons by a novel porcine reproductive and respiratory syndrome virus isolate. Virology. 2012;432:261–270. doi: 10.1016/j.virol.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nan Y, Ma Z, Wang R, Yu Y, Kannan H, Fredericksen B, Zhang YJ. Enhancement of interferon induction by ORF3 product of hepatitis E virus. J Virol. 2014;88:8696–8705. doi: 10.1128/JVI.01228-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nan Y, Ma Z, Kannan H, Stein DA, Iversen PI, Meng XJ, Zhang YJ. Inhibition of hepatitis E virus replication by peptide-conjugated morpholino oligomers. Antiviral Res. 2015;120:134–139. doi: 10.1016/j.antiviral.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan S, Lin N, Shan G, Zuo P, Cui L. Safflower yellow for acute ischemic stroke: A systematic review of randomized controlled trials. Complement Ther Med. 2014;22:354–361. doi: 10.1016/j.ctim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhou MX, Fu JH, Zhang Q, Wang JQ. Effect of hydroxy safflower yellow A on myocardial apoptosis after acute myocardial infarction in rats. Genet Mol Res. 2015;14:3133–3141. doi: 10.4238/2015.April.10.24. [DOI] [PubMed] [Google Scholar]
- 29.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritthipichai K, Nan Y, Bossis I, Zhang Y. Viral FLICE inhibitory protein of rhesus monkey rhadinovirus inhibits apoptosis by enhancing autophagosome formation. PLoS One. 2012;7:e39438. doi: 10.1371/journal.pone.0039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 32.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- 33.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 34.Li LJ, Li YM, Qiao BY, Jiang S, Li X, Du HM, Han PC, Shi J. The Value of Safflower Yellow Injection for the Treatment of Acute Cerebral Infarction: A Randomized Controlled Trial. Evid Based Complement Alternat Med. 2015;2015:478793. doi: 10.1155/2015/478793. [DOI] [PMC free article] [PubMed] [Google Scholar]