Abstract
This study is to investigate the effect of subconjunctival injection of in vitro induced regulatory T cells (iTregs) on the survival of corneal allografts. iTregs were expanded by culturing CD4+T cells with TGF-β in vitro. Foxp3, LAP and GARP were analyzed and the suppression ability of iTregs was assayed by co-culturing with effective T cells. Allogeneic transplantations in mice were modeled and randomly classified into PBS control, iTregs and TA groups. The allografts were observed for 60 days. CD25, Foxp3, LAP and GARP in CD4+T cells were analyzed on day 21 after the surgery. Inflammatory cells infiltrated in allografts were detected by flow cytometry and histopathological examination. Expressions of Foxp3, GARP and LAP in iTregs were high. iTregs suppressed the proliferation of effective T cells in vitro. The corneal allograft survival time for PBS, iTregs and TA groups was (18 ± 1.73) days, (38.6 ± 1.14) days and (60 ± 0) days, respectively. The corneal allograft survival time in iTregs group was significantly prolonged compared with PBS group (P < 0.05), but shorter than that in TA group (P < 0.05). No significant difference was observed in expressions of CD25, Foxp3, LAP or GARP in CD4+T cells (P > 0.05). Finally, CD3+CD4+T cell infiltration and fewer inflammatory cells were reduced in allografts in iTregs and TA groups compared with PBS group. The survival time of allografts were prolonged in mice after subconjunctival injection of iTregs. Local immune modulation might be involved in the mechanism.
Keywords: Subconjunctival injection, transforming growth factor-β-induced regulatory T cells, corneal transplantation, allograft, immune tolerance
Introduction
Corneal allograft rejection is a complicated process related with multiple factors. The specific immunological mechanisms for corneal allograft rejection are not clear yet [1]. With the development of researches on immune system, more and more concern is focused on the important roles of regulatory T cells (Tregs) in maintaining the body’s immune tolerance and immune response to homeostasis [2]. Tregs can be divided into two categories: natural Tregs (nTregs) that arise from thymus, and induced Tregs (iTregs) that exist in periphery tissues. nTregs account for 5-10% of the peripheral blood lymphocytes in healthy human bodies [3]. iTregs are cells induced by interleukin (IL)-10, immature dendritic cells or transforming growth factor (TGF)-β [4,5]. Transcription factor forkhead box P3 (Foxp3) is known as a specific marker expressed in the nucleus of CD4+CD25+ Tregs [6]. Recently, a surface marker, glycoprotein-A repetitions predominant protein (GARP) that was a toll-like receptor composed of repeated leucine sequences, was found to be selectively expressed on the surface of activated Tregs [7]. Another marker, latency-associated peptide (LAP), was found to be expressed on the surface of activated Tregs, exerting immunosuppressive function by binding with membrane TGF-β [8]. In this research, we study the function of iTregs cultured in vitro and investigate the effect of iTregs administered via subconjunctival injection on the local and systemic immune system by measuring the allogeneic corneal graft survival time after allograft transplantation.
Materials and methods
Animals
Balb/c (H-2d) mice were used as recipients and C57BL/6 (H-1b) mice were used as donors. All mice were male and aged 6-8 weeks. The animals were purchased from the Experimental Center of Capital Medical University, Beijing, China, and housed in a specific pathogen-free facility. All animals used in this study were handled in accordance with the Association for Research in Vision and Ophthalmology Resolution on Use of Animals in Research.
The recipient mice were randomly divided into 3 groups of 12 mice as follows (C57BL/6→Balb/c): phosphate buffered saline (PBS) group, 10 μL PBS was injected to the recipients at the end of operation via subconjunctival injection; iTregs group, 10 μL cultured iTregs (1×105) were injected in the same manner as PBS group; and triamcinolone acetonide (TA) group, 10 μl TA (0.4 mg) were injected in the same manner as PBS group. BALB/c mice that received syngeneic (Balb/c→Balb/c) grafts were used as control.
Reagents and instruments
Recombinant human TGF-β1 and recombinant mouse IL-2 were purchased from BD Biosciences, USA. Anti-CD3/28 mAb-coated Dynabeads were obtained from Invitrogen, USA. Carboxyfluorescein succinimidyl ester (CFSE) was from Molecular Probes, USA. Allophycocyanin (APC)-labeled anti-mouse CD4, phycoerythrin (PE)-labeled anti-mouse CD25, Alexa488 (A488)-labeled anti-mouse Foxp3, PE-labeled anti-mouse GARP, Peridinin Chlorophyll protein (PerCP)-labeled anti-mouse LAP were purchased from BD Biosciences, USA. Collagenase type IV was obtained from Sigma-Aldrich, USA. Fixation/permeabilization buffer was from BioLegend, USA. CD4+CD25+ Treg isolation kit was from Miltenyi Biotec, Germany. Optisol corneal storage medium was from Eyebank of Beijing Tongren Hospital, China. Ophthalmic operating microscope was purchased from Topcon, USA. FACS Caliber was from BD Biosciences, USA. Microinjector was purchased from Hamilton Bonaduz AG, Switzerland.
Purification and expansion of TGF-β-iTregs
CD4+T cells from the spleens of BALB/c mice were isolated by magnetic separation using magnetic cell sorting (MACS) system and CD4+CD25+ Treg isolation kit (Miltenyi Biotec, Germany). Then, 5×105 CD4+T cells were grown in flat-bottom plates containing 5 ml complete RPMI-1640 medium supplemented with 10 ng/ml recombinant human TGF-β1, 10 ng/ml recombinant mouse IL-2, and 10 μl anti-CD3/28 monoclonal antibody (mAb)-coated Dynabeads (Invitrogen, USA). Cells were incubated at 37°C in a humid atmosphere with 5% CO2 for 72 h. All cells were sorted again based on the expression of CD4 and CD25 using MACS system. The CD4+CD25+T cells were TGF-β-iTregs.
Flow cytometry
Freshly isolated and cultured cells were washed with PBS containing ethylene diamine tetraacetate acid (EDTA) and bovine serum albumin (BSA). For surface staining, the cells were labeled with appropriate APC-, PE-, or PerCP-conjugated antibodies for 10 min. For intracellular staining, surface-stained cells were fixed and permeabilized for 30 min before being incubated with appropriate A488-conjugated Foxp3. Stained cells were washed for three times and analyzed using flow cytometry according to manufacturer’s manual (FACS Caliber; BD Biosciences, USA).
Spleen, ipsilateral draining submandibular and cervical lymph nodes of 5 mice from each group were collected and single cell suspensions were prepared on day 21 after the surgery. This time point for T cell analysis was selected on the basis of survival time when all allografts in PBS group failed while corneal grafts in other groups induced no rejection. Single cell suspensions were stained and analyzed in the same way as described before in phenotypic analysis. The corneal grafts were taken and cut into small pieces, and then digested in 2 mg/ml collagenase IV under 37°C for 1 h. The well digested tissues were forced through a 70 μm nylon mesh to obtain single cell suspension. For surface staining, cells were labeled with appropriate PerCP-conjugated CD3 and APC-conjugated CD4 for 10 min and analyzed by flow cytometry according to manufacturer’s manual (FACS Caliber; BD Biosciences, USA).
Suppression assay
Suppression assays were performed in triplicate in 96-well plates. Freshly isolated naive CD4+CD25-T cells from Balb/c mice severed as effective T cells (Teff, 1×105/well) labeled with 0.5 μM CFSE (Molecular Probes, USA), and anti-CD3/28 mAb-coated Dynabeads severed as stimulators (2 ml/well) with or without indicated ratio of iTregs for 3 days. T cell proliferation was determined by CFSE dilution profile, and percent suppression was calculated using the following formula: % suppression = [(Teff proliferation without iTreg-Teff proliferation with iTreg)/(Teff proliferation without iTreg)] ×100%.
Corneal transplantation and assessment of graft
Standard protocol for murine orthotropic corneal transplantation was used [9]. Briefly, donor center corneas (2 mm in diameter) were excised from donor C57BL/6 mice and fixed in the recipient graft beds prepared by excising a site of 2 mm in diameter with 8 interrupted 11-0 polyamide sutures in the central cornea of recipient BALB/c mice. The eyelids were closed after operation with 8-0 nylon sutures. The eyelid sutures were removed after 72 h and the corneal sutures were removed on day 7 after the operation. All grafts were evaluated using a slit-lamp microscope three times a week after the surgery for 60 days. Grafts were defined as rejected when they became opaque and the iris details could not be recognized clearly using a standardized opacity grading (ranges 0-5) scheme [10,11]. For animals with opacity scores ≥ 2, the grafts were judged as rejected. Grafts with severe complications such as infection or hyphema were excluded from the study.
Histopathological staining
On day 21 after the surgery, 2 mice from each group were sacrificed. The operated eyeballs were taken and fixed in 10% formaldehyde solution, embedded in paraffin, sliced, and stained with hematoxylin and eosin for histopathological examination.
Statistical analysis
Student’s t test was used for comparison of mean values between groups. Allograft survival data were used to generate Kaplan-Meier survival curves, and comparisons between groups were performed by log-rank analysis. Data were presented as means ± SD and differences with P < 0.05 were considered statistically significant.
Results
TGF-β-induced Tregs exert inhibitory effect on Teff proliferation in vitro in a dose-dependent manner
To observe Tregs induction by TGF-β, the cells were cultured and induced by TGF-β in vitro for 72 hours, and visualized under an inverted microscope. The shapes of the cells appeared to be rod for division. The purity of CD4+CD25+T cells before and after cultivation was 10.01 ± 0.53% and 95.76 ± 1.39%, respectively. The expression of surface marker GARP before and after cultivation was 2.67 ± 0.20% and 75.78 ± 8.18%, respectively. The expression of surface marker LAP was 9.25 ± 3.12% and 85.76 ± 5.01%, respectively. In addition, the expression of Foxp3 before and after cultivation was 2.13 ± 0.47% and 74.2 ± 5.2%, respectively.
To test the suppressive properties in vitro, iTregs were assayed on day 7 in the culture using suppression assay and flow cytometry. The suppression assay showed that about 60% inhibition of Teff was achieved at iTregs/Teff ratio of 1:1, while only less than 7% inhibition of Teff was observed at iTreg/Teff ratio of 1:8 (Figure 1). These data demonstrated that TGF-β-induced Tregs had inhibitory effect on Teff proliferation in vitro in a dose-dependent manner.
Figure 1.
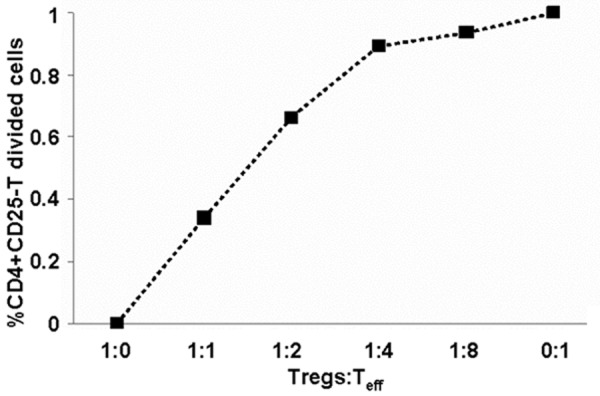
Teff proliferation rate in vitro at different ratios of Tregs: Teff. Flow cytometry analysis was used to measure Teff (CD4+CD25-T cells) proliferation. Freshly isolated naive CD4+CD25-T cells from Balb/c mice severed as effective T cells (Teff, 1×105/well) labeled with 0.5 μM CFSE (Molecular Probes, USA), and anti-CD3/28 mAb-coated Dynabeads severed as stimulators (2 ml/well) with or without indicated ratio of iTregs (Tregs:Teff represents the ratio of the number of Tregs induced by TGF-β (TGF-β-iTregs) to the number of Teff) for 3 days. T cell proliferation was determined by CFSE dilution profile, and percent suppression was calculated using the following formula: % suppression = [(Teff proliferation without iTreg-Teff proliferation with iTreg)/(Teff proliferation without iTreg)] ×100%. Data are means ± SD. P < 0.05 between any two data points.
Treatment with iTregs prolongs the survival time of corneal allografts
To investigate the effect of iTregs on the survival of allogeneic corneal grafts, we measured the survival time and observed all of the corneal grafts. Mean survival time (MST) of the grafts in PBS, iTreg and TA groups was 18 ± 1.73, 38.6 ± 1.1 and 60 ± 0 days, respectively (P < 0.05). Meanwhile, the MST of the syngeneic (control) group (60 ± 0 days) demonstrated that the influence of operation on the result of the experiments could be eliminated (Figure 2A). Mouse corneal graft survival curve showed that grafts in PBS group became rejected 17 days after allogeneic transplantation, with all of the grafts being rejected on day 21 after the surgery. By contrast, rejection of grafts in iTregs group began on day 37 after the operation, after which all grafts were rejected within 3 days. However, grafts in TA group were transparent within 60 days after the surgery (Figure 2B). On day 14 after the transplantation, grafts in PBS group exhibited mild edema, but the pupil and iris texture were clearly visible; on day 21, grafts became severely opaque, and edema with small neovessels grew to the center of the grafts, with the opacity score being ≥ 2. Grafts in iTregs group only showed slight edema on day 28, and the pupil and iris texture were clearly recognized (opacity score was 1). On the other hand, grafts in TA and syngeneic (control) group were clear till the end of the 60 day with little neovessel formation (Figure 3). These data suggested that iTregs treatment prolonged the survival time of corneal allografts.
Figure 2.
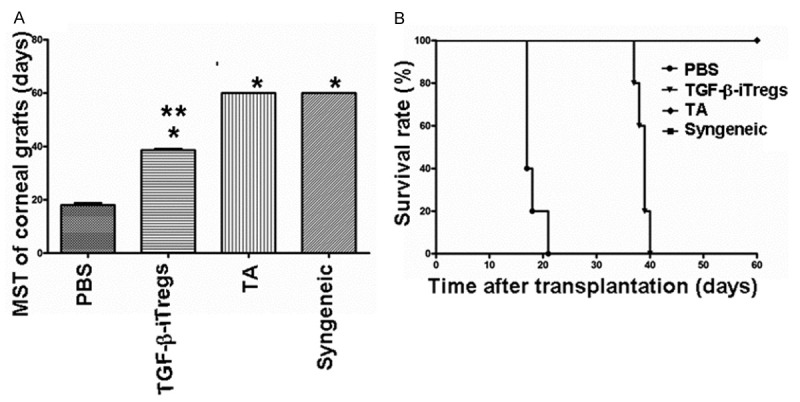
Survival analysis of corneal allografts. A: Mean survival time of corneal allografts in PBS, iTregs, TA and control groups. Data are means ± SD (n = 5) *P < 0.05 compared with PBS group; **P < 0.05 compared with TA group. B: Survival curve of corneal allografts in PBS, iTregs, TA and control groups.
Figure 3.
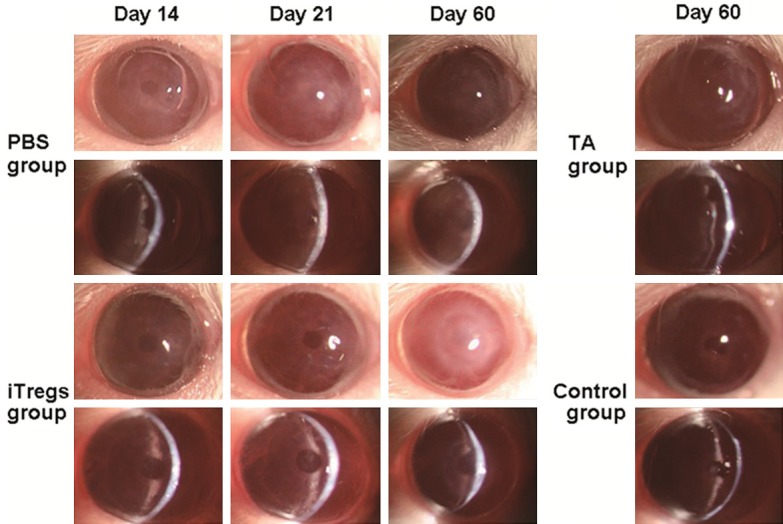
Slit lamp microscopic images after corneal transplantation in mice. Grafts were defined as rejected when they became opaque and the iris details could not be recognized clearly using a standardized opacity grading (ranges 0-5) scheme. For animals with opacity scores ≥ 2, the grafts were judged as rejected.
Conjunctival injection of iTregs does not affect the immune balance around the eye or in the systemic immune system
To study the expression of CD25, Foxp3, GARP and LAP, CD4+T cells in ipsilateral drainage lymph nodes and spleens of mice in the three groups were detected by flow cytometry on day 21 after the surgery. The results showed no significant difference in the expression of CD25, Foxp3, GARP and LAP in CD4+T cells from graft draining lymph nodes or spleen between allograft recipients in the three groups (P > 0.5) (Figure 4A-D). These data demonstrated that conjunctival injection of iTregs did not affect the immune balance around the eye or in the systemic immune system.
Figure 4.
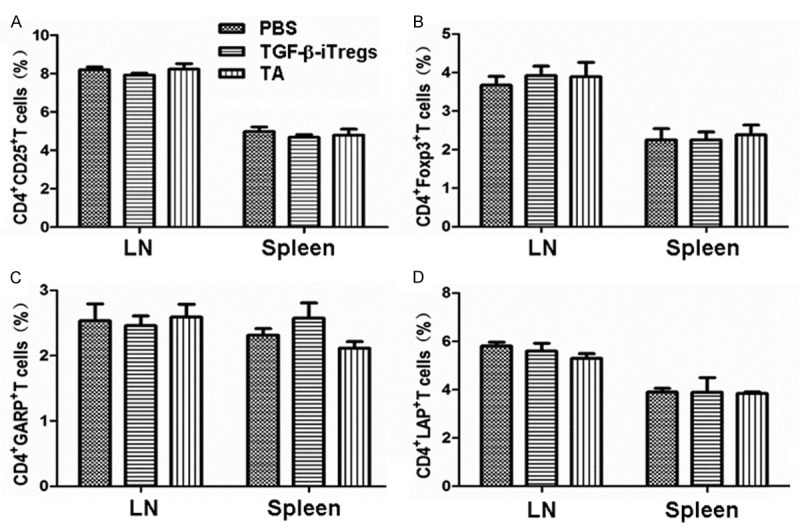
The expression of (A) CD25, (B) Foxp3, (C) GARP and (D) LAP in CD4+T cells from ipsilateral drainage lymph nodes and spleens on day 21 determined by flow cytometry. Data are means ± SD (n = 5).
Treatment with iTregs suppresses CD3+CD4+T cell infiltration and reduces the number of inflammatory cells in corneal allografts
To study the properties of corneal cells and grafts, flow cytometry and histopathological staining were performed. Corneal grafts of each group were taken on day 21 after the operation and digested to obtain single cell suspension, followed by staining. Flow cytometry showed that the percentage of CD3+CD4+T cells in corneal grafts of iTregs and TA groups was decreased significantly compared with PBS group (P < 0.05), with no significant difference being observed between iTregs group and TA group (P > 0.05) (Figure 5A). In addition, histopathological staining showed that, stromal fibers of allografts in the PBS group appeared to be disordered, with neovessels being formed and significant infiltrationof inflammatory cells beingobserved. However, only a few inflammatory cells and neovessels were observed in iTregs group, with limited graft thickening. Furthermore, stromal fibers of corneal graft layer in TA group were arranged neatly, with few neovessels and inflammatory cell infiltration (Figure 5B). These data suggested that treatment with iTregs suppressed CD3+CD4+T cell infiltration and reduced the number of inflammatory cells in corneal allografts.
Figure 5.
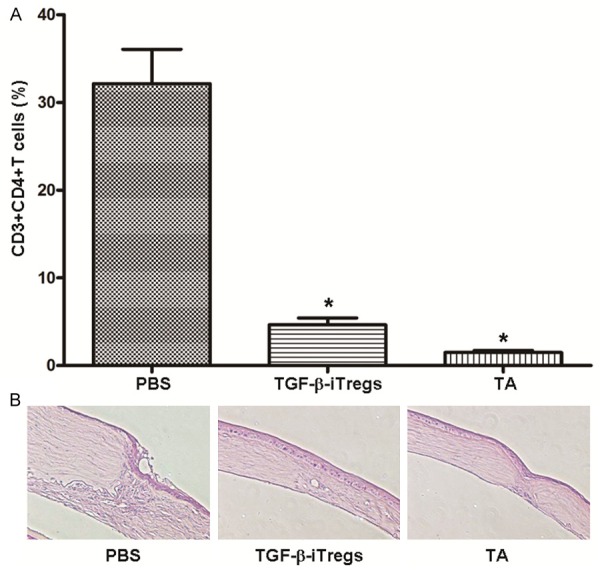
Analysis of corneal grafts. A: The percentage of CD3+CD4+T cells in corneal grafts on day 21. Data are means ± SD (n = 5). *P < 0.05 compared with PBS group. B: Histopathological examination of corneal grafts in PBS group, iTregs group and TA group on day 21 after the surgery. Hematoxylin and eosin staining was performed before microscopic investigation using original magnification of ×20.
Discussion
The discovery and application of natural CD4+CD25+ Treg cells (nTregs) produced in thymus opened our minds on the treatment of autoimmune diseases, organ transplantation rejection and tumor control. However, nTregs account for only 5-10% [3] of total CD4+T cells in human body and its amplification efficiency is not high and hence, limiting researches and clinical applications of nTregs to a large extent. The present research demonstrated that CD4+T cells can be induced to a large amount of CD4+CD25+ Treg cells in vitro. The proliferation of T cells can be stimulated by anti-CD3/28 monoclonal antibody non-specifically while its anergy can be interrupted by IL-2 [12]. However, a lot of other effective T cells can also be expanded at the same time in vitro in addition to regulatory T cells, when this kind of broad spectrum amplification method was used [13]. By contrast, TGF-β can induce T cells for differentiation into iTregs [8,14].
In this study, surface and intracellular markers in iTregs were tested by flow cytometry, and the results showed that specific markers such as GARP, LAP and Foxp3 were highly expressed in iTregs. Foxp3 was recognized as the specific marker of Tregs [15], but other researches discovered that some CD4+CD25-T cells also expressed Foxp3 in the nuclei without immunosuppressive function [16]. Another T cell marker, GARP, was proved to be expressed specifically on the surface of CD4+CD25+Foxp3+ Tregs, and could be used as an indicator for Tregs activation. Activated Tregs can specifically increase the expression of LAP, so the separation of LAP+Foxp3+ Tregs can improve the purity of Tregs [17]. This study also examined the inhibitory function of iTregs in vitro. The cultured cells inhibited the proliferation of T cells in vitro, with its inhibitory function being enhanced as the increase of the number of Tregs.
In previous studies, we demonstrated that adoptive transfer of TGF-β-iTregs through tail vein suppressed allogeneic corneal graft rejection in mice [18,19]. CFSE tracing showed that iTregs could migrate to local draining lymph nodes and spleens in the body, and exert immunosuppressive function through sustained expression of CD25 [18]. In the present study, we confirmed that the survival time of corneal allografts could also be extended in mice through subconjunctival injection of iTregs. We analyzed the local draining lymph nodes and spleens of recipient mice in rejection stage using flow cytometry, and found that subconjunctival injection of iTregs did not affect the state of local draining lymph nodes and systemic immune system in mice. Therefore, we speculated that iTregs could not enter the blood or lymph vessels to proliferate after subconjunctival injection, and only played the role of immune negative regulation locally by producing some cytokines around the operated eye or by cell-cell contact. Meanwhile, it was confirmed that the number of inflammatory cells infiltrated in allografts was decreased after subconjunctival injection of iTregs using flow cytometry and histopathological examination.
In this study, we injected iTregs in subconjunctival around the operated eye after in vitro induction and amplification, and discovered that iTregs did not interfere with the systemic immune system, but played a role of immunosuppression locally. The method used in this study can be applicable in clinical treatments, but the optimal concentration of injected iTregs, the response time and the specific mechanism how iTregs work locally around the eye still need further investigations.
Acknowledgements
This work was supported by the National Natural Scientific Foundation of China (Nos. 81170824 and 81070709) and Beijing Natural Scientific Foundation (No. 70802052).
Disclosure of conflict of interest
None.
References
- 1.Thompson RW Jr, Price MO, Bowers PJ, Price FW Jr. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. BGM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol. 2011;89:235–49. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-β–secreting cells inducing CD4+CD25+regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 2012;26:2253–76. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- 7.Probst-Kepper M, Balling R, Buer J. FOXP3: Required but not sufficient. The role of GARP (LRRC32) as a safeguard of the regulatory phenotype. Curr Mol Med. 2010;10:533–9. doi: 10.2174/1566524011009060533. [DOI] [PubMed] [Google Scholar]
- 8.Oida T, Weiner HL. TGF-b induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang EP, Schrunder S, Hoffmann F. Orthotopic corneal transplantation in the mouse-a new surgical technique with minimal endothelial cell loss. Graefes Arch Clin Exp Ophthalmol. 1996;234:714–9. doi: 10.1007/BF00292359. [DOI] [PubMed] [Google Scholar]
- 10.Jessup CF, Brereton HM, Sykes PJ, Thiel MA, Coster DJ, Williams KA. Local gene transfer to modulate rat corneal allograft rejection. Invest Ophthalmol Vis Sci. 2005;46:1675–81. doi: 10.1167/iovs.04-1140. [DOI] [PubMed] [Google Scholar]
- 11.Newman DK, Isaacs JD, Watson PG, Meyer PA, Hale G, Waldmann H. Prevention of immune-mediated corneal graft destruction with the anti-lymphocyte monoclonal antibody. Eye. 1995;9:564–9. doi: 10.1038/eye.1995.140. [DOI] [PubMed] [Google Scholar]
- 12.Zheng SG, Wang J, Horwitz DA. Cutting Edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-βare resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–6. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, Shi FD. Expansion of CD4+CD25+ regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental autoimmune myasthenia. Eur J Immunol. 2010;40:1577–89. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3+ regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 15.Chuhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–53. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3+ T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–33. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Jie Y, Ren D, Zeng H, Zhang Y, He Y, Pan Z. In vitro-expanded CD4+CD25highFoxp3+ regulatory T cells controls corneal allograft rejection. Hum Immunol. 2012;73:1061–7. doi: 10.1016/j.humimm.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Jie Y, Wang B, Zeng H, Zhang Y, Pan Z. Adoptive transfer of donor corneal antigenspecific regulatory T cells can prolong mice corneal grafts survival. Cornea. 2010;29:S25–31. doi: 10.1097/ICO.0b013e3181ea4999. [DOI] [PubMed] [Google Scholar]


