Abstract
Background: Genetic variation of SNPs in the PTGS2 gene is reported to be capable of creating tissue milieu favoring tumorigenesis. Some studies have implicated the common SNP rs20417 has association with PCa risk, while others showed reverse results. The study was performed with an aim to reconcile the existing controversy by performing a meta-analysis. Methods: We searched databases of Embase and PubMed and identified 8 publications fulfilling the specified inclusion criteria. X2-based Q-test and I2 statistic were quantified to measure the heterogeneity across studies. The pooled ORs and 95% CIs were calculated to estimate the association between SNP rs20417 and PCa risk. Results: Based on an enlarged sample size by combining the data from published meta-analyses and those missed in them, the results of the present meta-analysis revealed the association of SNP rs20417 and overall PCa risk was not significant. Subgroup analyses according to ethnicity and source of controls did not show any significant results, either. Conclusions: The meta-analysis suggests that the presence of SNP rs20417 is unlikely to be associated with PCa risk. Our understanding of the genetic risk for PCa should be further expanded and refined through future research in a much larger number of participants.
Keywords: PTGS2, polymorphism, rs20417, prostate cancer
Introduction
Prostate cancer (PCa) is one of the most frequently diagnosed malignancies and a major cause of morbidity and mortality in men worldwide, ranking the first in European countries and the last in Southern/Eastern Asia [1,2]. The aggressive malignancy favors the men who are over the age of fifty in particular [3]. Advances achieved in chemotherapy, radiotherapy and surgical techniques fail to effectively preclude the onset of PCa, for the cancer cells may metastasize from the prostate to other parts of the body, particularly the bones and lymph nodes and consequently become resistant to hormone treatment [4-6]. As compared with men with no family history, evidence from related reports reveals those with one first-degree relative and two first-degree relatives affected by PCa respectively have two times and five times higher risk of suffering such cancer [7]. These data suggest a central role of genetic susceptibility in PCa predisposition.
Prostaglandin endoperoxide synthase 2 (PTGS2, also known as cyclooxygenase-2, COX-2) at human chromosome 1q25.2-eq25.3 having 10 exons and spanning 8 kb is a candidate gene for PCa susceptibility [8,9]. PTGS2 catalyzes the rate-limiting steps in prostanoid biosynthesis and its expression is under the control of several extracellular stimuli including growth factors, cytokines and tumor promoters at both transcriptional and post-transcriptional levels [10]. A panel of investigations has documented the involvement of PTGS2 in cell proliferation, apoptosis, immune suppression, tumor progression, and metastasis [11-14]. PTGS2 over expresses in rheumatoid arthritis and various human cancers, such as colorectal cancer, breast cancer [10], head and neck carcinoma [12], and PCa [15-18] compared to benign tissue from the same patients.
To date, many common single nucleotide polymorphisms (SNPs) of PTGS2 (rs2745557, rs689470, rs5277, rs5275, rs20432, rs20417) have been studied in extensive research on PCa [19-21]. Of these, SNP rs20417 has received more favorable observations concerning the association between this SNP and PCa risk. A study of Caucasian and African descendants found an increased PCa risk in the latter population [22]. This discovery, however, was not replicated in a later case-control study, where a larger number of subjects were enrolled [23]. Similar inconsistent results were also presented in several other studies looking at the association [24-26].
Given the increasing attention directed to the association of rs20417 polymorphism and PCa risk, we undertook the present study with two aims: (1) to reconcile the existing controversy by performing a meta-analysis of comprehensive data gathered from published meta-analyses and electronic databases; (2) to identify whether there was significant heterogeneity undermining the association.
Materials and methods
Publication search
A literature search of Embase and PubMed was performed to identify all papers regarding PCa risk connected with SNP rs20417 using the keywords including prostaglandin endoperoxide synthase 2, PTGS2, cyclooxygenase-2, COX-2, -765G > C, -899G > C, rs20417, polymorphism, polymorphisms and prostate cancer. The publications available for this meta-analysis were not restricted to any language. To ensure all data on this topic were included, we additionally checked the citations of all potentially relevant papers.
Inclusion criteria
The studies were selected for the meta-analysis as long as they fulfilled the criteria that a case-control study assessing the association between SNP rs20417 and PCa risk and that the study was published before March 15, 2014 with available genotype frequency which could help infer the results of the papers.
Data extraction
Two independent investigators carried out the data extraction in duplicate using a standard protocol to ensure the accuracy of the data. The following characteristics collected from each study were first author, year of publication, ethnicity/race (Caucasian, Asian, African), country origin, source of controls (hospital-/population-based), genotyping methods, and genotype frequency. For the studies used the same case series, the most recent or the largest study with complete data was finally included.
Statistical methods
The strength of association between SNP rs20417 and PCa risk was estimated by odds ratios (ORs). Meanwhile, a sense of the precision of the estimate was presented by 95% confidence intervals (CIs). The pooled ORs and 95% CIs were calculated under an assumption of CC contrast GG, CC + GC contrast GG, CC contrast GC + GG, C contrast G and GC contrast GG. Analyses in total samples and subgroups of ethnicity and control source were independently performed for each comparison. The goodness-of-fit X2-test was used to examine deviation of genotype frequencies in controls from Hardy-Weinberg equilibrium (HWE), with a P-value < 0.05 being considered significant. All statistics of this meta-analysis were analyzed with Stata software (version 12.0, StataCorp LP, College Station, TX, USA).
X2-based Q-test was used to measure between-study heterogeneity. Also, I2 statistic was quantified to check the heterogeneity assumption [27]. A P-value greater than 0.05 and I2 less than 50% suggests absence of obvious heterogeneity across studies and the summary OR estimate of each study was calculated by the fixed-effects model (the Mantel-Haenszel method). On the contrary, the random-effects model (the DerSimonian and Laird method) was performed [28,29].
With an aim to check if there was any significant influence from each single study on total results, we conducted sensitivity analyses. Publication bias was estimated with funnel plots and Egger’s tests. An symmetric plot and P value > 0.05 for Egger’s tests indicate no significant publication bias [30].
Results
Characteristics of included studies
After electronic and manual search combined with rigorous selection according to pre-specified inclusion criteria, a total of 8 publications [22-26,31-34] were eligible for inclusion of this meta-analysis (Figure 1). Among these, Panguluri et al. and Cheng et al. [22,23] presented usable data for subjects of African and Caucasian ancestry, thus this meta-analysis included 10 studies in total, providing 12,326 PCa cases and 14,027 PCa-free controls.
Figure 1.
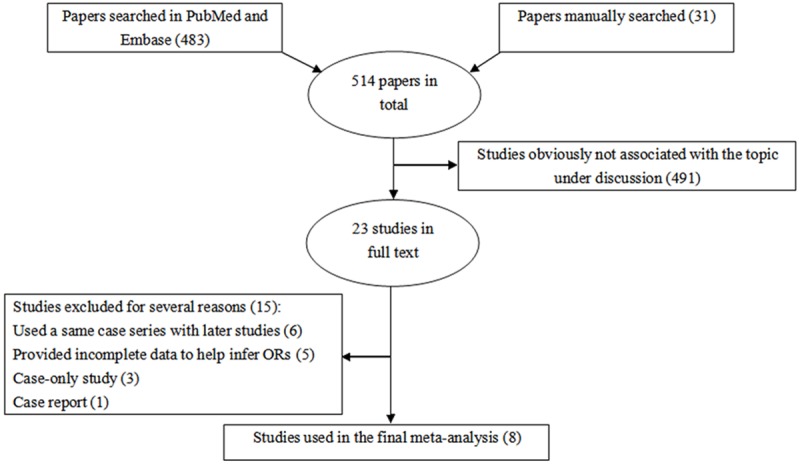
Flow chart shows literature search for relevant studies about the association between SNP rs20417 and risk of prostate cancer (PCa).
Table 1 lists the main characteristics extracted from the identified publications. Three different ethnic populations from ten studies consisting of two studies for Africans, two for Asians and six for Caucasians were collected to summarize the pooled results. Most studies were population-based, accounting for seventy percent. The genotype distribution for control subjects of three studies was in disagreement with HWE [22,24,32].
Table 1.
Main characteristics of all studies for PTGS2 rs20417 polymorphism included in meta-analysis
| First author | Year | Study population | Total sample | Genotyping method | Source | HWE | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cases | Controls | ||||||
| Panguluri | 2004 | USA/African | 270 | 271 | Direct sequencing | Population | < 0.10 |
| Panguluri | 2004 | USA/Caucasian | 90 | 90 | Direct sequencing | Hospital | NE |
| Cheng | 2007 | USA/African | 89 | 88 | TaqMan | Not detailed | 0.61 |
| Cheng | 2007 | USA/Caucasian | 416 | 417 | TaqMan | Population | 0.98 |
| Dossus | 2009 | USA/Caucasian | 7975 | 8566 | GoldenGate IBA | Population | 0.01 |
| Murad | 2009 | UK/Caucasian | 1592 | 3028 | PCR TaqMan | Population | 0.53 |
| Balistreri | 2010 | Italy/Caucasian | 50 | 125 | PCR-RFLP | Population | 0.19 |
| Mandal | 2011 | India/Asian | 195 | 250 | PCR-RFLP | Hospital | 0.01 |
| Wu | 2011 | China/Asian | 218 | 436 | PCR | Population | 0.06 |
| Catsburg | 2012 | USA/Caucasian | 1431 | 756 | TaqMan | Population | 0.21 |
PCR: polymerase chain reaction; PCR-RFLP: PCR-restriction fragment length polymorphism; IBA: Illumina BeadArrayTM; HWE: Hardy-Weinberg equilibrium; NE: not estimated.
Main meta-analysis results
As shown in Table 2, when pooling all studies into the meta-analysis, we did not find statistically significant results between SNP rs20417 and risk of PCa either at genotypical level or at allele level (CC vs. GG, OR, 1.00, 95% CI 0.87 to 1.15; CC + GC vs. GG, OR, 1.01, 95% CI 0.96 to 1.06; CC vs. GC + GG, OR, 0.99, 95% CI 0.86 to 1.14; C vs. G, OR, 1.01, 95% CI 0.96 to 1.06; GC vs. GG, OR, 1.01, 95% CI 0.96 to 1.07). Moreover, we found no statistical evidence that PCa risk was modified by SNP rs20417 when the general population was subgrouped according to ethnic origin and source of controls.
Table 2.
Results of meta-analysis for PTGS2 rs20417 polymorphism and PCa risk
| Study groups | Comparisons | Main effects of rs20417 polymorphism on PCa risk | Analysis model | Total (case/control) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | Ph | I2 (%) | ||||
| All | 10 | 12326/14027 | ||||
| CC vs. GG | 9 | 1.00 (0.87, 1.15) | 0.962 | 0 | Fixed | |
| CC + GC vs. GG | 10 | 1.01 (0.96, 1.06) | 0.585 | 0 | Fixed | |
| CC vs. GC + GG | 9 | 0.99 (0.86, 1.14) | 0.952 | 0 | Fixed | |
| C vs. G | 10 | 1.01 (0.96, 1.06) | 0.542 | 0 | Fixed | |
| GC vs. GG | 10 | 1.01 (0.96, 1.07) | 0.528 | 0 | Fixed | |
| Ethnicity | 359/359 | |||||
| African | 2 | |||||
| CC vs. GG | 2 | 0.93 (0.44, 1.95) | 0.616 | 0 | Fixed | |
| CC + GC vs. GG | 2 | 0.95 (0.66, 1.35) | 0.721 | 0 | Fixed | |
| CC vs. GC + GG | 2 | 0.89 (0.43, 1.82) | 0.528 | 0 | Fixed | |
| C vs. G | 2 | 0.94 (0.69, 1.29) | 0.919 | 0 | Fixed | |
| GC vs. GG | 0.94 (0.64, 1.39) | 0.564 | 0 | Fixed | ||
| Caucasian | 6 | 11554/12982 | ||||
| CC vs. GG | 5 | 1.01 (0.87, 1.17) | 0.821 | 0 | Fixed | |
| CC + GC vs. GG | 6 | 1.02 (0.96, 1.07) | 0.854 | 0 | Fixed | |
| CC vs. GC + GG | 5 | 1.00 (0.87, 1.16) | 0.848 | 0 | Fixed | |
| C vs. G | 6 | 1.01 (0.97, 1.06) | 0.789 | 0 | Fixed | |
| GC vs. GG | 6 | 1.02 (0.96, 1.07) | 0.858 | 0 | Fixed | |
| Asian | 2 | 413/686 | ||||
| CC vs. GG | 1 | 0.85 (0.34, 2.10) | . | . | Fixed | |
| CC + GC vs. GG | 2 | 0.88 (0.65, 1.19) | 0.031 | 78.6 | Fixed | |
| CC vs. GC + GG | 1 | 0.79 (0.32, 1.94) | . | . | Fixed | |
| C vs. G | 2 | 0.87 (0.66, 1.06) | 0.033 | 77.9 | Fixed | |
| GC vs. GG | 2 | 0.89 (0.64, 1.22) | 0.023 | 80.7 | Fixed | |
| Source of controls | ||||||
| Population | 7 | 11952/13599 | ||||
| CC vs. GG | 6 | 1.01 (0.87, 1.17) | 0.902 | 0 | Fixed | |
| CC + GC vs. GG | 7 | 1.01 (0.96, 1.06) | 0.424 | 0 | Fixed | |
| CC vs. GC + GG | 6 | 1.01 (0.87, 1.16) | 0.917 | 0 | Fixed | |
| C vs. G | 7 | 1.01 (0.96, 1.06) | 0.363 | 8.6 | Fixed | |
| GC vs. GG | 7 | 1.01 (0.95, 1.06) | 0.403 | 3.0 | Fixed | |
| Hospital | 2 | 285/340 | ||||
| CC vs. GG | 1 | 0.85 (0.34, 2.10) | . | . | Fixed | |
| CC + GC vs. GG | 2 | 1.19 (0.81, 1.75) | 0.349 | 0 | Fixed | |
| CC vs. GC + GG | 1 | 0.79 (0.32, 1.94) | . | . | Fixed | |
| C vs. G | 2 | 1.13 (0.80, 1.58) | 0.331 | 0 | Fixed | |
| GC vs. GG | 2 | 1.27 (0.84, 1.91) | 0.369 | 0 | Fixed | |
Heterogeneity test
Since the results from X2-based Q-test together with I2 statistic showed no significant heterogeneity among the studies, we performed the fixed-effects model for OR estimate of all studies (Table 2; Figures 2, 3).
Figure 2.
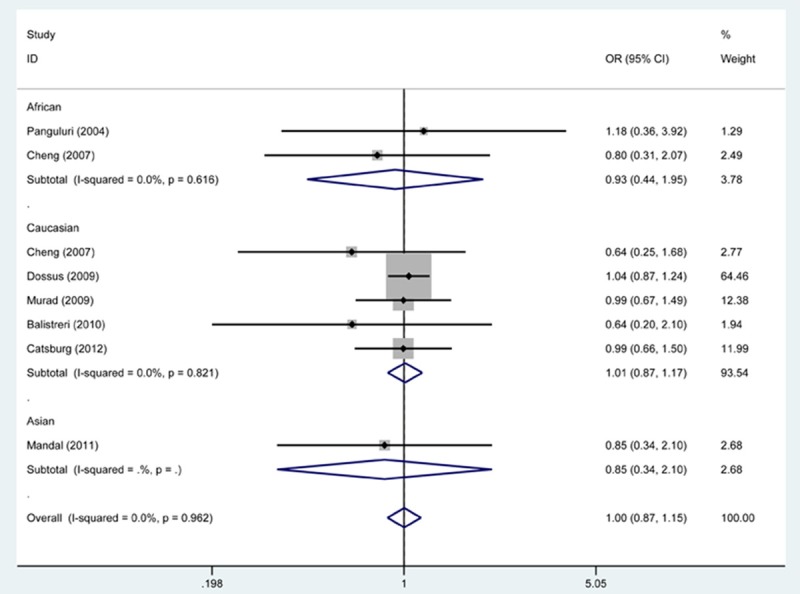
Forest plot shows association between SNP rs20417 and risk of PCa by CC vs. GG genetic model. CI: confidence interval; OR: odds risk.
Figure 3.
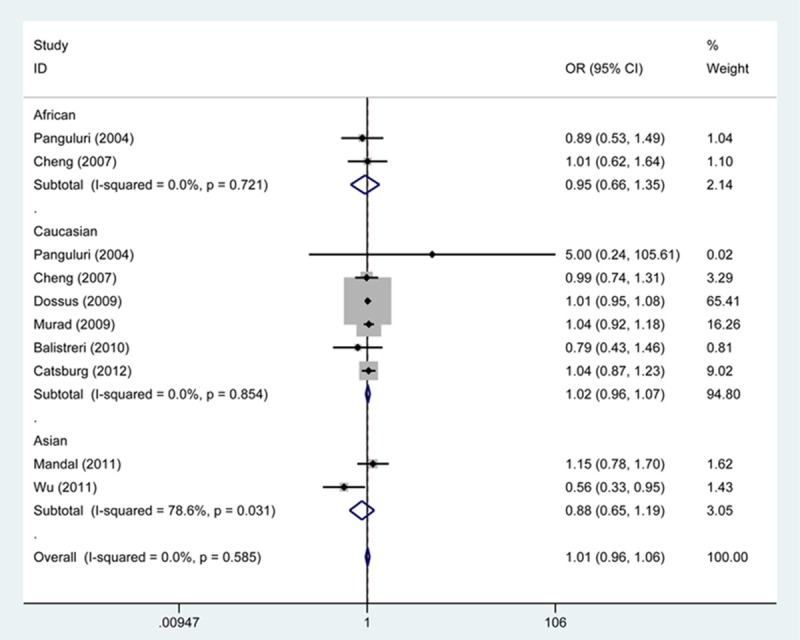
Forest plot shows association between SNP rs20417 and risk of PCa by CC + GC vs. GG genetic model. CI: confidence interval; OR: odds risk.
Sensitivity analyses
We performed sensitivity analyses by repeated meta-analyses based on the data composed of nine studies (one study was excluded in each analysis). Although significant departure was revealed in the control subjects of several studies, we did not observe any quantitative modification occurring in pooled results, suggesting our findings were statistically credible.
Publication diagnosis
We diagnosed the publication bias with Begg’s funnel plot and Egger’s test. The shapes of the funnel plots demonstrated no evidence of obvious asymmetry (Figure 4). In addition, Egger’s test provided statistical evidence of funnel plot symmetry (CC + GC vs. GG, Begg, P = 0.592; Egger, P = 0.915).
Figure 4.
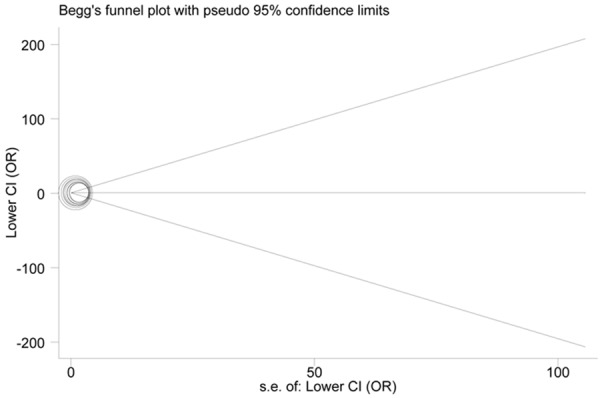
Funnel plots for association studies between SNP rs20417 and risk of PCa under CC + GC vs. GG genetic model.
Discussion
Genetic sequence variation resulting from SNPs is known to be a common event in human genome, with the total number of SNPs reported in public SNP databases currently exceeding 9 million [35]. The normal coding and splicing activities of sequences affected by the variation could lead to cancer susceptibility in populations of various ancestries [36,37]. The inducible enzyme PTGS2 converting arachidonic acid to prostaglandins is expressed during prostatic inflammation. Chronic inflammation has been suggested to be corrected with the pathophysiology of PCa. The development of the malignancy is promoted by an inflammatory microenvironment through up-regulating PTGS2, whose induction may be linked to pro-inflammatory cytokines released by adjacent inflammatory cells [38]. Genetic variation of SNPs in the PTGS2 gene determines pro-inflammatory genotypes, responsible for mediating the risk state of chronic inflammation and tissue damage and capable of producing tissue milieu favoring tumorigenesis [31].
The involvement of SNPs in the PTGS2 gene in inflammatory process directly associated with carcinogenesis has led a number of investigators to concentrate on their associations with PCa progression. A recent investigation conducted on 218 patients with prostate cancer and 436 healthy controls in central Taiwan demonstrated SNP rs20417 may be used as biomarker for early detection of PCa, with GG genotype conferring higher risk than GC genotype [26]. However, this finding was not supported by a subsequent larger replication study consisting of 1,431 cases and 756 controls [34]. In such a case, we decided to perform a meta-analysis to derive a summary estimate of the overall effect between SNP rs20417 and PCa risk.
In the present meta-analysis of eight SNP rs20417 publications totaling 12,326 PCa cases and 14,027 PCa-free controls, we found no significant association of SNP rs20417 and overall risk of PCa. The subsequent subgroup analyses also did not show this SNP was a risk factor for developing PCa. Nevertheless, a most recent study examining cancer risk in correlation with SNP rs20417 found the C allele was related to increased cancer susceptibility, especially gastric cancer in the Asian population [39]. Since the molecular mechanism differs substantially from cancer to cancer, and the inflammation status may be distinct in various cancers, leading to varying degrees of genetic predisposition of the same SNP to cancer.
Over the past decades, in an attempt to develop accurate, rapid, and cost-effective technologies for SNP analysis, a great deal of effort has been devoted. The detection and identification of SNPs are performed by using a variety of genotyping methods, which should be employed according to the number of subjects recruited and SNPs to be analyzed [35]. In our meta-analysis, the independent studies adopted various genotyping methods, some of which may inappropriately used when genotyping SNPs, consequently introducing heterogeneity and deviation from HWE. Although the two factors did not significantly alter the overall results in our meta-analysis, we cannot exclude the possibility that they have potential contributions to the null findings. In addition, this study analyzed the subjects of African descent, Asian descent and Caucasian descent, but the total samples of former two ethnicities are relatively small, which may limit the statistical power of the findings.
In spite of the limitations, based on an enlarged sample size by combining the data from published meta-analyses and those missed in them, our meta-analysis indicated that SNP rs20417 of the PTGS2 gene was not associated with PCa risk. Future research in a much larger number of participants is needed to further confirm the association between SNP rs20417 and PCa risk and to expand and refine our understanding of the genetic risk for PCa.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL Jr, Baker LH, Coltman CA Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury S, Burbridge S, Harper PG. Chemotherapy for the treatment of hormonerefractory prostate cancer. Int J Clin Pract. 2007;61:2064–2070. doi: 10.1111/j.1742-1241.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- 5.Thomas BM, Smith C, Evans J, Button MR, Kumar S, Palaniappan N, Staffurth J, Tanguay JS, Lester JF. Time to prostate specific antigen (PSA) nadir may predict rapid relapse in men with metastatic castration-resistant prostate cancer (CRPC) receiving docetaxel chemotherapy. Med Oncol. 2013;30:719. doi: 10.1007/s12032-013-0719-0. [DOI] [PubMed] [Google Scholar]
- 6.Zaorsky NG, Harrison AS, Trabulsi EJ, Gomella LG, Showalter TN, Hurwitz MD, Dicker AP, Den RB. Evolution of advanced technologies in prostate cancer radiotherapy. Nat Rev Urol. 2013;10:565–579. doi: 10.1038/nrurol.2013.185. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC. Family history and the risk of prostate cancer. Prostate. 1990;17:337–347. doi: 10.1002/pros.2990170409. [DOI] [PubMed] [Google Scholar]
- 8.Pairet M, Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: possible physiological and therapeutic implications. Fundam Clin Pharmacol. 1996;10:1–17. doi: 10.1111/j.1472-8206.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 9.Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem. 1994;221:889–897. doi: 10.1111/j.1432-1033.1994.tb18804.x. [DOI] [PubMed] [Google Scholar]
- 10.Hla T, Bishop-Bailey D, Liu CH, Schaefers HJ, Trifan OC. Cyclooxygenase-1 and -2 isoenzymes. Int J Biochem Cell Biol. 1999;31:551–557. doi: 10.1016/s1357-2725(98)00152-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 12.Lee DW, Sung MW, Park SW, Seong WJ, Roh JL, Park B, Heo DS, Kim KH. Increased cyclooxygenase-2 expression in human squamous cell carcinomas of the head and neck and inhibition of proliferation by nonsteroidal anti-inflammatory drugs. Anticancer Res. 2002;22:2089–2096. [PubMed] [Google Scholar]
- 13.Pruthi RS, Derksen E, Gaston K. Cyclooxygenase-2 as a potential target in the prevention and treatment of genitourinary tumors: a review. J Urol. 2003;169:2352–2359. doi: 10.1097/01.ju.0000047364.56051.74. [DOI] [PubMed] [Google Scholar]
- 14.Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Uotila P, Valve E, Martikainen P, Nevalainen M, Nurmi M, Harkonen P. Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol Res. 2001;29:23–28. doi: 10.1007/s002400000148. [DOI] [PubMed] [Google Scholar]
- 17.Kim BH, Kim CI, Chang HS, Choe MS, Jung HR, Kim DY, Park CH. Cyclooxygenase-2 overexpression in chronic inflammation associated with benign prostatic hyperplasia: is it related to apoptosis and angiogenesis of prostate cancer? Korean J Urol. 2011;52:253–259. doi: 10.4111/kju.2011.52.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Martinez AB, Carmena MJ, Arenas MI, Bajo AM, Prieto JC, Sanchez-Chapado M. Overexpression of vasoactive intestinal peptide receptors and cyclooxygenase-2 in human prostate cancer. Analysis of potential prognostic relevance. Histol Histopathol. 2012;27:1093–1101. doi: 10.14670/HH-27.1093. [DOI] [PubMed] [Google Scholar]
- 19.Amirian ES, Ittmann MM, Scheurer ME. Associations between arachidonic acid metabolism gene polymorphisms and prostate cancer risk. Prostate. 2011;71:1382–1389. doi: 10.1002/pros.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W, Wei BB, Shan X, Liu P. -765G > C and 8473T > C polymorphisms of COX-2 and cancer risk: a meta-analysis based on 33 casecontrol studies. Mol Biol Rep. 2010;37:277–288. doi: 10.1007/s11033-009-9685-1. [DOI] [PubMed] [Google Scholar]
- 21.Danforth KN, Hayes RB, Rodriguez C, Yu K, Sakoda LC, Huang WY, Chen BE, Chen J, Andriole GL, Calle EE, Jacobs EJ, Chu LW, Figueroa JD, Yeager M, Platz EA, Michaud DS, Chanock SJ, Thun MJ, Hsing AW. Polymorphic variants in PTGS2 and prostate cancer risk: results from two large nested case-control studies. Carcinogenesis. 2008;29:568–572. doi: 10.1093/carcin/bgm253. [DOI] [PubMed] [Google Scholar]
- 22.Panguluri RC, Long LO, Chen W, Wang S, Coulibaly A, Ukoli F, Jackson A, Weinrich S, Ahaghotu C, Isaacs W, Kittles RA. COX-2 gene promoter haplotypes and prostate cancer risk. Carcinogenesis. 2004;25:961–966. doi: 10.1093/carcin/bgh100. [DOI] [PubMed] [Google Scholar]
- 23.Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, Witte JS. COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer. 2007;97:557–561. doi: 10.1038/sj.bjc.6603874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dossus L, Kaaks R, Canzian F, Albanes D, Berndt SI, Boeing H, Buring J, Chanock SJ, Clavel-Chapelon F, Feigelson HS, Gaziano JM, Giovannucci E, Gonzalez C, Haiman CA, Hallmans G, Hankinson SE, Hayes RB, Henderson BE, Hoover RN, Hunter DJ, Khaw KT, Kolonel LN, Kraft P, Ma J, Le Marchand L, Lund E, Peeters PH, Stampfer M, Stram DO, Thomas G, Thun MJ, Tjonneland A, Trichopoulos D, Tumino R, Riboli E, Virtamo J, Weinstein SJ, Yeager M, Ziegler RG, Cox DG. PTGS2 and IL6 genetic variation and risk of breast and prostate cancer: results from the Breast and Prostate Cancer Cohort Consortium (BPC3) Carcinogenesis. 2010;31:455–461. doi: 10.1093/carcin/bgp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murad A, Lewis SJ, Smith GD, Collin SM, Chen L, Hamdy FC, Neal DE, Donovan J, Martin RM. PTGS2-899G > C and prostate cancer risk: a population-based nested case-control study (ProtecT) and a systematic review with metaanalysis. Prostate Cancer Prostatic Dis. 2009;12:296–300. doi: 10.1038/pcan.2009.18. [DOI] [PubMed] [Google Scholar]
- 26.Wu HC, Chang CH, Ke HL, Chang WS, Cheng HN, Lin HH, Wu CY, Tsai CW, Tsai RY, Lo WC, Bau DT. Association of cyclooxygenase 2 polymorphic genotypes with prostate cancer in taiwan. Anticancer Res. 2011;31:221–225. [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balistreri CR, Caruso C, Carruba G, Miceli V, Campisi I, Listi F, Lio D, Colonna-Romano G, Candore G. A pilot study on prostate cancer risk and pro-inflammatory genotypes: pathophysiology and therapeutic implications. Curr Pharm Des. 2010;16:718–724. doi: 10.2174/138161210790883877. [DOI] [PubMed] [Google Scholar]
- 32.Mandal RK, Mittal RD. Polymorphisms in COX-2 gene influence prostate cancer susceptibility in a northern Indian cohort. Arch Med Res. 2011;42:620–626. doi: 10.1016/j.arcmed.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, John EM, Ingles SA, Stern MC. Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer: results from a multiethnic case-control study. Carcinogenesis. 2012;33:2108–2118. doi: 10.1093/carcin/bgs242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catsburg C, Joshi AD, Corral R, Lewinger JP, Koo J, John EM, Ingles SA, Stern MC. Polymorphisms in carcinogen metabolism enzymes, fish intake, and risk of prostate cancer. Carcinogenesis. 2012;33:1352–1359. doi: 10.1093/carcin/bgs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Misra A. SNP genotyping: technologies and biomedical applications. Annu Rev Biomed Eng. 2007;9:289–320. doi: 10.1146/annurev.bioeng.9.060906.152037. [DOI] [PubMed] [Google Scholar]
- 36.Liu F, Li B, Wei Y, Chen X, Ma Y, Yan L, Wen T. P21 codon 31 polymorphism associated with cancer among white people: evidence from a meta-analysis involving 78,074 subjects. Mutagenesis. 2011;26:513–521. doi: 10.1093/mutage/ger010. [DOI] [PubMed] [Google Scholar]
- 37.Lin BK, Clyne M, Walsh M, Gomez O, Yu W, Gwinn M, Khoury MJ. Tracking the epidemiology of human genes in the literature: the HuGE Published Literature database. Am J Epidemiol. 2006;164:1–4. doi: 10.1093/aje/kwj175. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61:60–72. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- 39.Wang XF, Huang MZ, Zhang XW, Hua RX, Guo WJ. COX-2-765G > C polymorphism increases the risk of cancer: a meta-analysis. PLoS One. 2013;8:e73213. doi: 10.1371/journal.pone.0073213. [DOI] [PMC free article] [PubMed] [Google Scholar]


