Abstract
Aim: To investigate the correlations of MMP-2 and TIMP-2 polymorphisms with the risk and prognosis of gastric cancer (GC). Methods: With an incorporation of 254 GC patients in the case group and 250 healthy subjects enrolled as the control group, this experiment was conducted. Denaturing high performance liquid chromatography method was adopted to detect all genotypes under partial denaturation, haloptype analysis was completed with the Shesis software. Serum MMP-2 and TIMP-2 levels were determined by the immunohistochemical semi-quantitative analysis. The follow-up examination was conducted after the patients had completed systemic therapy and discharged. Results: The distribution frequencies of MMP-2-735C/T locus genotypes between groups were compared (P > 0.05). However, the distributions of alleles and genotype frequencies of MMP-2-1306C/T, TIMP-2-303G/A and -418G/C all exhibited statistical difference (all P < 0.05). The gene polymorphism of MMP-2-1306C/T was statistically correlated with the expression of MMP-2 protein (P < 0.05); the same result was also found regarding TIMP-2-303G/A (P < 0.05). The haplotype analysis revealed that the CGC frequency of the case group was apparently higher than that of the control group (P < 0.05), the positive survival rate of CGC was apparently lower than the negative one. Conclusion: MMP-2-1306C/T, TIMP-2-303G/A and -418G/C variants might be correlated with GC susceptibility. Serum MMP-2 and TIMP-2 protein levels might be associated with GC susceptibility; Additionally, CGC haplotype might be the risk factor of GC.
Keywords: GC, MMP-2, TIMP-2, gene polumorphism, immunohistochemistry, haplotype analysis, prognosis, survival rate
Introduction
Gastric cancer (GC), with its morbidity ranking fourth and mortality ranking second among all malignant tumors in the world, is the most common malignant tumor in digestive tract, which seriously threats human health and life [1]. The majority of the patients lack typical symptoms in the early course of the illness, if any, only nausea, emesis, or the upper gastrointestinal tract symptom similar to canker occurs, which is easy to be ignored. Herein, 70-80% of the patient exhibit locally advanced GC or cancer metastasis when they are diagnosed [2]. To date, specific pathogenesis of GC is not fully understood, the occurrence, development and prognosis was suggested to be closely related to the alteration of structure and expression of various proto-oncogenes and suppressor genes [3].
In recent years, the effect of matrix metalloproteinases in the process of tumor invasion has received continuous attention. A mass of known evidences indicate that matrix metalloproteinase, especially matrix metalloproteinase-2 (MMP-2), play an important role in the degradation of extracellular matrix mediated by tumor cells [4]. MMP-2 gene is located on chromosome 16q21 and composed of 12 introns and 13 exons, the total length of structural genes is 27 kb [5]. Unlike other family members of metalloproteinase, the promoter region of 5’ flanking sequence in MMP-2 gene contains two GC boxes instead of TATA boxes [6]. The tissue inhibitor of metalloproteinase is natural specific inhibitor of matrix metalloproteinases, via a specifically combination with the corresponding matrix metalloproteinase or active enzyme to suppress the activity and occurrence of matrix metalloproteinase, they are critical in coordinating the synthesis and degradation processes of the components in extracellular matrix and maintaining environment stabilization and structural integrity inside of the extracellular matrix [7]. There were previous studies showed that polymorphisms of MMP-2 and TIMP-2 can affect their protein or mRNA expression and tumor invasion by altering the transcriptional activity of its own genes, and eventually involving in the development of breast cancer, lung cancer, esophageal cancer and colon cancer [8-11]. However, there barely exists study which reports its correlation with GC. In our present work, the case-control study was performed to investigate the correlations of MMP-2 and TIMP-2 genetic polymorphisms with the risk and prognosis of GC.
Materials and methods
Clinical data
This study included 254 GC patients (162 men and 92 women, 26-75 years of age, mean age 53.35 ± 9.35 years) operated on at our hospital during the periods from March 2010 to June 2013. The number of lymph node metastasis and non-lymph node metastasis were 186 and 86, respectively. There were 75 subjects who did not break through the serosa layer and other 179 ones did. According to the TNM staging standard set by the Union for International Cancer Control (UICC) in 2009, the number of patients in phase I, II, III and IV were 74, 77, 80 and 23, respectively. The tumor diameters of 95 patients were shorter than 5 cm, other 159 patients exhibited diameters of no shorter than 5 cm. Inclusion criteria: New cases diagnosed by the histopathologists and received no radiotherapy or chemotherapy before operation. Exclusion criteria: patients received radiotherapy or chemotherapy before operation; non-primary GC patients.
Additionally, control group consisted of 250 healthy subjects who had received physical examination. Inclusion criteria: The two groups had the same nationality and sex ratio, age difference was within ± 3 years old. The individuals had no neoplastic or digestive system diseases, or blood relationship with each other. Exclusion criteria: subjects who showed different nationalities with the ones in the case group, age difference > 3 years old, with a blood relationship, or having neoplastic or digestive system diseases. The age and sex difference of the two groups showed no statistic significance (P > 0.05). The questionnaire and medical record were employed to collect the age, sex and relevant clinical data of all objects, who have signed the informed consent form.
DNA extraction and genotyping
Fasting hemospasia was conducted among GC patients and the healthy subjects (for contrast) in the morning, and the content of peripheral blood was set as 5 ml. EDTA anticoagulation was operated according to the specification inside the DNA kit (Qiagen Company, Germany). DNA content was detected by Ultraviolet Spectrometry Photometer. The absorbance values (A value) A260 and A280 were determined and all the ratios were between 1.8 and 2.0. The four SNP locus: MMP-2-1306C/T, MMP-2-735C/T, TIMP-2-303G/A and TIMP-2-418G/C were adopted. The Primer Premier 5.0 software was employed to design and verify the PCR amplifier aiming at each locus of MMP-2 and TIMP-2. The primer was synthesized by Shanghai Biological Engineering Technology Co. Ltd. The primer sequence and PCR conditions was shown in Table 1.
Table 1.
Primers design for polymorphic locus of MMP-2 and TIMP-2 genes
| Genes | Primers | Location | Product Length |
|---|---|---|---|
| MMP-2-1306C/T | Forward 5’-ACCAGACAAGCCTGAACTTGTCTGA-3’ | Promoter | 295 bp |
| Reverse 5’-TGTGACAACCGTCTCTGAGGAATG-3’ | |||
| MMP-2-735C/T | Forward 5’-GGATTCTTGGCTTGGCGCAGGA-3’ | Promoter | 391 bp |
| Reverse 5’-GGGGGCTGGGTAAAATGAGGCTG-3’ | |||
| TIMP-2-303C/T | Forward 5’-TAGGAACAGCCCCACTTCTG-3’ | Exon 3 ser101 | 119 bp |
| Reverse 5’-CCTCCTCGGCAGTGTGTG-3’ | |||
| TIMP-2-418G/C | Forward 5’-CGTCTCTTGTTGGCTGGTCA-3’ | Promotor | 304 bp |
| Reverse 5’-CCTTCAGCTCGACTCTGGAG-3’ |
PCR reaction conditions: 94°C 2 min, 94°C 1 min, 52°C 1 min, 72°C 1 min, for 35 cycles, following an extension of 5 min at 72°C, the product was then preserved at 4°C. All the genotypes were detected with the denaturing high performance liquid chromatography (DHPLC) method under the condition of partial degeneration. The column temperature was 59.3°C, the flow velocity of mobile phase was 0.9 ml/min. The genotyping of the fragment length of MMP-2-1306C/T was accomplished by two steps (seen in Figure 1). The bimodal morphology detected by DHPLC in the first step was the heterozygote CT genotype (Figure 1A); DHPLC was conducted after the PCR sample with unimodal morphology was equivalently mixed with the CC genotype sample (confirmed by sequencing). The unimodal and bimodal ones were CC and TT genotypes, respectively (Figure 1B). C, T mutation was confirmed by sequencing. The genotyping of the fragment length of TIMP-2-418G/C was shown in Figure 2. The unimodal ones were GG genotype (Figure 2A) and CC genotype (Figure 2B), respectively. The bimodal one was GC genotype (Figure 2C).
Figure 1.
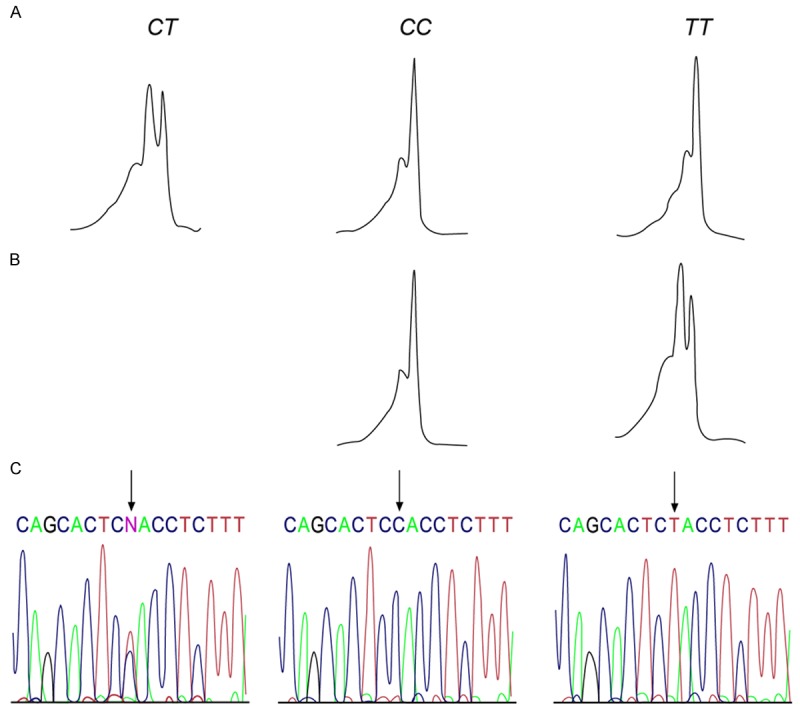
The analysis atlas and sequencing result of Polymerase Chain Reaction (PCR) product of MMP-2-1306C/T genotype. A. Heterozygote CT genotype, with its bimodal morphology detected by DHPLC; B. DHPLC was conducted after the unimodal PCR sample was equivalently mixed with the CC genotype sample (confirmed by sequencing), the unimodal one was CC genotype and the bimodal one was TT genotype; C. Sequencing-confirmed C, T mutation.
Figure 2.
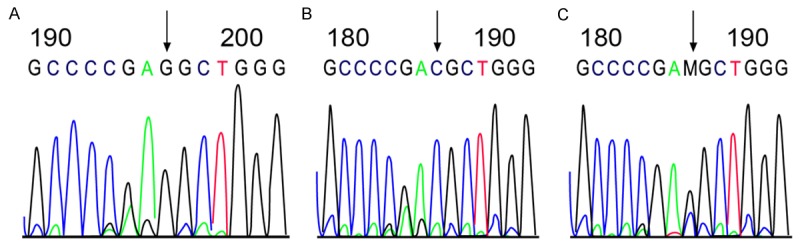
The analysis atlas and sequencing result of Polymerase Chain Reaction (PCR) product of TIMP-2-418G/C genotype. A. GG genotype with unimodal morphology; B. CC genotype with unimodal morphology; C. GC genotype with bimodal morphology.
Immunohistochemistry method
The tissue section after operation or the bioptic tissue section before operation was chosen and all the specimens were fixed by the neutral formalin and embedded by routine paraffin. Serial sectioning with 4 μm and the immunohistochemistry method were conducted for the paraffin. The paraffin section was baked to avoid detachment, the phosphate buffer saline (PBS, pH = 7.4) solution was used to wash the routine paraffin for three times after hydration. The tissue section was put into the citric acid antigen retrieval solution and high-temperature antigen retrieval was conducted in microwave oven for 12 minutes. H2O2 was added to the washed PBS to remove the activity of peroxidase, 50 μL endogenous biological blocker (solution A) was then added and incubated for 13 minutes to seal histones. Solution A was toppled and the remaining solution was mixed with 50 μL goat serum (solution B) and incubated for 12 minutes to throw away the serum. The 50 μL mouse anti-human MMP-2 monoclonal antibody (clone CA-4001, Oncogene, Cambridge, MA; cloneCA-4001) was added to each section. The antibody was diluted in the TBS (containing 2.5% Bovine Serum Albumin [BSA]) with a ratio of 1:100 (v/v). TIMP-2 adopted the anti-human TIMP-2 monoclonal antibody and the antibody was diluted in clone 67-4H11 (Diagnostic International, Schriesheim, Germany) with a ration of 1:200. All the sections were put into the citrate buffer (pH = 6.0) and kept in 700 W microwave oven for 10 minutes. The DAB solution was used as color developing agent and the hematoxylin was employed to conduct counterstaining after a coloration of 3-10 minutes.
Semi-quantitative analysis on the expression of MMP-2 and TIMP-2 proteins
The judgment standards for MMP-2 and TIMP-2 proteins were the same. The positive staining mainly aimed at the cytoplasm, with the peripheral interstitial partially colored. The evenly colored integrated cells were regard as the positive cells (Figure 3). The semi-quantitative analysis was adopted and the judgment was conducted according to the number and staining intensity of positive cells in tumor cells. Five high power fields (×400) were randomly selected to count the total number of tumor cells and positive cells. The percentage of positive cells was then obtained. When areas with different differentiation existed in the GC tissues, the tumor area with advantageous differentiation in the same section of GC tissue was chosen to conduct counting statistics. Four groups were formed according to the amount of positive tumor cells: Negatives (score 0); no more than 30% tumor cells (score 1); 30-70% tumor cells (score 2); more than 70% tumor cells (score 3) [12].
Figure 3.
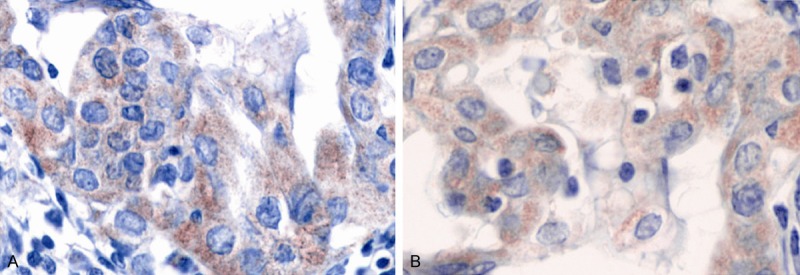
Positive immunohistochemistry of MMP-2 and TIMP-2 in gastric cancer (A. MMP-2; B. TIMP-2). A and B shared the same judgment standard: the positive staining mainly aimed at the cytoplasm, with the peripheral interstitial partially colored; the evenly colored integrated cell was regarded as the positive cell.
Post-operation follow-up examination among GC patients
The follow-up records for all patients should be established after admission, they should have integrated clinical, pathological and discharge data. Reexamination in the outpatient department, telephone follow-up examination or the follow-up examination in patients’ home was adopted for GC patients. The start time of follow-up examination was set as the discharge time after the accomplishment of systemic therapy for patients and the deadline was December 2014. The process was conducted as the post-operation follow-up examination required: once every three months in the first year after discharging; once every three to six months in the second year; once every six months after the third year. The survival time was calculated by months. The death of patients was regarded as the end point of the follow-up examination.
Statistical analysis
Statistical treatment on data was completed with the application of SPSS 19.0 (SPSS Inc, Chicago, IL, USA) statistical software. The enumeration data was expressed as a percentage or rate. Chi-square method was employed to compare the discrepancy of genotype frequencies in each group and verify that whether the genotype frequencies were consistent with Hardy-Weinberg equilibrium. The odds ratio (OR) and its 95% confidence interval (CI) were used to denote the risk level of GC in each genotype. The t-test was employed to conduct the comparison between the two groups. The haplotype frequency analysis was accomplished with Shesis analysis software. The survival rate calculation was examined by Kaplan-Meier curve. The discrepancy showed statistical significance when P < 0.05.
Results
The correlation of MMP-2 and TIMP-2 polymorphisms with the risk of GC
The distributions of genes and genotypes in the case and control groups were consistent with Hardy-Weinberg equilibrium (P > 0.05), indicating that the experimental crowd had a group representative. As shown in Table 2, the distribution frequencies of CC, CT and TT in MMP-2 1306C/T gene in the case group were 78.74%, 20.08% and 1.18%, respectively. As for the control group, the frequencies were 68.80%, 29.20% and 2.00%, respectively. The discrepancy between the two groups exhibited statistical significance (P = 0.039). The risk of GC for patients carried with C allele (CC+CT) was 1.803 times higher than the ones carried with TT genotype (95% CI = 0.649-5.004). The distribution frequencies of GG, AG and AA in TIMP-2 303G/A gene in the case group were 56.69%, 38.58% and 4.93%, respectively. As for the control group, the frequencies were 53.20%, 36.00% and 10.80%, respectively. The discrepancy between the two groups also exhibited statistical significance (P = 0.039). The risk of GC for patients carried with G allele (GG+GA) was 2.35 times higher than the ones carried with AA genotype (95% CI = 1.161-4.743). The distribution frequencies of GG, GC and CC in TIMP-2-418G/C gene in the case group were 58.66%, 33.07% and 8.27%, respectively. As for the control group, the frequencies were 68.40%, 29.60% and 2.00%, respectively. The discrepancy between the two groups exhibited statistical significance (P = 0.003). The risk of GC for patients carried with G allele (GG+GC) was 0.219 times lower than the ones carried with CC genotype (95% CI = 0.108-0.4419). However, there showed no statistical significance (P > 0.05) when the comparison was conducted on the distribution frequencies of MMP-2-735C/T locus genotype between the case and control groups.
Table 2.
The correlation of MMP-2 and TIMP-2 gene polymorphisms with the risk of gastric cancer
| Genotype | Case group (n = 254) | Control group (n = 250) | χ2 | P |
|---|---|---|---|---|
| MMP-2-1306C/T | ||||
| CC | 200 (78.74%) | 172 (68.80%) | 6.479 | 0.039 |
| CT | 51 (20.08%) | 73 (29.20%) | ||
| TT | 3 (1.18%) | 5 (2.00%) | ||
| TT+CT | 54 (21.26%) | 78 (31.20%) | 6.44 | 0.011 |
| CC | 200 (78.74%) | 172 (68.80%) | ||
| C | 451 (88.78%) | 417 (83.40%) | 6.097 | 0.014 |
| T | 57 (11.22%) | 83 (16.60%) | ||
| MMP-2-735C/T | ||||
| CC | 166 (65.35%) | 165 (66.00%) | 0.041 | 0.98 |
| CT | 76 (29.92%) | 74 (29.60%) | ||
| TT | 12 (4.72%) | 11 (4.4%) | ||
| CT+TT | 88 (34.65%) | 85 (34.00%) | 0.023 | 0.879 |
| CC | 166 (65.35%) | 165 (66.00%) | ||
| C | 408 (80.31%) | 404 (80.80%) | 0.038 | 0.846 |
| T | 100 (19.69%) | 96 (19.20%) | ||
| TIMP-2-303G/A | ||||
| GG | 144 (56.69%) | 133 (53.20%) | 6.515 | 0.039 |
| AG | 98 (38.58%) | 90 (36.00%) | ||
| AA | 12 (4.72%) | 27 (10.80%) | ||
| AA+AG | 110 (43.31%) | 117 (46.80%) | 0.621 | 0.431 |
| GG | 144 (56.69%) | 133 (53.20%) | ||
| G | 386 (75.98%) | 356 (71.20%) | 2.969 | 0.085 |
| A | 122 (24.02%) | 144 (28.80%) | ||
| TIMP-2-418G/C | ||||
| GG | 149 (58.66%) | 171 (68.40%) | 11.962 | 0.003 |
| GC | 84 (33.07%) | 74 (29.60%) | ||
| CC | 21 (8.27%) | 5 (2.00%) | ||
| GC+CC | 105 (41.34%) | 79 (31.60%) | 5.155 | 0.023 |
| GG | 149 (58.66%) | 171 (68.40%) | ||
| C | 382 (75.20%) | 416 (83.20%) | 9.786 | 0.002 |
| G | 126 (24.80%) | 84 (16.80%) |
The correlation of MMP-2-1306C/T and TIMP-2-303G/A polymorphisms with clinicopathologic features of GC
The case group was divided into two groups according to the situation of tumor infiltration: the group broke through the serosal layer (179 cases) and the group did not break through the serosal layer (75 cases). The distribution frequencies of GG+AG, AA genotypes in TIMP-2-303G/A were 87.76% and 12.24% for the group did not break through the serosal layer, respectively. As for the group which broke through the serosal layer, the frequencies were 96.10% and 3.9%, respectively. The discrepancy exhibited statistical significance (χ2 = 5.285, P = 0.022). The case group was divided into four phases according to TNN staging criteria: Phase I (74 cases), Phase II (77 cases), Phase III (80 cases) and Phase IV (23 cases). The distribution frequencies of AA+AG, GG genotypes in TIMP-2-303G/A were 40.54%, 59.45% for Phase I, respectively. As for Phase II, the frequencies were 35.06%, 64.94%, respectively. When it came to Phase III and Phase IV, the frequencies were 56.25%, 43.75% and 34.78%, 65.22%, respectively. The discrepancy among these groups also exhibited statistical significance (χ2 = 8.500, P = 0.037). However, the genotype distribution of the locus showed no correlation with tumor diameter and lymphatic metastasis (P > 0.05) (Table 3). The genotype distribution of MMP-2-1306C/T and TIMP-2-418G/C also exhibited no correlation with tumor diameter, the depth of tissue infiltration and lymphatic metastasis (P > 0.05) (Tables 4, 5).
Table 3.
The correlation of TIMP-2-303G/A polymorphism with clinicopathological features of gastric cancer
| Clinicopathological features | Cases | P | GG+AG* (n) | P | AA+AG** (n) | P | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| GG | AG | AA | ||||||
| Tumor diameter | ||||||||
| < 5 cm | 58 | 35 | 2 | 0.243 | 93 | 0.128 | 37 | 0.278 |
| ≥ 5 cm | 86 | 63 | 10 | 149 | 73 | |||
| Infiltration depth | ||||||||
| Unbroken-through serosal layer | 26 | 17 | 6 | 0.071 | 43 | 0.022 | 23 | 0.611 |
| Broken-through serosal layer | 117 | 80 | 8 | 197 | 88 | |||
| Lymphatic metastasis | ||||||||
| No metastasis | 104 | 74 | 8 | 0.742 | 178 | 0.599 | 82 | 0.679 |
| Metastasis existed | 40 | 24 | 4 | 64 | 28 | |||
| TNM staging of tumor | ||||||||
| 0-І | 44 | 27 | 3 | 0.121 | 71 | 0.799 | 30 | 0.037 |
| II | 50 | 24 | 3 | 74 | 27 | |||
| III | 35 | 41 | 4 | 76 | 45 | |||
| IV | 15 | 6 | 2 | 21 | 8 | |||
compared with AA;
compared with GG;
TNM, tumor node metastasis.
Table 4.
The correlation of MMP-2-1306C/T polymorphism with clinicopathological features of gastric cancer
| Clinicopathological features | Cases | P | CC+CT* (n) | P | CT+TT** (n) | P | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CC | CT | TT | ||||||
| Tumor diameter | ||||||||
| < 5 cm | 75 | 19 | 0 | 0.41 | 94 | 0.182 | 19 | 0.755 |
| ≥ 5 cm | 125 | 32 | 3 | 157 | 35 | |||
| Infiltration depth | ||||||||
| Unbroken-through serosal layer | 39 | 10 | 1 | 0.836 | 49 | 0.55 | 11 | 0.887 |
| Broken-through serosal layer | 161 | 41 | 2 | 202 | 43 | |||
| Lymphatic metastasis | ||||||||
| No metastasis | 51 | 13 | 2 | 0.271 | 64 | 0.106 | 15 | 0.735 |
| Metastasis existed | 149 | 38 | 1 | 187 | 39 | |||
| TNM staging of tumor | ||||||||
| 0-І | 28 | 6 | 0 | 0.952 | 34 | 0.897 | 6 | 0.712 |
| II | 45 | 9 | 1 | 54 | 10 | |||
| III | 59 | 15 | 1 | 74 | 16 | |||
| IV | 68 | 21 | 1 | 89 | 22 | |||
compared with TT;
compared with CC;
TNM, tumor node metastasis.
Table 5.
The correlation of TIMP-2-418G/C polymorphism with clinicopathologic features of GC
| Clinicopathological features | Cases | P | CC+CT* (n) | P | CT+TT** (n) | P | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| GG | GC | CC | ||||||
| Tumor diameter | ||||||||
| < 5 cm | 55 | 30 | 7 | 0.944 | 85 | 0.7738 | 37 | 0.785 |
| ≥ 5 cm | 94 | 54 | 14 | 148 | 68 | |||
| Infiltration depth | ||||||||
| Unbroken-through serosal layer | 30 | 16 | 6 | 0.618 | 46 | 0.3369 | 22 | 0.874 |
| Broken-through serosal layer | 119 | 68 | 15 | 187 | 83 | |||
| Lymphatic metastasis | ||||||||
| No metastasis | 57 | 28 | 8 | 0.747 | 85 | 36 | 0.518 | |
| Metastasis existed | 92 | 56 | 13 | 148 | 0.8831 | 69 | ||
| TNM staging of tumor | ||||||||
| 0-І | 8 | 3 | 2 | 0.099 | 11 | 0.057 | 5 | 0.677 |
| II | 29 | 17 | 9 | 46 | 26 | |||
| III | 99 | 59 | 7 | 158 | 66 | |||
| 13 | 8 | 1 | 21 | 9 | ||||
compared with CC;
compared with GG;
TNM, tumor node metastasis.
Haplotype analysis of MMP-2-1306C/T, TIMP-2-303G/A and TlMP2-418G/C locus between the case and control groups
The haplotypes of MMP-2-1306C/T, TIMP-2-303G/A and -418G/CS2 locus were shown in the Table 6. The haplotype analysis of different locus in MMP-2, TIMP-2 genes in the two groups was conducted with Shesis analysis software. The haplotypes with frequencies little than 3% were neglected. The results demonstrated that the frequencies of haplotype CGC in case group were apparently higher than the ones in control group (χ2 = 4.506, P = 0.034).
Table 6.
Haplotype analysis of MMP-2-1306C/T, TIMP-2-303G/A and TIMP-2-418G/C locus between the case and control groups
| Haplotypes | Case group (n = 254) | Control group (n = 250) | χ2 | P | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MMP-2-1306C/T | TIMP-2-303G/A | TIMP-2-418G/C | |||||
| C | A | C | 13 (5.29%) | 10 (4.04%) | 0.362 | 0.548 | 1.295 (0.557-3.010) |
| C | A | G | 41 (16.04%) | 50 (19.98%) | 1.268 | 0.260 | 0.770 (0.488-1.215) |
| C | G | C | 43 (17.00%) | 25 (9.98%) | 5.183 | 0.023 | 1.834 (1.082-3.109) |
| C | G | G | 129 (51.00%) | 124 (49.40%) | 0.071 | 0.790 | 1.049 (0.740-1.487) |
| T | A | C | 2 (1.00%) | 2 (0.80%) | 0.001 | 0.987 | 0.984 (0.138-7.045) |
| T | A | G | 5 (2.00%) | 10 (3.98%) | 1.801 | 0.180 | 0.482 (0.162-1.431) |
| T | G | C | 5 (2.11%) | 5 (1.99%) | 0.001 | 0.98 | 0.984 (0.281-3.442) |
| T | G | G | 16 (6.00%) | 25 (9.83%) | 2.309 | 0.129 | 0.605 (0.315-1.163) |
The correlation of the genotype frequencies of MMP-2 and TIMP-2 with the expression of their proteins
As plotted in Table 7, MMP-2-1306C/T gene polymorphism was statistically correlated with the expression of MMP protein (P < 0.05), TIMP-2-303G/A gene polymorphism was also statistically related to the expression of TIMP protein (P < 0.05). However, TIMP-2-303G/A and TIMP-2-418G/C exhibited no statistic correlation with the expression of MMP-2 and TIMP-2 proteins (P > 0.05).
Table 7.
The correlation of the genotype frequencies of MMP-2 and TIMP-2 with the expression of their proteins
| MMP-2/TIMP-2 tissue status (score 0-3) | P | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| All | 0 | 1 | 2 | 3 | ||
| MMP-2-1306C/T | ||||||
| CC | 200 | 37 | 80 | 65 | 18 | 0.043 |
| CT/TT | 54 | 8 | 31 | 8 | 4 | |
| MMP-2-735C/T | ||||||
| CC | 166 | 28 | 69 | 59 | 10 | 0.110 |
| CT/TT | 88 | 17 | 37 | 22 | 12 | |
| TIMP-2-303G/A | ||||||
| GG | 144 | 37 | 61 | 34 | 12 | 0.042 |
| AA+AG | 110 | 22 | 44 | 41 | 3 | |
| TIMP-2-418G/C | ||||||
| GG | 149 | 29 | 60 | 50 | 10 | 0.460 |
| CC/GC | 105 | 21 | 44 | 28 | 12 | |
Post-operation survival analysis of GC patients
For the 254 patients, the follow-up examination was accomplished by telephone or registering the situation of reexamination in the outpatient department, the follow-up examination data was integrated. The time for follow-up examination lasted for 10-40 months, with a median time of 26 months, the follow-up examination rate was 97.24%. The number of patients died from tumor relapse was 124, the survival rate within 3 years was 52.76% for the patients received the examination. Among the 247 patients who received follow-up examination after the operation of GC, the positive survival rate of CGC was apparently lower than the negative one (χ2 = 5.174, P = 0.023). The Kaplan-Meier method was employed to draw the overall survival curve (Figure 4).
Figure 4.
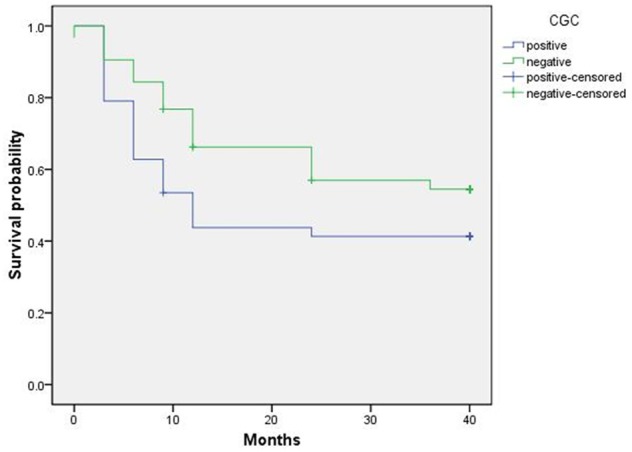
Kaplan-Meier survival curve of positive and negative CGC of gastric cancer patients.
Discussion
In present work, the correlations of MMP-2 and TIMP-2 gene locus polymorphisms with the risk and prognosis of GC were investigated. The study result showed that MMP-2-1306C/T, TIMP-2-303G/A and -418G/C variants might be correlated with GC susceptibility. The two SNP locus, -1306C/T and -735C/T locus located in the upstream of MMP-2 transcription start site, whose variation might destroy the binding site of Sp1, resulting in the decrement of gene transcription level and eventually, in the influence on the expression of protein [13]. In this study, the distribution frequencies of CC, CT and TT locus of MMP-2-1306C/T in the two groups demonstrated obvious discrepancy. The risk of GC for patients carried with C allele was 1.803 times higher than the ones carried with TT genotype. Associated study related to lung cancer, which was reported by Zhou et al., also demonstrated that the risk of lung cancer for the carriers with MMP-2-1306C/T genotype was two times higher than those who didn’t, indicating that MMP-2-1306C/T variant was the risk factor that affected the occurrence and development of diseases [9]. Additionally, this study also confirmed that the distribution frequencies of MMP-2-735C/T locus genotype in case group exhibited no obvious discrepancy with the ones in control group. Rollin et al. also discovered that the distribution of polymorphic 735C/T genotype in non-small cell lung cancer group and control group showed no obvious discrepancy. The result and tendency was consistent with our study, indicating that the correlation of the polymorphism of MMP-2-735C/T locus with the occurrence and development of GC needs further study [14]. The -303G/A locus polymorphism of TIMP-2 may destroy the combination of transcription factor Spl, which may decrease the transcriptional activity of genes [15]. In current work, the distribution frequencies of GG, AG and AA genes of TIMP-2-303G/A genotype in case and control groups demonstrated apparent discrepancy. The risk of GC for patients carried with G allele was 2.35 times higher than the ones carried with AA genotype. Xiandong Lin et al reported that the risk of GC for patients carried with G allele was 1.94 times higher than the ones carried with AA genotype (for the -303G/A locus), whose conclusion and tendency were also consistent with our result, indicating that the locus was closely correlated with the occurrence and development of GC [16]. In the meantime, the correlation of TIMP-2-303G/A locus with clinicopathologic features of GC was also investigated in this study, the result showed that the locus polymorphism of TIMP-2-303G/A was correlated with tumor infiltration degree of GC and clinico-pathologic staging. The distribution frequencies of GG, GC and CC locus in TIMP-2-418G/C gene in case and control groups exhibited obvious discrepancy, the risk of GC for patients carried with G allele was 0.219 times higher than the ones carried with CC genotype, indicating that TIMP-2-418G/C, C/C genotypes might increase the risk of GC when compared with G/C genotype. There were other studies, whose result was consistent with that in present study, also believed that patients carried with C genotype can increase the risk of diseases [17,18].
In addition, the haplotypes of MMP-2-1306C/T, TIMP-2-303G/A and -418G/C locus in case and control groups were also analyzed in this study. The result showed that the frequency of haplotype CGC in case group was apparently higher than that in control group. Meanwhile, CGC was closely correlated with the survival time of GC, the survival time of positive CGC was obviously shorter than negative one. Herein, we further proved that the -1306C/T locus of MMP-2 gene, -303G/A and -418G/C locus of TIMP-2 genes might be correlated with the susceptibility of GC. -1306C/T locus carried with C allele, -303G/A locus of TIMP-2 gene carried with G allele and -418G/C locus carried with C allele would increase the risk of GC. Therefore, the three loca can be used as indexes of poor prognosis.
In our study, the correlation of genotype frequencies of MMP-2 and TIMP-2 with the expression of their proteins was also analyzed, the result demonstrated that MMP-2 1306C/T and TIMP-2-303G/A polymorphisms affected the expression of MMP-2 and TIMP-2, respectively. -1306C/T variant can regulate the transcriptional activity of its own genes and eventually, alter the expression of protein. In this study, patients carried with CC allele possessed higher positive protein expression of MMP-2 when compared with the ones carried with CT+TT genotype, indicating that -1306C/T gene polymorphism can express high-level MMP-2. The overexpression and increment in activity of MMP-2 exhibited close correlation with the occurrence, development, invasion and metastasis of GC [19]. TIMP-2-303G/A located in the third exon of TIMP-2 gene, which belongs to synonymous mutation. Although synonymous mutation do not alter coded amino acid, nucleotide sequence was changed, which might have an influence on post-transcriptional processing, cut-grafting order of signal and important bases in promoter region. All the above-mentioned ones might affect the expression of protein in the end. In this work, patients carried with GG allele possessed higher positive protein expression of TIMP-2 when compared with the ones carried with AA+AG genotype, indicating that the polymorphism of TIMP-2-303G/A could improve the expression level of TIMP-2 protein.
In conclusion, this work proved that MMP-2-1306C/T, TIMP-2-303G/A and -418G/C polymorphisms were correlated with GC susceptibility. Serum MMP-2 and TIMP-2 levels might be associated with GC susceptibility. Additionally, CGC haplotype might be the risk factor of GC. The occurrence and development of GC involved not only the role of single-gene, but the combined effect of polygenes. Therefore, the role of different genes independently or jointly in the genetic susceptibility of GC needs to be further investigated for the confirmation of the in vivo mechanism of GC and prophylaxis and treatment of GC.
Acknowledgements
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article.
Disclosure of conflict of interest
None.
References
- 1.Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C, Huang D, Chen X, Zhang H, Zhuang R, Deng T, Liu H, Yin J, Wang S, Zen K, Ba Y, Zhang CY. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784–791. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Baba H, Kuwabara K, Ishiguro T, Kumamoto K, Kumagai Y, Ishibashi K, Haga N, Ishida H. Prognostic factors for stage IV gastric cancer. Int Surg. 2013;98:181–187. doi: 10.9738/INTSURG-D-12-00027.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Y, Yue Y, Pan M, Sun J, Chu J, Lin X, Xu W, Feng L, Chen Y, Chen D, Shin VY, Wang X, Jin H. Histone deacetylase 3 inhibits new tumor suppressor gene DTWD1 in gastric cancer. Am J Cancer Res. 2015;5:663–673. [PMC free article] [PubMed] [Google Scholar]
- 4.Kesanakurti D, Chetty C, Dinh DH, Gujrati M, Rao JS. Role of MMP-2 in the regulation of IL-6/Stat3 survival signaling via interaction with alpha5beta1 integrin in glioma. Oncogene. 2013;32:327–340. doi: 10.1038/onc.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000;19:623–629. doi: 10.1016/s0945-053x(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu RR, Li MD, Li T, Tan Y, Zhang M, Chen JC. Matrix metalloproteinase 2 (MMP2) protein expression and laryngeal cancer prognosis: a meta analysis. Int J Clin Exp Med. 2015;8:2261–2266. [PMC free article] [PubMed] [Google Scholar]
- 7.Meschiari CA, Marcaccini AM, Santos Moura BC, Zuardi LR, Tanus-Santos JE, Gerlach RF. Salivary MMPs, TIMPs, and MPO levels in periodontal disease patients and controls. Clin Chim Acta. 2013;421:140–146. doi: 10.1016/j.cca.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Waleh NS, Murphy BJ, Zaveri NT. Increase in tissue inhibitor of metalloproteinase-2 (TIMP-2) levels and inhibition of MMP-2 activity in a metastatic breast cancer cell line by an antiinvasive small molecule SR13179. Cancer Lett. 2010;289:111–118. doi: 10.1016/j.canlet.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Bourboulia D, Han H, Jensen-Taubman S, Gavil N, Isaac B, Wei B, Neckers L, Stetler-Stevenson WG. TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/betacatenin complex expression in A549 lung cancer cells. Oncotarget. 2013;4:163–173. doi: 10.18632/oncotarget.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groblewska M, Mroczko B, Kozlowski M, Niklinski J, Laudanski J, Szmitkowski M. Serum matrix metalloproteinase 2 and tissue inhibitor of matrix metalloproteinases 2 in esophageal cancer patients. Folia Histochem Cytobiol. 2012;50:590–598. doi: 10.5603/20327. [DOI] [PubMed] [Google Scholar]
- 11.Kapral M, Wawszczyk J, Jurzak M, Dymitruk D, Weglarz L. Evaluation of the expression of metalloproteinases 2 and 9 and their tissue inhibitors in colon cancer cells treated with phytic acid. Acta Pol Pharm. 2010;67:625–629. [PubMed] [Google Scholar]
- 12.Alakus H, Grass G, Hennecken JK, Bollschweiler E, Schulte C, Drebber U, Baldus SE, Metzger R, Holscher AH, Monig SP. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–923. doi: 10.14670/HH-23.917. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava P, Pandey S, Mittal B, Mittal RD. No Association of Matrix Metalloproteinase [MMP] -2 (-735C > T) and Tissue Inhibitor of Metalloproteinase [TIMP] -2 (-418G > C) Gene Polymorphisms with Cervical Cancer Susceptibility. Indian J Clin Biochem. 2013;28:13–18. doi: 10.1007/s12291-012-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava P, Kapoor R, Mittal RD. Association of single nucleotide polymorphisms in promoter of matrix metalloproteinase-2, 8 genes with bladder cancer risk in Northern India. Urol Oncol. 2013;31:247–254. doi: 10.1016/j.urolonc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Palei AC, Sandrim VC, Amaral LM, Machado JS, Cavalli RC, Duarte G, Tanus-Santos JE. Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Exp Mol Pathol. 2012;92:217–221. doi: 10.1016/j.yexmp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, Li Y, Feng J, Wang X, Hao B, Shi R, Zhang G. A functional polymorphism T309G in MDM2 gene promoter, intensified by Helicobacter pylori lipopolysaccharide, is associated with both an increased susceptibility and poor prognosis of gastric carcinoma in Chinese patients. BMC Cancer. 2013;13:126. doi: 10.1186/1471-2407-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu LX, Wang Y, Xia ZG, Xi B, Mao C, Wang JL, Wang BY, Lv FF, Wu XH, Hu LQ. miR-196a2 C allele is a low-penetrant risk factor for cancer development. Cytokine. 2011;56:589–592. doi: 10.1016/j.cyto.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Yari K, Rahimi Z, Moradi MT, Rahimi Z. The MMP-2 -735 C allele is a risk factor for susceptibility to breast cancer. Asian Pac J Cancer Prev. 2014;15:6199–6203. doi: 10.7314/apjcp.2014.15.15.6199. [DOI] [PubMed] [Google Scholar]
- 19.Guo MM, Hu LH, Wang YQ, Chen P, Huang JG, Lu N, He JH, Liao CG. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1. Med Oncol. 2013;30:542. doi: 10.1007/s12032-013-0542-7. [DOI] [PubMed] [Google Scholar]


