Abstract
The pathogenesis of Chlamydia-induced inflammation is poorly understood. pORF5 is the only secreted protein encoded by Chlamydial plasmid. This study aims to investigate the effects of pORF5 on the production of interleukin-1β (IL-1β) and interleukin-18 (IL-18) and the underlying mechanisms of these effects. THP-1 (a human acute monocytic leukemia cell line) cells were stimulated by pORF5 with or without pretreatment with Natch domain, Leucine-rich repeat and PYD-containing protein 3 (NALP3) siRNA, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) siRNA, cysteine aspartate-specific protease-1 (caspase-1) specific inhibitor and p38 mitogen-activated protein kinase (p38 MAPK) inhibitor. IL-1β, IL-18 and caspase-1 expression was detected through both ELISA and qRT-PCR. NALP3 and ASC expression was detected by qRT-PCR. The expression of caspase-1 and phosphorylated-p38 MAPK was detected by western blot analysis. pORF5 induced IL-1β, IL-18, caspase-1 and NALP3 inflammasome expression in THP-1 cells. Caspase-1 inhibitor significantly reduced pORF5-induced IL-1β and IL-18 expression. The siRNAs for NALP3 inflammasome significantly reduced pORF5-induced IL-1β, IL-18 and caspase-1 expression. Furthermore, p38 MAPK inhibitor significantly reduced pORF5-induced IL-1β, IL-18, caspase-1 and NALP3 inflammasome expression. pORF5 could induce production of IL-1β and IL-18 via NALP3 inflammasome activation and p38MAPK pathway. pORF5 protein might play an important role in Chlamydia pathogenesis. This study provides a new insight into the molecular pathogenesis of Chlamydial diseases.
Keywords: pORF5, NALP3 inflammasome, Interleukin-1β, Interleukin-18, p38MAPK
Introduction
Chlamydia trachomatis (Ct) is an obligate intracellular bacterial pathogen associated with a variety of human diseases worldwide. It is not only the main cause of blindness in developing countries, but also the leading cause of sexually transmitted bacterial diseases in developed countries. Ct causes various diseases depending on the serovar involved. Serovars A to C are associated with trachoma. Serovars D to K are associated with genital tract disease, conjunctivitis and infant pneumonia. And the lymphogranuloma venereum (LGV) serovars L1, L2, L2a and L3 are associated with an invasive disease known as LGV [1-3]. In addition, Ct correlates with an increased risk for the transmission or acquisition of HIV and is a risk factor for the development of invasive cervical carcinoma [4-7]. Most chlamydia-positive patients are asymptomatic or with minimal clinical symptoms and the infected individuals can serve as important reservoirs for new infections. It is important to identify the virulence factors and pathogenesis of Ct for the control and prevention of Chlamydial diseases.
Inflammation is the major cause of Chlamydial diseases. Ct can activate a variety of immune cells and induce the release of multiple inflammatory cytokines including interleukin-1β (IL-1β), interleukin-18 (IL-18), tumor necrosis factor (TNF) and so on in vivo and in vitro [8-10]. IL-1β and IL-18 play important roles in Ct induced inflammation [11]. They are produced as inactive cytoplasmic precursors (pro-IL-1β and pro-IL-18) that must be cleaved by cysteine aspartate-specific protease-1 (caspase-1) to generate the mature active form. However, the precise mechanisms on how chlamydia triggers such a response and which factor(s) stimulate the production of IL-1β and IL-18 remain unclear.
NALP3 (Natch domain, Leucine-rich repeat and PYD-containing protein 3, NALP3) inflammasome is a cytosolic multiprotein complex that serves as a platform for activating IL-1β and IL-18 via caspase-1 cleavage [12,13]. NALP3 contains a PYD domain that mediates the interaction with apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) which acts as a bridge to recruits caspase-1. NALP3 inflammasome mediates IL-1β and IL-18 production in response to the NALP3 agonist and host danger signal [14-17]. It has been reported that Ct could induce IL-1â and IL-18 production through NALP3 inflammasome [18,19]. However, little is known about the mechanisms on Ct induced NALP3 inflammasome activation.
Besides a highly conserved genome, all Ct serovars also contain a 7.5 kb cryptic plasmid that encodes 8 open reading frames designated as pORF1 to 8. Plasmid-deficient chlamydia fails to cause pathological changes of genital tract [20,21]. It seems that the chlamydial plasmid plays an important role in the pathophysiology of Ct associated diseases. The pORF5 protein, encoded by the open reading frame 5 of the Chlamydial cryptic plasmid [22], is the only secreted plasmid protein and mainly locates in the cytosol of Ct-infected cells. This suggests that pORF5 might participate in the crosstalk of Ct with host cells. Our precious studies have shown that pORF5 plasmid protein can induce monocytes to produce inflammatory cytokines such as interleukin-6 (IL-6), IL-8 and TNF-á partly via p38 mitogen-activated protein kinase (p38 MAPK) signal pathway [23], indicating that pORF5 plasmid protein may be responsible for the occurrence and development of chlamydia-induced pathologies.
In this study, the effects of pORF5 on IL-1β and IL-18 production and their underlying mechanisms were investigated. pORF5 could induce the production of IL-1β and IL-18 via NALP3 inflammasome activation and p38 MAPK pathway. These results suggest that pORF5 protein is an important virulence factor of Ct.
Materials and methods
Cell culture and stimulation
THP-1 cells (No. TIB-202, ATCC) were cultured in RPMI 1640 medium (HyClone, Logan, Utah, USA) containing 10% heat-inactivated fetal bovine serum (FBS, GIBCO BRL, Rockville, MD, USA), 100 U⁄ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified CO2 atmosphere (air with 5% CO2). For stimulation experiments, THP-1 cells were plated in 12-well flat-bottom plate at a density of 5×105/well, and differentiated into macrophages with 160 nM phorbol 12-myristate 13-acetate (PMA, Sigma, St. Louis, MO, USA) for 24 hours. The pORF5 protein, prepared as previously described [23], was added to cultures as stimulus at indicated concentrations and times. The E. coli lipopolysaccharide (LPS; Sigma) was used as a positive control. In some experiments, THP-1 cells were transfected with NALP3 or ASC siRNA at 24 hours before pORF5 stimulation. In some experiments, THP-1 cells were pretreated with caspase-1 specific inhibitor Z-YVAD-FMK (Sigma) or p38 inhibitor SB202190 (Sigma) for 30 min before pORF5 stimulation.
Enzyme-linked immunosorbent assay (ELISA)
At the end of culture, THP-1 cells were lysed by two consecutive cycles of freezing/thawing to yield maximal recovery of total cytokines, which included both intracellular cytokines and those released into the cell culture medium. The concentrations of IL-1β, IL-18, TNF-α and caspase-1 were measured by commercial human ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturers’ protocols.
Quantitative Real-time PCR (qRT-PCR) assay
Total RNA was extracted from THP-1 cell pellets using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and quantified by a spectrophotometer. A DNase digestion step was included to eliminate contamination with genomic DNA. cDNA was synthesized by the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA, USA). The five primer sets were shown in Table 1. qRT-PCR was performed as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 20 sec. The samples were analyzed in duplicate. The expression levels of the target genes were normalized to the endogenous control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression and expressed as fold changes compared with the control group.
Table 1.
Primer sequences used for qRT-PCR
| Gene | Size (bp) | Sense primer | Anti-sense primer |
|---|---|---|---|
| NALP3 | 169 | 5’-AACAGCCACCTCACTTCCAG-3’ | 5’-CCAACCACAATCTCCGAATG-3’ |
| ASC | 75 | 5’-CTCCTCAGTCGGCAGCCAAG-3’ | 5’-TTGTGACCCTCGCGATAAGC-3’ |
| Caspase-1 | 146 | 5’-GCACAAGACCTCTGACAGCA-3’ | 5’-TTGGGCAGTTCTTGGTATTC-3’ |
| IL-1β | 107 | 5’-AACCTCTTCGAGGCACAAGG-3’ | 5’-GGCGAGCTCAGGTACTTCTG-3’ |
| IL-18 | 142 | 5’-GATTACTTTGGCAAGCTTGAA-3’ | 5’-ATATGGTCCGGGGTGCATTA-3’ |
| GAPDH | 112 | 5’-GAAGGTGAAGGTCGGAGTC-3’ | 5’-GTCAATGAAGGG GTCATT-3’ |
Note: NALP3, Natch domain, Leucine-rich repeat and PYD-containing protein 3; ASC, apoptosis-associated speck-like protein containing a CARD; Caspase-1, Cysteiny aspartate-specific protease-1; IL, interleukin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis
THP-1 cells were lysed in lysis buffer. The lysates were subjected to electrophoresis on 12% SDS-PAGE gels. Then proteins were transferred to nitrocellulose membranes for western blot analysis. After blocking with 5% non-fat milk for 1 hour at room temperature, the membranes were incubated overnight with rabbit anti-phosphorylated-p38 antibody (Cell Signaling Technology, Beverly, MA, USA) or rabbit anti-caspase-1 p20 antibody (Cell Signaling Technology). After washing, the membrane was then incubated with mouse anti-rabbit HRP-conjugated secondary antibody (Jackson Immunologicals, Westgrove, PA, USA). Bound antibodies were detected using enhanced chemiluminescence (ECL) kit (Santa Cruz Biotech, Santa Cruz, CA, USA). β-actin was used as an internal control and the mouse anti-β-actin antibody was purchased from Santa Cruz Biotech. The mean normalized optical density (OD) of caspase-1 p20 or phosphorylated-p38 protein band relative to the OD of β-actin band from the same sample was calculated using the ImageTool 3.0 gray scale scanning software. The expression levels of caspase-1 p20 and phosphorylated-p38 were expressed as fold changes compared with the control group.
RNA interference
THP-1 cells (1×106 cells/well in 6 well plates) were transfected with 100 nM siRNA for NALP3, ASC or negative control using Lipofectamine 2000 transfection reagent (Invitrogen). Control non-silencing siRNA, NALP3 siRNA and ASC siRNA were purchased from RiboBio Co. LTD (Guangzhou, China). The siRNA sequences targeting NALP3 were 5’-GAAAUGGAUUGAAGUGAAA-3’ (sense) and 5’-UUUCACUUCAAUCCAUUUC-3’ (antisense), siRNA sequences targeting ASC were 5’-GAUGCGGAAGCUCUUCAGU-3’ (sense) and 5’-ACUGAAGAGCUUCCGC AUC-3’ (antisense). Twenty-four hours after transfection, the cells were incubated in medium containing 24 μg/ml pORF5 protein for 24 hours.
Statistical analysis
Data were expressed as mean ± SD. All experiments were performed at least three times. Quantitative data (gene expression levels and cytokine levels) were analyzed by Student’s t test and one-way analysis of variance (ANOVA). The differences with P values less than 0.05 were considered statistically significant.
Results
pORF5 induces IL-1β and IL-18 production from THP-1 cells
To demonstrate the effects of pORF5 on IL-1â and IL-18 production, THP-1 cells were treated with pORF5 at the concentrations of 3, 6, 12, 24 and 36 μg/ml for 24 hours. PBS was used as the negative control, and LPS (100 ng/ml) was used as the positive control. Mature IL-1â and IL-18 protein levels in the samples containing both intracellular cytokines and those released into the cell culture medium were measured using ELISA. As expected, IL-1â and IL-18 expression was very low in the negative control group while significantly increased in the positive control group. As the concentration of pORF5 protein increased from 3, 6, 12, to 24 μg/ml, IL-1â expression increased from 165.2 ± 22.3, 242.1 ± 29.2, 330.9 ± 40.5, to 491.3 ± 52.5 pg/ml and IL-18 expression increased from 47.4 ± 5.1, 79.8 ± 8.2, 145.2 ± 15.2, to 186.1 ± 20.5 pg/ml. IL-1â and IL-18 production decreased as the concentration of pORF5 reached 36 μg/ml. pORF5 protein at each indicated concentration except at 3 μg/ml led to significantly increased IL-1â and IL-18 production compared with those in the negative control group (P < 0.01). These results indicate that pORF5 induces IL-1â and IL-18 production from THP-1 cells in a concentration-dependent manner (Figure 1A).
Figure 1.
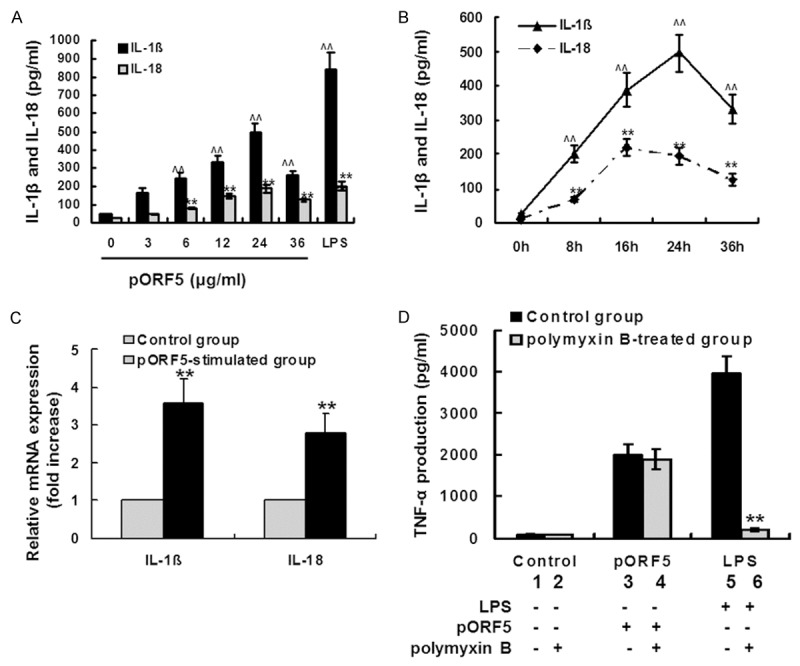
pORF5 induces IL-1β and IL-18 production in THP-1 cells. A. THP-1 cells were treated with pORF5 at the indicated concentrations for 24 hours. PBS was used as the negative control, and LPS (100 ng/ml) was used as the positive control. Mature IL-1â and IL-18 protein levels in the samples containing both intracellular cytokines and those released into the cell culture medium were measured using ELISA. The data represents mean ± SD of three independent experiments. Student’s t test, ^^P < 0.01 versus IL-1â protein levels in the control group and **P < 0.01 versus IL-18 protein levels in the control group. B. THP-1 cells were exposed to pORF5 (24 μg/ml) for 0, 8, 16, 24 and 36 hours. Mature IL-1â and IL-18 protein levels were measured using ELISA. The data represents mean ± SD of three independent experiments. Student’s t test, ^^P < 0.01 versus IL-1â protein levels in the control group and **P < 0.01 versus IL-18 protein levels in the control group. C. THP-1 cells were treated with pORF5 for 24 hours at 24 μg/ml. PBS was used as the negative control. IL-1â and IL-18 mRNA expression was detected using qRT-PCR. The expression levels of the target genes were normalized to the endogenous control GAPDH expression and expressed as fold changes compared with the control group. The data represents mean ± SD of three independent experiments. Student’s t test. **P < 0.01. D. THP-1 cells were treated with PBS (lane 1), PBS pretreated with 50 μg/ml polymyxin B (lane 2), pORF5 (24 μg/ml, lane 3), pORF5 pretreated with 50 μg/ml polymyxin B (lane 4), LPS (100 ng/ml, lane 5) and LPS pretreated with 50 μg/ml polymyxin B (lane 6) for 24 hours. The concentrations of TNF-α were determined by ELISA. The data represents mean ± SD of three independent experiments. ANOVA, **P < 0.01 compared with polymyxin-treated THP-1 cells.
Then THP-1 cells were treated with pORF5 at 24 μg/ml and the expression kinetics of IL-1β and IL-18 were detected using ELISA. As the time of treatment prolonged from 0, 8, 16 to 24 hours, IL-1â expression increased from 23.2 ± 3.3, 203.4 ± 25.3, 387.6 ± 49.6, to 495.1 ± 55.5 pg/ml. After that, IL-1â expression decreased to 331.2 ± 42.5 pg/ml (36 hours). As the time of treatment prolonged from 0, 8 to 16 hours, IL-18 expression increased from 11.3 ± 1.2, 69.4 ± 8.1 to 220.2 ± 25.8 pg/ml. After that, IL-18 expression decreased to 195.4 ± 25.2 pg/ml (24 hours) and 125.1 ± 18.6 pg/ml (36 hours). At each indicated time point, IL-1β and IL-18 production were significantly higher than those in the control group (0 hour). These results indicate that pORF5 induces IL-1â and IL-18 production from THP-1 cells in a time-dependent manner (Figure 1B).
Since pORF5 could induce mature IL-1â and IL-18 protein production from THP-1 cells, we wonder whether such was also the case of IL-1â and IL-18 mRNA expression. THP-1 cells were treated with 24 μg/ml pORF5 for 24 hours. IL-1â and IL-18 mRNA expression were detected using qRT-PCR. In pORF5 treated group, IL-1â expression was 3.6-fold higher and IL-18 expression was 2.2-fold higher when compared with those in the control group (Figure 1C). These results indicate that pORF5 could also induce IL-1â and IL-18 mRNA expression in THP-1 cells.
To exclude the possibility that the effect of pORF5 was due to LPS contamination, pORF5 (24 μg/ml) was preincubated with 50 μg/ml polymyxin B for neutralization of endotoxin. LPS (100 ng/ml) preincubated with polymyxin B was used as a control. We tested the effect of polymyxin B on pORF5-induced TNF-α secretion using ELISA. As shown in Figure 1D, preincubation with polymyxin B completely abrogated LPS-induced TNF-α production (P < 0.01), but had no significant effect on pORF5-induced TNF-α production by THP-1 cells (P > 0.05). These results confirmed that the effect of pORF5 was not due to LPS contamination.
pORF5 induces IL-1β and IL-18 production in a caspase-1-dependent manner
Since caspase-1 plays a major role in the cleavage of the inactive precursors of IL-1β and IL-18 into bioactive cytokines, we examined its influence on pORF5-induced IL-1β and IL-18 production in THP-1 cells. THP-1 cells were treated with pORF5 for 24 hours at 24 μg/ml. Then the supernatant and the cell pellet were collected. Caspase-1 is synthesized as an inactive zymogen (45 kDa) that becomes activated by cleavage to produce an enzymatically active heterodimer composed of 10 and 20 kDa subunits [24]. The expression of the cleaved p20 subunit of caspase-1 in the supernatant of THP-1 cells was detected using western blot analysis. In pORF5 treated group, caspase-1 p20 subunit expression was 3.2-fold higher when compared with that in the control group (Figure 2A and 2B). Furthermore, caspase-1 mRNA expression was detected using qRT-PCR. In pORF5 treated group, caspase-1 mRNA expression was 4.2-fold higher when compared with that in the control group (Figure 2C). These results indicate that pORF5 could induce both caspase-1 activation and caspase-1 mRNA expression in THP-1 cells.
Figure 2.
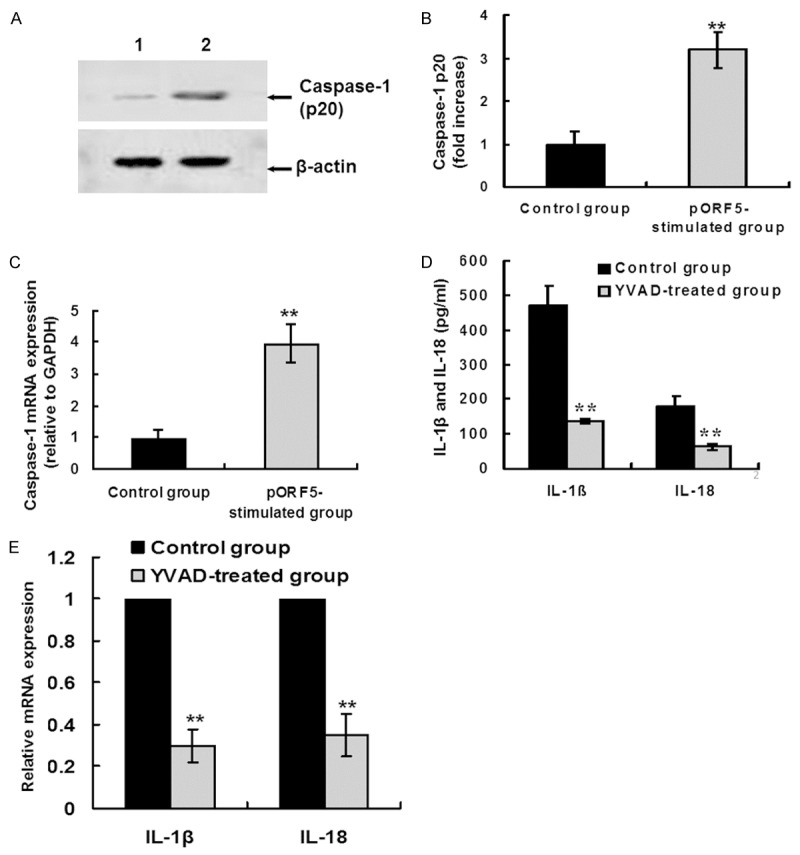
pORF5 induces IL-1β and IL-18 production in a caspase-1-dependent manner. (A) THP-1 cells were stimulated with PBS (lane 1) and pORF5 (24 μg/ml, lane 2) for 24 hours. Cleaved caspase-1 p20 subunit was detected by western blot analysis. β-actin was used as an internal control. The representative results of three independent experiments were shown. (B) Quantitative comparison of caspase-1 p20 subunit expression by qRT-PCR. Data were derived from three independent experiments as in (A). The mean normalized optical density (OD) of caspase-1 p20 protein band relative to the OD of β-actin band from the same sample was calculated using the ImageTool 3.0 gray scale scanning software. The expression levels of caspase-1 p20 were expressed as fold changes compared with the control group. Data were expressed as mean ± SD. Student’s t test, **P < 0.01. (C) THP-1 cells were treated as the same in (A). Caspase-1 mRNA levels were measured by qRT-PCR. The expression levels of the target genes were normalized to the endogenous control GAPDH expression and expressed as fold changes compared with the control group. The data represents mean ± SD of three independent experiments. Student’s t test. **P < 0.01. (D) THP-1 cells were treated with pORF5 (24 μg/ml) only for 24 hours (control group) or pretreated with Z-YVAD-FMK (a specific caspase-1 inhibitor, 20 μM) for 30 min before pORF5 treatment (YVAD-treated group). Mature IL-1â and IL-18 protein expression was detected using ELISA. The data represents mean ± SD of three independent experiments. ANOVA, **P < 0.01 relative to the control group. (E) THP-1 cells were treated as the same in (D). IL-1â and IL-18 mRNA expression was detected using qRT-PCR. The expression levels were obtained as the same in (C). The data represents mean ± SD of three independent experiments. ANOVA, **P < 0.01 versus the control group.
To determine whether caspase-1 is required for pORF5-induced IL-1â and IL-18 production, THP-1 cells were pretreated with Z-YVAD-FMK (20 μM) for 30 min before pORF5 (24 μg/ml) treatment. Twenty-four hours after pORF5 treatment, samples were collected for IL-1â and IL-18 detection using both ELISA and qRT-PCR. In Z-YVAD-FMK pretreated group, mature IL-1â and IL-18 protein expression were significantly lower than those in the group treated with pORF5 only (135.3 ± 8.2 pg/ml versus 470.1 ± 56.3 pg/ml, P < 0.01; 61.2 ± 10.1 pg/ml versus 180.4 ± 28.0 pg/ml, P < 0.01, respectively) (Figure 2D). Furthermore, In Z-YVAD-FMK pretreated group, IL-1â mRNA expression was 30% and IL-18 mRNA expression was 35% of that in the group treated with pORF5 only (Figure 2E). These results indicate that pORF5 could induce IL-1β and IL-18 production in a caspase-1-dependent manner.
NALP3 inflammasome is required for caspase-1-mediated IL-1β and IL-18 production induced by pORF5
Then the molecular mechanisms regulating caspase-1 mediated production of IL-1β and IL-18 were investigated. Activation of caspase-1 is mediated by a multi-protein complex known as the NALP3 inflammasome, which is comprised of three proteins including NLRP3, ASC and procaspase-1 [25]. NALP3 and ASC mRNA expression was detected using qRT-PCR. pORF5 produced a 4.3-fold induction of NALP3 mRNA level and a 3.5-fold induction of ASC mRNA level compared with those in the control group (Figure 3A). These results indicate that pORF5 could induce NALP3 and ASC mRNA expression in THP-1 cells.
Figure 3.
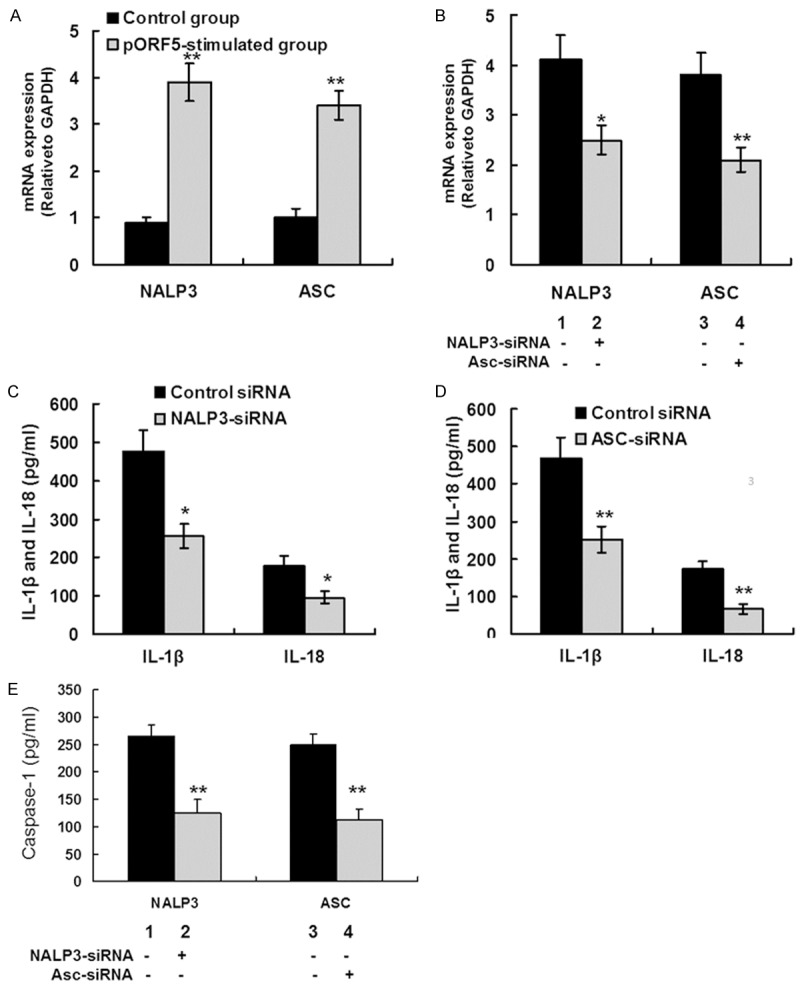
pORF5 triggers IL-1β and IL-18 production via the NALP3 inflammasome. A. THP-1 cells were treated with pORF5 for 24 hours at 24 μg/ml. NALP3 and ASC mRNA expression was detected using qRT-PCR. The expression levels of the target genes were normalized to the endogenous control GAPDH expression and expressed as fold changes compared with the control group. The data represents mean ± SD of three independent experiments. Student’s t test. **P < 0.01. B. THP-1 cells were transfected with NALP3 (lane 2), ASC (lane 4), and negative control (lane 1 and 3) siRNA for 24 hours, then treated with 24 μg/ml pORF5 for an additional 24 hours. NALP3 and ASC mRNA expression was detected using qRT-PCR. The expression levels were obtained as the same in (A). The data represents mean ± SD of three independent experiments. ANOVA, *P < 0.05; **P < 0.01. C. THP-1 cells were transfected with NALP3 and negative control siRNA for 24 hours, followed by 24 μg/ml pORF5 treatment for 24 hours. Mature IL-1â and IL-18 protein expression was detected using ELISA. The data represents mean ± SD of three independent experiments. ANOVA, *P < 0.05. D. THP-1 cells were transfected with ASC and negative control siRNA for 24 hours, followed by 24 μg/ml pORF5 treatment for 24 hours. Mature IL-1â and IL-18 protein expression was detected using ELISA. The data represents mean ± SD of three independent experiments. ANOVA, **P < 0.01. E. THP-1 cells were transfected with NALP3 (lane 2), ASC (lane 4), and negative control (lane 1 and 3) siRNA for 24 hours, then treated with 24 μg/ml pORF5 for 24 hours. The conditioned supernatant was collected and caspase-1 protein expression was detected using ELISA. The data represents mean ± SD of three independent experiments. ANOVA, **P < 0.01 compared with control group.
The role of NALP3 inflammasome in pORF5-induced IL-1β and IL-18 production was further analyzed. In each group, THP-1 cells were transfected with NALP3, ASC, or negative control siRNA for 24 hours, and then treated with 24 μg/ml pORF5 for an additional 24 hours. SiRNA-mediated gene silence was detected using qRT-PCR. Compared with the siRNA for negative control, siRNA for NALP3 and ASC led to 43% reduction in NALP3 mRNA expression and 35% reduction in ASC mRNA expression, respectively (Figure 3B). The effects of siRNA treatment on mature IL-1â and IL-18 protein expression and caspase-1 activation were detected using ELISA. The negative control siRNA had no effect on mature IL-1â and IL-18 protein production in pORF5-stimulated THP-1 cells. In the group treated with NALP3 siRNA, the production of IL-1â and IL-18 was significantly lower than that in the control group (477.1 ± 55.0 pg/ml versus 255.3 ± 32.2 pg/ml, P < 0.05; 178.2 ± 24.8 pg/ml versus 96.4 ± 14.6 pg/ml, P < 0.05, respectively) (Figure 3C). In the group treated with ASC siRNA, IL-1â and IL-18 expression was significantly lower than that in the control group (466.9 ± 56.3 pg/ml versus 252.9 ± 35.2 pg/ml, P < 0.01; 172.4 ± 20.0 pg/ml versus 65.7 ± 13.1 pg/ml, P < 0.01, respectively) (Figure 3D). Furthermore, in the group treated with NALP3 siRNA or ASC siRNA, caspase-1 protein expression was significantly lower than that in the control group (124.9 ± 20.2 pg/ml versus 266.4 ± 24.6 pg/ml, P < 0.01; 112.4 ± 19.8 pg/ml versus 249.3 ± 21.1 pg/ml, P < 0.01, respectively) (Figure 3E). These results indicate that NALP3 inflammasome is required for caspase-1-mediated IL-1β and IL-18 secretion in response to pORF5 stimulation.
p38 MAPK signaling pathway modulates IL-1β and IL-18 production by regulating NALP3 inflammasome activation in THP-1 cells
We previously reported that pORF5 induced IL-1β secretion via p38MAPK signaling pathway in THP-1 cells [23]. To determine the potential role of p38MAPK signaling pathway in pORF5-induced NALP3 inflammasome expression, THP-1 cells were pretreated with p38MAPK inhibitor (SB202190 ) for 30 min prior to pORF5 (24 μg/ml) stimulation for an additional 24 hours. The expression of NALP3, ASC and caspase-1 mRNA was analyzed using qRT-PCR. In SB202190 pretreated group, NALP3, ASC and caspase-1 mRNA expression was significantly lower than that in the group treated with pORF5 only (2.8 ± 0.4 versus 4.3 ± 0.5, P < 0.05; 2.4 ± 0.3 versus 3.9 ± 0.5; P < 0.05; 2.0 ± 0.3 versus 3.5 ± 0.6, P < 0.05, respectively (Figure 4A). The expression of mature IL-1β and IL-18 protein was analyzed using ELISA. In SB202190 pretreated group, IL-1β and IL-18 protein expression was significantly lower than that in the group treated with pORF5 only (214.4 ± 34.9 pg/ml versus 457.1 ± 64.7 pg/ml, P < 0.01; 89.1 ± 11.6 pg/ml versus 185.2 ± 26.4 pg/ml; P < 0.05, respectively) (Figure 4B).
Figure 4.
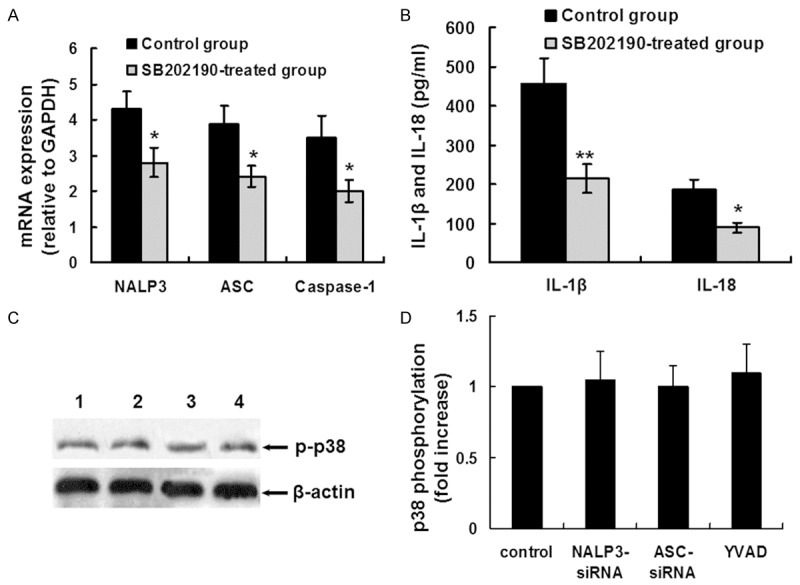
Relationship between p38MAPK signaling pathway and NALP3 inflammasome activation. (A) THP-1 cells were pretreated with SB202190 (p38MAPK inhibitor, 30 μM) for 30 min prior to pORF5 (24 μg/ml) for an additional 24 hours. NALP3, ASC and caspase-1 mRNA expression was detected using qRT-PCR. The expression levels of the target genes were normalized to the endogenous control GAPDH expression and expressed as fold changes compared with the control group. The data represents mean ± SD of three independent experiments. ANOVA, *P < 0.05. (B) THP-1 cells were treated as the same in (A). Mature IL-1â and IL-18 protein expression was detected using ELISA. The data represents mean ± SD of three independent experiments. ANOVA, *P < 0.05; **P < 0.01. (C) THP-1 cells were treated with NALP3 siRNA for 24 hours (lane 2), ASC siRNA for 24 hours (lane 3) or Z-YVAD-FMK (YVAD, 20 μM) for 30 min (lane 4), followed by stimulation with pORF5 protein at 24 μg/ml for 24 hours. THP-1 cells treated with pORF5 (24 μg/ml) only were used as the control (lane 1). P38 phosphorylation was detected using western blot analysis. β-actin was used as an internal control. The representative results of three independent experiments were shown. (D) Quantitative comparison of p38 phosphorylation. Data were derived from three independent experiments as in (C). The mean normalized optical density (OD) of p38 phosphorylation band relative to the OD of β-actin band from the same sample was calculated using the ImageTool 3.0 gray scale scanning software. The expression levels of p38 phosphorylation were expressed as fold changes compared with the control group. Data were expressed as mean ± SD.
THP-1 cells were treated with NALP3 siRNA for 24 hours, ASC siRNA for 24 hours or Z-YVAD-FMK (20 μM) for 30 min, followed by stimulation with pORF5 protein for 24 hours, and p38 phosphorylation was detected using western blot analysis. As shown in Figure 4C and 4D, there were no significant differences in p38 phosphorylation among the NALP3 siRNA treated group, the ASC siRNA treated group, the YVAD treated group and the control group, indicating p38 phosphorylation was not the downstream signaling of NALP3 inflammasome. Altogether, these results suggest that NALP3 inflammasome activation induced by pORF5 is partially dependent on p38MAPK signaling pathway.
Discussion
Ct infection induces inflammatory pathologies in the urogenital tract, which may contribute to long-term sequelae, such as pelvic inflammatory disease, infertility and ectopic pregnancy [26,27]. Clinical and experimental evidence of immunopathogenic basis for chlamydial diseases implicates both acute and chronic inflammation in the pathologic process of urogenital damage. It is important to investigate the molecular basis of chlamydial infection-induced inflammatory damage. pORF5 is the only secreted protein encoded by the Chlamydial plasmid. In this study, the effects of pORF5 on IL-1β and IL-18 production and the underlying mechanisms of these effects were investigated.
Many inflammatory cytokines, including IL-1 family members IL-1β and IL-18, have been detected in chlamydia-infected cells [8-10,28,29]. Excessive IL-1β and IL-18 secretion plays a role in pathology associated with chlamydial infection [11]. In this study, pORF5 was capable of inducing robust IL-1β and IL-18 production in a time- and concentration-dependent manner in THP-1 cells. Production of IL-1β and IL-18 during infection by many bacteria requires both microbial products from the pathogen and an exogenous danger signal. Our recent study showed that TLR2 was involved in the production of inflammatory cytokines induced by pORF5 [23]. Therefore, pORF5 provides both the pathogen-associated molecular pattern and danger signals necessary for IL-1β and IL-18 synthesis and secretion from human monocytes. These data suggest that pORF5 is an important virulence factor responsible for Ct-related pathogenesis.
The NALP3 inflammasome containing NALP3, ASC and caspase-1, is an important innate immune pathway that regulates secretion of the proinflammatory cytokines IL-1β and IL-18. The NALP3 inflammasome is activated by a wide variety of particles, crystals, bacterial toxins [30-32]. Its activation induces oligomerization of NALP3 and leads to recruitment of ASC. In turn, ASC forms large speck-like structures and recruits pro-caspase-1, leading to autocatalytic activation of caspase-1. The activated caspase-1 then induces the processing of pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18. In this study, we provided evidence for the first time that pORF5 contributes to caspase-1 activation for production of IL-1β and IL-18 through a process requiring the NALP3 inflammasome.
In this study, p38MAPK was a critical player in pORF5-induced IL-1β and IL-18 production in THP-1 cells. These data was in line with our previous studies, indicating that pORF5 triggers the activation of p38 MAPK signal pathway [23]. In this study, pORF5-induced IL-1β and IL-18 production was dependent on p38 MAPK signal pathway. Blocking p38 MAPK signal pathway with p38 inhibitor displayed partial abrogated IL-1β and IL-18 production as well as the expression of NALP3 inflammasome complex in pORF5-stimulated THP-1 cells, indicating that the p38MAPK may be involved in NALP3 activation. However, as we did not observe a complete inhibition of NALP3 inflammasome using p38 inhibitor in THP-1 cells, it is plausible that p38 and/or other signaling molecules may be involved in the NALP3 inflammasome activation of THP-1 cells in response to pORF5.
Taken together, the production of IL-1β and IL-18 in pORF5-treated THP-1 monocytes relied on the NALP3 inflammasome. Moreover, p38MAPK signaling pathway in pORF5-stimulated THP-1 cells can influence NALP3 inflammasome activation. However, potassium efflux, reactive oxygen species (ROS) production and cathepsin B release may also be involved in activation of NALP3 inflammasome [33-35]. Additional studies are needed to determine which pathways are responsible for pORF5-rnduced IL-1β and IL-18 production and the roles of IL-1β and IL-18 during Ct-induced inflammatory pathologies.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81102230 and 31470277), the Innovation Platform of Open Fund Project for Universities in Hunan Province (No. 13K081), High Medical Talent Project “225” Program in Hunan Province (No. 2013-13), Construct Program of the Key Discipline in Hunan Province (No. 2011-76), Hunan Provincial Key Laboratory for Special Pathogens Prevention and Control (No. 2014-5) and Hunan Province Cooperative innovation Center for Molecular Target New Drug Study (No. 2014-405).
Disclosure of conflict of interest
None.
References
- 1.Dal Conte I, Mistrangelo M, Cariti C, Chiriotto M, Lucchini A, Vigna M, Morino M, Di Perri G. Lymphogranuloma venereum: an old, forgotten re-emerging systemic disease. Panminerva Med. 2014;56:73–83. [PubMed] [Google Scholar]
- 2.Pendle S, Gowers A. Reactive arthritis associated with proctitis due to Chlamydia trachomatis serovar L2b. Sex Transm Dis. 2012;39:79–80. doi: 10.1097/OLQ.0b013e318235b256. [DOI] [PubMed] [Google Scholar]
- 3.Spaargaren J, Schachter J, Moncada J, de Vries HJ, Fennema HS, Peña AS, Coutinho RA, Morré SA. Slow epidemic of lymphogranuloma venereum L2b strain. Emerg Infect Dis. 2005;11:1787–8. doi: 10.3201/eid1111.050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis SC, Ao TT, Vanobberghen FM, Chilongani J, Hashim R, Andreasen A, Watson-Jones D, Changalucha J, Kapiga S, Hayes RJ. Epidemiology of curable sexually transmitted infections among women at increased risk for HIV in northwestern tanzania: inadequacy of syndromic management. PLoS One. 2014;9:e101221. doi: 10.1371/journal.pone.0101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiridou M, Vriend HJ, Lugner AK, Wallinga J, Fennema JS, Prins JM, Geerlings SE, Rijnders BJ, Prins M, de Vries HJ, Postma MJ, van Veen MG, Schim van der Loeff MF, van der Sande MA. Modelling the impact of Chlamydia screening on the transmission of HIV among men who have sex with men. BMC Infect Dis. 2013;13:436. doi: 10.1186/1471-2334-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavares MC, de Macêdo JL, de Lima Júnior SF, de Andrade Heráclio S, Amorim MM, de Mascena Diniz Maia M, de Souza PR. Chlamydia trachomatis infection and human papillomavirus in women with cervical neoplasia in Pernambuco-Brazil. Mol Biol Rep. 2014;41:865–74. doi: 10.1007/s11033-013-2927-2. [DOI] [PubMed] [Google Scholar]
- 7.Silva J, Cerqueira F, Medeiros R. Chlamydia trachomatis infection: implications for HPV status and cervical cancer. Arch Gynecol Obstet. 2014;289:715–23. doi: 10.1007/s00404-013-3122-3. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz KR, Stephens RS. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell Microbiol. 2006;8:1768–79. doi: 10.1111/j.1462-5822.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 9.Sellami H, Said-Sadier N, Znazen A, Gdoura R, Ojcius DM, Hammami A. Chlamydia trachomatis infection increases the expression of inflammatory tumorigenic cytokines and chemokines as well as components of the Toll-like receptor and NF-κB pathways in human prostate epithelial cells. Mol Cell Probes. 2014;28:147–54. doi: 10.1016/j.mcp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Gervassi A, Alderson MR, Suchland R, Maisonneuve JF, Grabstein KH, Probst P. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect Immun. 2004;72:7231–9. doi: 10.1128/IAI.72.12.7231-7239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hvid M, Baczynska A, Deleuran B, Fedder J, Knudsen HJ, Christiansen G. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol. 2007;9:2795–803. doi: 10.1111/j.1462-5822.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFNgamma production. Nature. 1997;386:619–23. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–9. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 14.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith JW, Sun T, McIntosh MT, Bucala R. Pure Hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol. 2009;183:5208–20. doi: 10.4049/jimmunol.0713552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010;12:188–98. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavathas PB, Boeras CM, Mulla MJ, Abrahams VM. Nod1, but not the ASC inflammasome, contributes to induction of IL-1β secretion in human trophoblasts after sensing of Chlamydia trachomatis. Mucosal Immunol. 2013;6:235–43. doi: 10.1038/mi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdul-Sater AA, Saïd-Sadier N, Padilla EV, Ojcius DM. Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome. Microbes Infect. 2010;12:652–61. doi: 10.1016/j.micinf.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connell CM, Ingalls RR, Andrews CW Jr, Scurlock AM, Darville T. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol. 2007;179:4027–34. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 21.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun. 2014;82:983–92. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–28. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, Huang Q, Li Z, Wu Y, Xie X, Ma K, Cao W, Zhou Z, Lu C, Zhong G. PORF5 plasmid protein of Chlamydia trachomatis induces MAPK-mediated pro-inflammatory cytokines via TLR2 activation in THP-1 cells. Sci China Life Sci. 2013;56:460–6. doi: 10.1007/s11427-013-4470-8. [DOI] [PubMed] [Google Scholar]
- 24.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 25.Inoue M, Shinohara ML. NLRP3 Inflammasome and MS/EAE. Autoimmune Dis. 2013;2013:859145. doi: 10.1155/2013/859145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price MJ, Ades AE, De Angelis D, Welton NJ, Macleod J, Soldan K, Simms I, Turner K, Horner PJ. Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: Analysis of prospective studies with a multistate model. Am J Epidemiol. 2013;178:484–92. doi: 10.1093/aje/kws583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen CR, Brunham RC. Pathogenesis of Chlamydia induced pelvic inflammatory disease. Sex Transm Infect. 1999;75:21–4. doi: 10.1136/sti.75.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jha R, Vardhan H, Bas S, Salhan S, Mittal A. Chlamydia trachomatis heat shock proteins 60 and 10 induce apoptosis in endocervical epithelial cells. Inflamm Res. 2011;60:69–78. doi: 10.1007/s00011-010-0237-x. [DOI] [PubMed] [Google Scholar]
- 29.Lu H, Shen C, Brunham RC. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J Immunol. 2000;165:1463–9. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- 30.Ganz M, Csak T, Nath B, Szabo G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol. 2011;17:4772–8. doi: 10.3748/wjg.v17.i43.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77:1981–91. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–9. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 34.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–9. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 35.Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–9. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]


