Abstract
Excess mesangial extracellular matrix (ECM) and mesangial cell (MC) proliferation is the major pathologic feature of diabetic nephropathy. Kruppel-like factor 15 (KLF15) is a member of the KLF transcription factor family that plays a critical role in regulating renal fibrosis. However, the role of KLF15 in diabetic nephropathy remains poorly understood. This study was conducted to explore the role of KLF15 in the development and progress of diabetic nephropathy in high glucose (HG)-stimulated human MCs. Here, we found down-regulated expression of KLF15 in MCs induced by HG. Overexpression of KLF15 significantly inhibited MCs proliferation and ECM production induced by HG. Moreover, overexpression of KLF15 inhibited HG-induced ERK1/2 phosphorylation in MCs. In summary, our data demonstrate that KLF15 can suppress HG-induced cell proliferation and ECM protein fibronectin expression in human MCs via ERK1/2 MAPK signaling. The results provide evidence that KLF15 might be a potential molecular target for the treatment of diabetic nephropathy.
Keywords: Kruppel-like factor 15 (KLF15), diabetic nephropathy, extracellular matrix (ECM, mesangial cells (MCs)
Introduction
Diabetic nephropathy is an important diabetic microvascular complication and the major cause of disability and death. It is characterized by albuminuria, glomerular hypertrophy, and progressive accumulation of glomerular matrix, culminating in glomerulosclerosis, tubulointerstitial fibrosis, and progressive loss of renal function [1]. It has been reported that the mesangial cells (MCs) play important roles in diabetic nephropathy, being responsible for the accumulation of ECM and mesangial expansion. Furthermore, there is increasing evidence that high glucose (HG) is one of the major factors in the development of diabetic nephropathy, and it promotes MC proliferation and increased matrix synthesis in vitro [2]. Thus, inhibition of HG-induced MC proliferation and ECM accumulation is beneficial in the treatment of diabetic nephropathy.
Kruppel-like factors (KLFs) are DNA-binding transcriptional regulators that contain three conserved zinc fingers within the carboxyl terminus that bind a putative consensus 5’-C(A/T)CCC-3’ motif in the promoters and enhancers of various genes [3,4]. Increasing evidence indicates that this family was involved in a variety of cellular processes, such as cell differentiation, cardiac remodeling, hematopoiesis, angiogenesis and stem cell-fate determination [5-8]. KLF15 is a member of the KLF transcription factor family that plays a critical role in regulating stress response, cardiac hypertrophy and fibrosis [9-12]. Recently, one study showed that overexpression of KLF15 repressed basal and transforming growth factor-β1 (TGF-β1)-induced extracellular matrix in rat renal fibroblasts [13]. In addition, Hong et al. reported that the MC proliferation was reduced in MCs overexpressing KLF15 in a classic rat anti-Thy1 mesangial proliferative nephritis model [14]. However, the role of KLF15 in diabetic nephropathy remains poorly understood. This study was conducted to explore the role of KLF15 in the development and progress of diabetic nephropathy in HG-stimulated human MCs.
Materials and methods
Cell culture
Normal human MC line was purchased from the Shanghai Academy of Life Sciences (Shanghai, China). The cells were cultured in MEM medium containing 5% fetal bovine serum (FBS) and 1 μg/ml of gentamicin (Sigma, St. Louis, MO, USA) and maintained at 37°C in 5% CO2 and 95% air. Cells were sub-cultured when 70-90% confluent and used for experiments between passages 5-7. At subconfluence, MCs were incubated with serum-free MEM medium for 24 h and then divided into four groups as follows: (a) control, where cells were kept in MEM medium without FBS, containing normal glucose concentration of 5.6 mM (NG group); (b) high glucose, where cells were cultured in MEM medium without FBS containing 25 mM glucose (HG group); (c) empty vector group; and (d) KLF15 overexpression (KLF15 OE) in 25 mM glucose condition. Cells were exposed to above conditions for 24 h.
KLF15 plasmid and cell transfection
A cDNA containing the full-length open reading frame of KLF15 (pcDNA3.1-KLF15 FLAG) was subcloned into an expression vector as previously described [15]. Empty vector control and pcDNA3.1-KLF15 FLAG adenoviral vectors were prepared by Shanghai Sangon Co., Ltd (Shanghai, China).
For in vitro transfection, MCs were seeded in each well of 24-well microplates, grown for 24 h to reach 50% confluence, and transfected with EV or KLF15 OE using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions.
Cell proliferation assay
Cell proliferation was determined using Cell Counting Kit-8 (CCK-8) (Beyotime Inst. Biotech, China) according to manufacturer’s instructions. In brief, MCs transfected with empty vector or KLF15 OE were seeded into 96-well plates (1 × 104 cells/well) and incubated with 25 mM glucose (HG) for 24 h, then incubated with WST-8 dye at 37°C. The optical density (OD) value of each well was measured at 450 nm.
Real-time quantitative PCR
Total RNA was extracted from the cells using the TRIzol reagent kit (Invitrogen, Carlsbad, CA) and treated with DNAse. 1 μg of treated RNA was utilized to synthesize cDNA using the SuperScript First-Stand Synthesis system (Invitrogen, Carlsbad, CA). PCR amplification was carried out by ABI PRISM 7900 thermocycler using SYBR Premix Taq (Applied Biosystems). The following primer pairs were used to PCR amplification: KLF15, forward 5’-GTTGGGTATCTGGGTGATAGGC-3’, reverse 5’-TGAGAGTCGGGACTGGAACAG-3’; GADPH, forward 5’-ATCCCATCACCATCTTCCAG-3’, reverse 5’-CCATCACGCCACAGTTTCC-3’. The reaction conditions was as follows: an initial denaturation step at 94°C for 2 min, followed by 40 cycles at 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 2 min, and a final elongation step at 72°C for 10 min. Relative levels of gene expression was expressed relative to β-actin and calculated using the 2-∆∆CT method [16].
Western blot analysis
The total proteins were extracted from MCs using the RIPA buffer (Beytime, Shanghai, China). The concentration of protein was determined using a BCA™ Protein Assay Kit according to the manufacturer’s instructions (Pierce, Rockford, USA). Equal amounts of protein (50 μg of protein/lane) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. The membranes were incubated overnight at 4°C with anti-KLF15, p21Cip1, p27Kip1, fibronectin, p-ERK1/2, ERK1/2 and GAPDH antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Then, the membrane was washed and incubated for 1 h at room temperature with a secondary antibody for 1 h at room temperature and then visualized by enhanced chemiluminescence detection reagents. Relative intensities of protein bands were analyzed by Image J Software.
Statistical analysis
Experiments were carried out at least in triplicate, and the results are expressed as mean ± SD. The differences were analyzed by the Student’s t test or one-way analysis of variance and Student’s t test. A p value of < 0.05 was considered to be statistically significant.
Results
Expression of KLF15 in mesangial cells under HG condition
To understand the role of KLF15 in the development of diabetic nephropathy, we examined the expression pattern of KLF15 in MCs induced by HG for 6, 12, 24, and 48 h. As shown in Figure 1A, KLF15 mRNA expression was significantly down-regulated in MCs after HG treatment, suggesting that the decreased level of KLF15 mRNA by HG was time-dependent. Western blot analysis also revealed that HG obviously decreased the expression of KLF15 protein in MCs. These results suggest that KLF15 is down-regulated in MCs induced by HG (Figure 1B).
Figure 1.
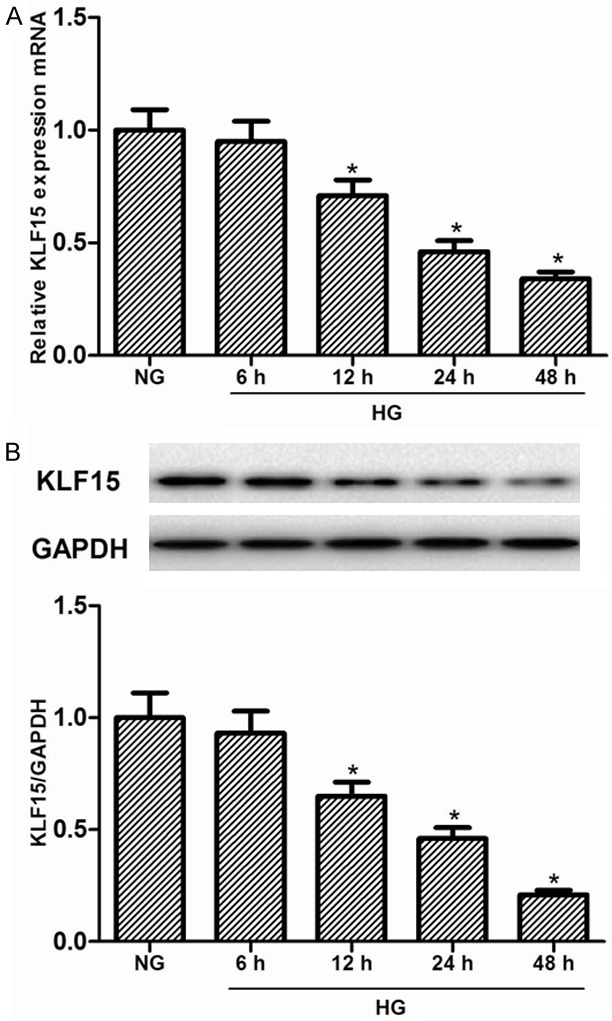
Expression of KLF15 in mesangial cells under high glucose condition. MCs were incubated with 25 mM glucose (HG) at the indicated times (0-48 h). A. The expression of KLF15 mRNA was detected by RT-qPCR. B. The expression of KLF15 protein was analyzed by Western blot. Data represent means ± SD. of three experiments. *P < 0.05 vs. NG group. NG, normal glucose.
KLF15 overexpression inhibits cell proliferation in MCs induced by HG
To gain further insight into the role of KLF15 in HG-stimulated MCs, we constructed KLF15 overexpressing MCs. The transfection efficiency was confirmed by RT-qPCR and western blot. We found that the expression levels of KLF15 were significantly increased in MCs after KLF-15 transfection (Figure 2A and 2B). Then, the mesangial cells proliferation was evaluated using CCK-8 assay. We found that HG could markedly enhance the cell proliferation of MCs, as compared with the NG group. However, KLF15 overexpression dramatically suppressed MC proliferation induced by HG (Figure 3A). In addition, we also examined the effects of KLF15 on HG-regulated expression of cell regulatory molecules. Western blot analysis revealed that exposure to HG resulted in a significant reduction of p21Waf1/Cip1 and p27Kip1, compared with NG control. Whereas, KLF15 overexpression significantly reversed HG-inhibited expression of p21Waf1/Cip1 and p27Kip1 (Figure 3B).
Figure 2.
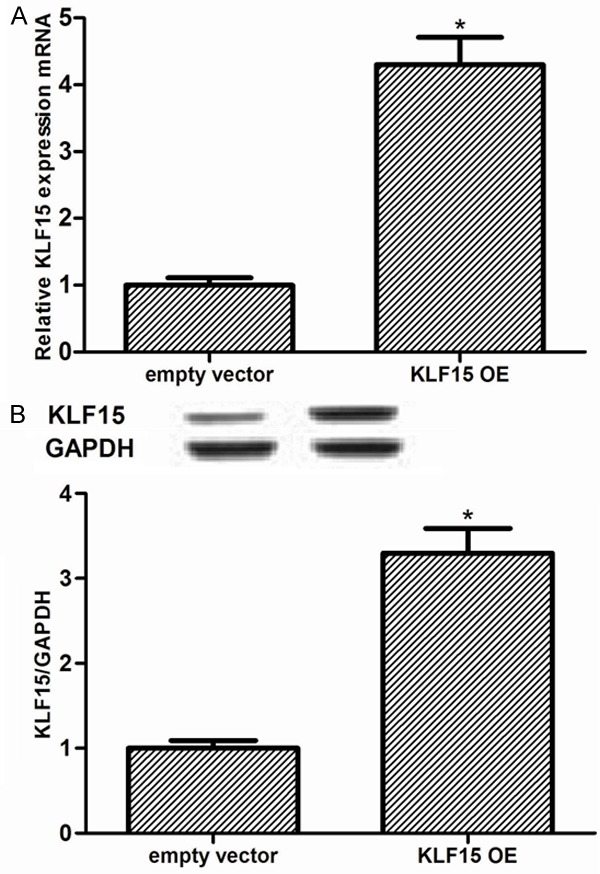
Determination of transfection efficiency in control MCs (empty vector) and KLF15 overexpressing MCs. MCs were transfected with empty vector or KLF15 OE for 24 h. The corresponding transfection efficiency was detected by RT-qPCR and Western blot. A. KLF15 mRNA expression in MCs. B. KLF15 protein expression in MCs. Data represent means ± SD. of three experiments. *P < 0.05 vs. empty vector group. OE, overexpression.
Figure 3.
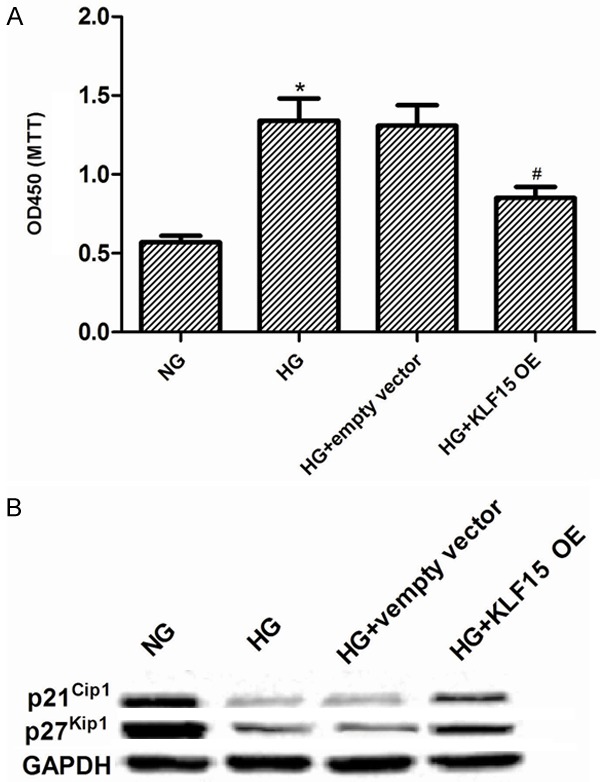
KLF15 overexpression inhibits cell proliferation in MCs induced by HG. MCs transfected with empty vector or KLF15 OE and incubated with 25 mM glucose (HG) for 24 h. A. Cell proliferation was determined by CCK-8 assay. B. The protein levels of p21Waf1/Cip1 and p27Kip1 were detected with Western blot. Data represent means ± SD. of three experiments. *P < 0.05 vs. NG group, #P < 0.05 vs. HG group. NG, normal glucose; OE, overexpression.
KLF15 overexpression reduces the expression of fibronectin in MCs induced by HG
Fibronectin upregulation is a molecular hallmark of ECM accumulation in diabetic nephropathy, so, we also detected the expression of fibronectin in MCs induced by HG. As indicated in Figure 4, we observed that the MCs treated with HG showed higher level of fibronectin than those cultured under NG condition at 24 h. Compared to HG-induced overexpression of fibronectin in the MCs transfected with empty vector, overexpression of KLF15 significantly decreased the expression of fibronectin in MCs.
Figure 4.
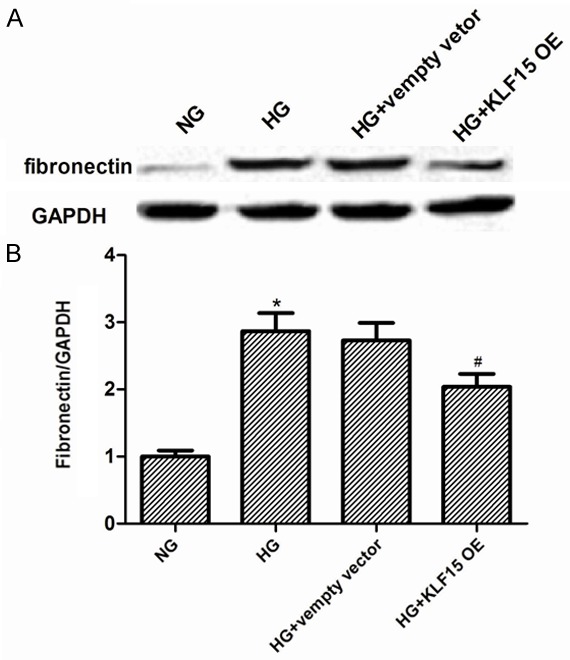
KLF15 overexpression reduces the expression of fibronectin in MCs induced by HG. MCs transfected with empty vector or KLF15 OE and incubated with 25 mM glucose (HG) for 24 h. A. Expression of fibronectin was analyzed by Western blot. B. Quantitative data. Data represent means ± SD. of three experiments. *P < 0.05 vs. NG group, #P < 0.05 vs. HG group. NG, normal glucose; OE, overexpression.
Effect of KLF15 on activation of the ERK1/2 MAPK pathway in MCs
Extracellular signal-regulated kinase 1/2 (ERK1/2) can be activated in MCs exposed to HG [17], therefore, we investigated the effect of KLF5 on the ERK1/2 MAPK pathway in MCs, we transfected KLF15 overexpression into MCs exposed to HG medium for 24 h and evaluated p-ERK1/2 levels. As indicated in Figure 5, the level of p-ERK1/2 increased after HG stimulation, however, p-ERK1/2 expression was decreased in MCs transfected with KLF15 overexpression.
Figure 5.
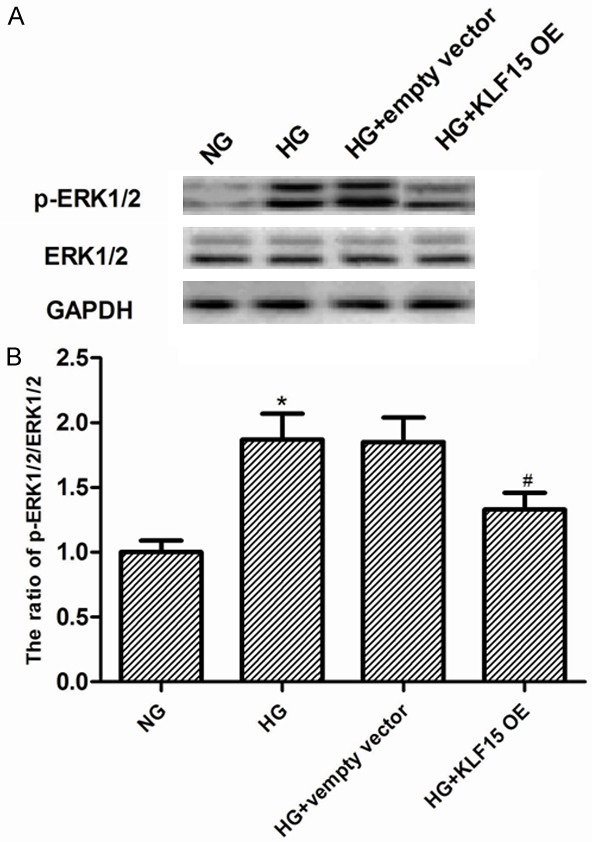
Effect of KLF15 on activation of the ERK1/2 MAPK pathway in MCs. A. MCs transfected with empty vector or KLF15 OE and incubated with 25 mM glucose (HG) for 24 h, and the ERK1/2 phosphorylation was determined using phospho-ERK1/2 specific antibody. B. The ratio of p-ERK1/2/ ERK1/2. Data represent means ± SD. of three experiments. *P < 0.05 vs. NG group, #P < 0.05 vs. HG group. NG, normal glucose; OE, overexpression.
Discussion
Here, we found down-regulated expression of KLF15 in MCs induced by HG. Overexpression of KLF15 significantly inhibited MCs proliferation and ECM production induced by HG. Moreover, overexpression of KLF15 inhibited HG-induced ERK1/2 phosphorylation in MCs. KLF15 may be involved in diabetic nephropathy by regulating MC proliferation and ECM accumulation via ERK1/2 MAPK signaling.
Previous studies found that KLF15, a DNA-binding transcription factor, is highly expressed in endothelial and MCs of the kidney, and the expression of KLF15 was lower during MC proliferation period in a rat anti-Thy1 mesangial proliferative nephritis model [14]. Likewise, our data revealed that HG time-dependently down-regulates mRNA and protein levels of KLF15 in MCs, suggesting that KLF15 is implicated in the pathogenesis of diabetic nephropathy.
Accumulating amounts of evidence suggest that MCs proliferation, which is a characteristic of mesangial cell activation, occurs in diabetic nephropathy [18-20]. Furthermore, a HG concentration has been shown to contribute mainly to uncontrolled cell proliferation in MCs, distal tubular epithelial cells and vascular smooth muscle cells in diabetic nephropathy [21]. Consistent with these results, in the present study, we found that HG enhanced MCs proliferation, whereas, overexpression of KLF15 ameliorated HG-induced MCs proliferation. Moreover, emerging studies have indicated that p21Waf1/Cip1 and p27Kip1 were critically involved in the G1-phase cell cycle arrest in MCs while the cells were exposed to HG in diabetic mice [22]. Similarly, in our experiments, the results demonstrated that overexpression of KLF15 reversed the downregulation of p21Waf1/Cip1 and p27Kip1 by HG. These data indicate that KLF15 inhibited HG-stimulated cell proliferation and hypertrophy by inhibiting cell cycle progression.
ECM production is a key event in the progression of diabetic nephropathy. When exposed to a variety of HG, MCs synthesize ECM proteins, which lead to the development of progressive renal disease and finally glomerulosclerosis in humans and animal diabetic models [23]. Similarly, in our experiments, the results demonstrated that HG significantly increased the expression of fibronectin. Overexpression of KLF15 ameliorated HG-induced the expression of fibronectin in MCs, indicating that KLF15 plays a significant role in ECM accumulation in MCs.
ERK1/2 is known as an important kinase in the regulation of cell proliferation and protein synthesis [24]. As a member of the MAPK family, it has been reported that ERK1/2 is activated in glomeruli of diabetic rats as well as in MCs cultured under high-glucose conditions [17,25]. The activation of the ERK1/2 pathway is also necessary for HG-induced production of TGF-β1 and connective tissue growth factor (CTGF) in MCs [26]. Besides, several agents reportedly could attenuate MCs proliferation and ECM synthesis by inhibiting ERK1/2 MAPK signaling pathway, such as fenofibrate [27], gremlin [28] and eicosapentaenoic acid [29] in the glomeruli of diabetic rats or stimulated in MCs upon exposure to HG. Therefore, the blockade of ERK1/2 MAPK signaling pathway may represent a new therapeutic target for preventing development of diabetic nephropathy. In the present study, we found that high concentrations of extracellular glucose stimulated the phosphorylation level of ERK1/2 MAPK in MCs. However, overexpression of KLF15 inhibited HG-induced ERK1/2 phosphorylation in MCs. These results suggest that the inhibitory effects of KLF15 to HG-induced the expression of fibronectin may be via inhibiting the activation of the ERK1/2 MAPK signaling pathway.
In summary, our data demonstrate that KLF15 can suppress HG-induced cell proliferation and ECM protein fibronectin expression in human MCs. The results provide evidence that KLF15 might be a potential molecular target for the treatment of diabetic nephropathy.
Disclosure of conflict of interest
None.
References
- 1.Valk EJ, Bruijn JA, Bajema IM. Diabetic nephropathy in humans: pathologic diversity. Curr Opin Nephrol Hypertens. 2011;20:285–289. doi: 10.1097/MNH.0b013e328345bc1c. [DOI] [PubMed] [Google Scholar]
- 2.Oh JH, Ha H, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-&bgr; 1 and fibronectin synthesis. Kidney Int. 1998;54:1872–1878. doi: 10.1046/j.1523-1755.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller I, Bieker JJ. A novel, erythroid cellspecific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieker JJ. Krüppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 5.Haldar SM, Ibrahim OA, Jain MK. Kruppellike Factors (KLFs) in muscle biology. J Mol Cell Cardiol. 2007;43:1–10. doi: 10.1016/j.yjmcc.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkins AC, Gardiner MR. Zebrafish KLF4 Is Essential for Primitive Haematopoiesis. ASH. 2005:106. [Google Scholar]
- 7.Bhattacharya R, SenBanerjee S, Lin Z, Mir S, Hamik A, Wang P, Mukherjee P, Mukhopadhyay D, Jain MK. Inhibition of vascular permeability factor/vascular endothelial growth factormediated angiogenesis by the Kruppel-like factor KLF2. J Biol Chem. 2005;280:28848–28851. doi: 10.1074/jbc.C500200200. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 9.Jung DY, Chalasani U, Pan N, Friedline RH, Prosdocimo DA, Nam M, Azuma Y, Maganti R, Yu K, Velagapudi A. KLF15 is a molecular link between endoplasmic reticulum stress and insulin resistance. PLoS One. 2013;8:e77851. doi: 10.1371/journal.pone.0077851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B, Haldar SM, Lu Y, Ibrahim OA, Fisch S, Gray S, Leask A, Jain MK. The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol. 2008;45:193–197. doi: 10.1016/j.yjmcc.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leenders JJ, Wijnen WJ, Van Der Made I, Hiller M, Swinnen M, Vandendriessche T, Chuah M, Pinto YM, Creemers EE. Repression of cardiac hypertrophy by KLF15: underlying mechanisms and therapeutic implications. PLoS One. 2012;7:e36754. doi: 10.1371/journal.pone.0036754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Mitch WE. Proteins and renal fibrosis: low-protein diets induce Kruppel-like factor-15, limiting renal fibrosis. Kidney Int. 2011;79:933–934. doi: 10.1038/ki.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Wu G, Gu X, Fu L, Mei C. Kruppel-like factor 15 modulates renal interstitial fibrosis by ERK/MAPK and JNK/MAPK pathways regulation. Kidney Blood Press Res. 2013;37:631–640. doi: 10.1159/000355743. [DOI] [PubMed] [Google Scholar]
- 14.Hong Q, Li C, Xie Y, Lv Y, Liu X, Shi S, Ding R, Zhang X, Zhang L, Liu S. Kruppel-like factor-15 inhibits the proliferation of mesangial cells. Cell Physiol Biochem. 2012;29:893–904. doi: 10.1159/000178518. [DOI] [PubMed] [Google Scholar]
- 15.Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007;104:7074–7079. doi: 10.1073/pnas.0701981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Haneda M, Araki SI, Togawa M, Sugimoto T, Motohide I, Kikkawa R. Mitogen-activated protein kinase cascade is activated in glomeruli of diabetic rats and glomerular mesangial cells cultured under high glucose conditions. Diabetes. 1997;46:847–853. doi: 10.2337/diab.46.5.847. [DOI] [PubMed] [Google Scholar]
- 18.Nahman N, Leonhart KL, Cosio FG, Hebert CL. Effects of high glucose on cellular proliferation and fibronectin production by cultured human mesangial cells. Kidney Int. 1992;41:396–402. doi: 10.1038/ki.1992.55. [DOI] [PubMed] [Google Scholar]
- 19.Ahn J, Morishita R, Kaneda Y, Kim H, Kim Y, Lee H, Lee K, Park J, Kim Y, Park K. Transcription factor decoy for AP-1 reduces mesangial cell proliferation and extracellular matrix production in vitro and in vivo. Gene Ther. 2004;11:916–923. doi: 10.1038/sj.gt.3302236. [DOI] [PubMed] [Google Scholar]
- 20.Girolami J, Ouardani M, Bascands J, Pécher C, Bompart G, Leung-Tack J. Comparison of B1 and B2 receptor activation on intracellular calcium, cell proliferation, and extracellular collagen secretion in mesangial cells from normal and diabetic rats. Can J Physiol Pharmacol. 1995;73:848–853. doi: 10.1139/y95-116. [DOI] [PubMed] [Google Scholar]
- 21.Sodhi CP, Phadke SA, Batlle D, Sahai A. Hypoxia and high glucose cause exaggerated mesangial cell growth and collagen synthesis: role of osteopontin. Am J Physiol-Renal Physiol. 2001;280:F667–F674. doi: 10.1152/ajprenal.2001.280.4.F667. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Wada J, Hida K, Eguchi J, Hashimoto I, Baba M, Yasuhara A, Shikata K, Makino H. Thiazolidinediones ameliorate diabetic nephropathy via cell cycle-dependent mechanisms. Diabetes. 2006;55:1666–1677. doi: 10.2337/db05-1285. [DOI] [PubMed] [Google Scholar]
- 23.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 24.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awazu M, Ishikura K, Hida M, Hoshiya M. Mechanisms of mitogen-activated protein kinase activation in experimental diabetes. J Am Soc Nephrol. 1999;10:738–745. doi: 10.1681/ASN.V104738. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Hu GY, Shen H, Peng ZZ, Ning WB, Tao LJ. Fluorofenidone inhibits TGF-β1 induced CTGF via MAPK pathways in mouse mesangial cells. Pharmazie. 2009;64:680–684. [PubMed] [Google Scholar]
- 27.Zeng R, Xiong Y, Zhu F, Ma Z, Liao W, He Y, He J, Li W, Yang J, Lu Q. Fenofibrate attenuated glucose-induced mesangial cells proliferation and extracellular matrix synthesis via PI3K/AKT and ERK1/2. PLoS One. 2013;8:e76836. doi: 10.1371/journal.pone.0076836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Huang H, Li Y, Liu M, Shi Y, Chi Y, Zhang T. Gremlin induces cell proliferation and extra cellular matrix accumulation in mouse mesangial cells exposed to high glucose via the ERK1/2 pathway. BMC Nephrol. 2013;14:33. doi: 10.1186/1471-2369-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, Kaneko S, Itoh T, Gohda T, Horikoshi S. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant. 2006;21:605–615. doi: 10.1093/ndt/gfi208. [DOI] [PubMed] [Google Scholar]


