Abstract
Autoimmune disease systemic lupus erythematosus (SLE) is associated with increased expression of pro-inflammatory cytokines such as interferons (IFNs) and specific interleukins (ILs), which are induced by toll-like receptors (TLRs). The present study aimed to examine the serum levels of cytokines, the activation of TLR-7 and TLR-8 of peripheral blood mononuclear cells (PBMCs) from pediatric SLE patients, and to investigate the response of those PBMCs to viral RNA via the TLR-7 and TLR-8 signaling. Results demonstrated that pediatric SLE patients had increased serum concentrations of interleukin (IL)-1β, IL-6, IL-8, IL-10, and IFN-α, and promoted activation of TLR-7 and TLR-8, compared to control subjects. Moreover, the peripheral blood mononuclear cells (PBMCs) from pediatric SLE patients were more sensitive to the stimulation by the transfection with viral RNA from influenza virus, with a promoted activation of TLR-7 and TLR-8 signaling. In conclusion, pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, IL-10, and IFN-α were promoted in pediatric SLE patients, with an increased activation of TLR-7 and TLR-8 to the stimuli, such as virus infection. It implies the TLR-7 and TLR-8 activation by virus infection might play an important role in the pathogenesis of pediatric SLE.
Keywords: Toll-like receptors-7 and -8, peripheral blood mononuclear cells, cytokines, pediatric systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a prototypic systemic autoimmune disease, which can result in skin rashes, arthritis, leukopenia, nephritis, and inflammation of the nervous system [1]. Immunologically, SLE is characterized by the high B cell reactivity and producing autoantibodies to nuclear and cytoplasmic components [2]. And sustained production of autoantibodies leads to the accumulation of immune complex in kidney [3], brain [4] and other organs. SLE begins with the self-tolerance loss and the presence of auto-reactive lymphocytes in the peripheral blood, due to both environmental and genetic factors [5,6]. Multiple types of cells in the adaptive and innate immune system have been confirmed to contribute to SLE pathogenesis.
Cytokines exerts a key regulatory role in controlling the immune responses in SLE patients [7]. There is an imbalance in the cytokine production of SLE patients [8,9], which exert orchestrated interactions in the SLE [10,11]. Previous studies suggested that serum IFN-α level was promoted in SLE patients and correlated with SLE Disease Activity Index (SLEDAI) scores, organ involvement, the levels of anti-dsDNA antibodies and complement activation [12]. And such clinical manifestations as fever, rash and lymphopenia correlated with elevated serum IFN-α in SLE patients [13]. The major functions of IL-1α and IL-1β, are to act as mediators of the host inflammatory reaction to infections [14]. High serum IL-1 levels tend to correlate with disease activity of lupus nephritis [15]. And two open-label trials with a recombinant form of IL-1Ra, suggested limited but positive effects [13]. IL-6 is produced mainly by macrophages, but also by many other cell types. IL-6 is upregulated in the serum and to higher degree in the urine of some lupus patients and in murine lupus models, implying the role for IL-6 in lupus nephritis [16,17]. And IL-6 upregulation is also recognized in kidney biopsies of SLE patients with renal disease [18]. A trial with the specific antibody to the IL-6R, tocilizumab for lupus patients demonstrated decreased disease activity [19]. Therefore, cytokines play important regulatory roles in SLE.
Toll-like receptors (TLRs) are a family of conserved pattern-recognition receptors in innate immune system and play crucial roles in the innate defense against foreign agents [20]. These protein receptors are characterized by their ability to promptly respond to invading pathogens including flagella in and lipopolysaccharide (LPS) of bacteria, nucleic acids derived from viruses and zymosan of fungi [21]. Such TLRs as TLR-3, TLR-7 and TLR-9 which are in B cells, plasmacytoid dendritic cells (pDCs) are recognized to play important roles in lupus pathogenesis via mediating the recognition and binding of self nucleic acids and related immune complexes [22]. Approximately half of SLE patients are defective in the clearance of dying cellular debris, which is rapidly cleaned up by macrophages in healthy individuals [23], and such defect was reconfirmed in an SLE murine model [24]. The accumulating cellular debris leads to an increased release of host DNA and RNA, which promote the production of autoantibodies [23]. Moreover, TLRs have been confirmed to mediate the host DNA/RNA recognition and the production of pathogenic autoantibodies in SLE [20].
In the present study, we examined the serum levels of pro-inflammatory cytokines such as IL-1β, IL-6, IFN-α and IL-8 in pediatric SLE patients, then we determined the expression levels of TLR-2, TLR-4, TLR-7, TLR-8 and TLR-9 in the PBMCs from these pediatric SLE patients, and investigated the sensitivity of the two groups of PBMCs to the stimulation with single-stranded RNA (ssRNA) or TLR-7/8 agonist. The present study implies the key regulatory role of TLR-7/8 in the upregulation of pro-inflammatory cytokines in PBMCs from pediatric SLE patients.
Materials and methods
Pediatric systemic lupus erythematosus patients and control subjects
Total of 22 pediatric patients with SLE and 17 healthy subjects were enrolled from Jan 2013 to Jan 2015 in the Department of Pediatrics, Affiliated Hospital of Inner Mongolia Medical University. Detailed characteristics of these pediatric SLE patients and healthy control subjects were described in Table 1. The healthy volunteers as control subjects had no history of autoimmune disorders or other inflammatory diseases, without recent (3 month) acute or lasting chronic infection with viruses or bacteria. All SLE patients were matched as closely as possible for age, sex, lifestyle and geographical lineage with control subjects, excepting that they were suffering from SLE. Diagnosis for SLE was made according to the criteria proposed by the American Rheumatism Association for the classification of SLE [25]. Written consent was obtained from each individual before the study. And the study was approved by the Ethics Committee of the affiliated Hospital of Inner Mongolia Medical University.
Table 1.
Demographic and clinical characteristics of patients with pediatric systemic lupus erythematosus
| Characteristics | PSLE (n = 22) | Control (n = 17) | P value |
|---|---|---|---|
| Age (years) | 13.6 (8-17) | 14.2 (7-20) | 0.4060 |
| Gender | 0.5099 | ||
| Female | 21 | 16 | |
| Male | 1 | 1 | |
| SLE Duration (years) | 4.2 ± 1.85 | / | / |
| SLEDAI | 6.727 (0-24) | / | / |
| SDI | 0-3 | / | / |
| Major manifestations | / | / | |
| Cerebrovascular accident | 4 | / | / |
| Deep vein thrombosis | 2 | / | / |
| Neuropsychiatric SLE | 2 | / | / |
| Pulmonary hypertension | 2 | / | / |
| Significant thrombocytopenia | 4 | / | / |
| Lupus nephritis | 8 | / | / |
Enzyme-linked immunosorbent assay (ELISA) for cytokines
Serum or supernatant levels of IL-1β, IL-6, IFN-α and IL-8 were quantified using the solid phase enzyme-linked immunosorbent assay (ELISA) kit (Abcam, Cambridge, UK) according to the product’s manual. In brief, the precoated microplates (with monoclonal mouse anti-human antibody against IL-1β, IL-6, IFN-α or IL-8) were firstly blocked with 1% Bovine Serum Albumin (BSA; Ameresco, Framingham, MA, USA) overnight at 4°C, and then were added with the standard samples, with the serially-diluted serum or with cell supernatant samples for a incubation at 37°C for 1 hour. Then each well in the plate was aspirated and were washed with phosphate buffered saline with Tween 20 (PBST) for three times. And then the detection antibody (polyclonal antibody against IL-1β, IL-6, IFN-α or IL-8 conjugated with horseradish peroxidase), was added to each well and incubated for 2 h at room temperature. Again after washing, substrate solution was added for another incubation of 20 min at room temperature. Finally, the stop solution was added, and the optical density of each well was determined immediately at 450 nm.
RNA isolation and quantitative real-time RT-PCR
Total mRNA from the PBMCs was extracted using the Trizol reagent (Life Technologies, Grand Island, NY, USA) under the guidance of the product’s manual. Quantitative real-time RT-PCR (RT-qPCR) was performed using QuantiFast SYBR Green PCR Kit (Qiagen, GmbH, Hilden, Germany) according to the kit’s manual, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control. The data were normalized to GAPDH and expressed as the fold change over control and calculated with the ∆∆Ct method [26].
Stimulation of PBMCs with ssRNA or with TLR-7/8 agonist
PBMCs were isolated from pediatric SLE patients using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) from blood of pediatric SLE patients or healthy volunteers and were resuspended in RPMI 1640 (GIBCO, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin, and 100 U/ml streptomycin (both from CSPC, Shijiazhuang, China). For the stimulation with ssRNA, PBMC cells were suspended with a titer of 1 × 105/ml and were transfected with ssRNA, which was synthesized by Integrated DNA Technologies (IDT) with high-pressure liquid chromatography (HPLC) purification (Ambion, Austin, TX, USA), and then were incubated at 37°C for 8 hours, and then were assayed for IL-1β, IL-6, IFN-α or IL-8 in the supernatant. For the stimulation with TLR7/8 agonist, 1 × 105/ml PBMCs were treated with R848 (Invitrogen, San Diego, CA, USA) with a final concentration of 0.5 or 1 μg/mL, for 6 hours, and then were incubated at 37°C for 8 hours, and then were assayed for IL-1β, IL-6, IFN-α or IL-8 in the supernatant.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 Software (GraphPad, San Diego, CA, USA). The results are presented as means ± standard deviation (SD), and the difference between the two groups was examined by the Mann-Whitney test.
Results
Upregulation of serum cytokines in patients with pediatric SLE
There were 22 pediatric SLE patients and 17 normal subjects were involved in this study. The demographic and clinical characteristics of these patients and subjects were shown in Table 1. We have examined the serum levels of pro-inflammatory cytokines such as IL-1β, IL-6, IFN-α and IL-8 in the two groups of subjects. As shown in Figure 1, the serum level of IL-1β was 1.599 ± 0.1957 ng/mL in pediatric SLE patients, significantly higher than 1.110 ± 0.07064 ng/mL (P<0.05) in normal subjects. And the serum levels of IL-6, IFN-α and IL-8 were also significantly higher in pediatric SLE patients, than in normal subjects (4.272 ± 0.4222 ng/mL vs 2.848 ± 0.2344 ng/mL for IL-6 with a p value of 0.0099, 3.597 ± 0.3220 ng/mL vs 1.917 ± 0.1908 ng/mL for IFN-α, with a p value of 0.0002, and 14.55 ± 1.873 vs 3.944 ± 0.3602 for IL-8, with a p value of less than 0.0001). Thus, we recognized the upregulation of pro-inflammatory cytokines such as IL-1β, IL-6, IFN-α and IL-8 in serum of pediatric SLE patients.
Figure 1.
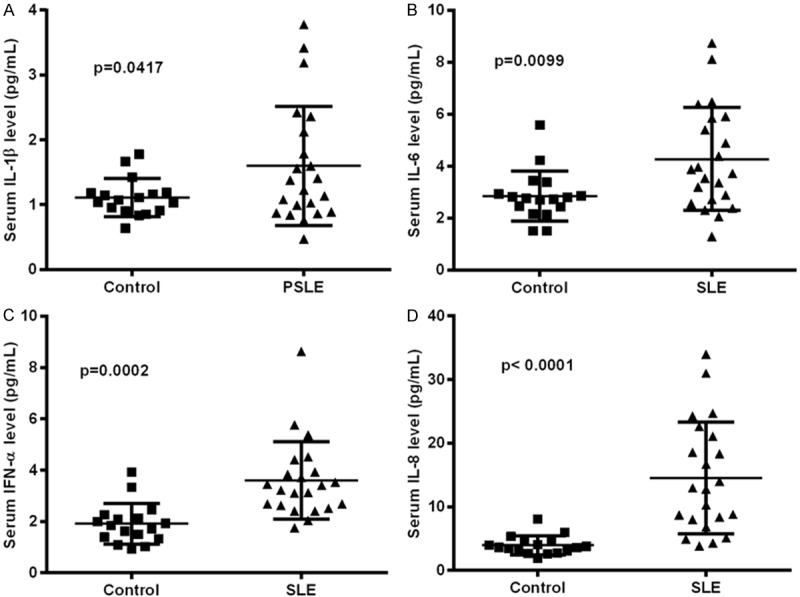
Serum levels of IL-1β, IL-6, IFN-α and IL-8 in pediatric SLE patients. Serum levels in the pediatric SLE patients (N = 22) or in normal subjects of IL-1β (A), IL-6 (B), IFN-α (C) or IL-8 (D) was assayed with the ELISA kit for each cytokine. The significance was calculated by the Mann-Whitney test, and the p-value was indicated accordingly.
TLRs level in PBMCs from patients with pediatric SLE
To deduce the mechanism into the promotion to pro-inflammatory cytokines in pediatric SLE patients, we then investigated the expression of TLRs in the peripheral blood mononuclear cells (PBMCs) of pediatric SLE patients. Firstly, the percentage of PBMCs with the positivity of various type of TLR in each subject was counted. Table 2 indicated that there was no significant difference in the TLR-2- or TLR-4-positivity between pediatric SLE patients and normal subjects (P = 0.066 for TLR-2 or P = 0.066 for TLR-4), whereas there were significantly more TLR-7-, TLR-8- or TLR-9-positive PBMCs in the pediatric SLE patients than in normal subjects (P<0.0001 for TLR-7-positivity, P<0.001 for TLR-8-positivity or P<0.01 for TLR-9-positivity). And then we quantitatively examined the expression levels of the above-mentioned TLRs in the PBMCs from pediatric SLE patients and normal subjects. Though the levels of TLR-2 and TLR-4 were higher in the pediatric SLE group, there was no significant difference between the two groups (P = 0.0725 for TLR-2 or P = 0.1523 for TLR-4; Figure 2A and 2B). The level of TLR-7 (P = 0.0003), TLR-8 (P = 0.0007) and TLR-9 (P<0.0236) were recognized to be significantly upregulated in pediatric SLE patients than in normal subjects (Figure 2C-E).
Table 2.
TLR-7 or TLR-8 positivity of PBMCs in PSLE patients
| TLR types | % positive cells (Mean ± SD) | P value | |
|---|---|---|---|
|
| |||
| PSLE (n = 22) | Control (n = 17) | ||
| TLR-2 | 8.46 ± 3.35 | 6.46 ± 2.35 | 0.066 |
| TLR-4 | 4.50 ± 1.47 | 5.37 ± 1.86 | 0.194 |
| TLR-7 | 28.28 ± 7.56 | 5.71 ± 2.13 | <0.0001 |
| TLR-8 | 22.83 ± 5.35 | 6.38 ± 2.72 | <0.001 |
| TLR-9 | 14.46 ± 2.68 | 8.52 ± 1.66 | <0.01 |
Figure 2.

Levels of TLR-2, -4, -7, -8 and -9 in PBMCs from pediatric SLE patients or normal subjects. Expression TLR-2 (A), -4 (B), -7 (C), -8 (D) and -9 (E) at mRNA levels in the PBMCs from pediatric SLE patients and normal subjects was quantified by real-time quantitative PCR, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control. The significance was calculated by the Mann-Whitney test, and the p-value was indicated accordingly.
PBMCs from pediatric SLE patients were sensitive to stimulation ssRNA or TLR-7/8 agonist R848
TLR-7, -8 and -9 are multiple pathogen recognition sensors that detect viral or other original RNAs [27]. The endosome-localized TLR-7/8/9 and their ligands determine the production of inflammatory cytokines. Multimeric RNA/DNA aggregates elicits the production of IFN-I and other cytokines [28,29]. To associate the high production of serum cytokines in pediatric SLE patients with the activation of TLRs in PBMCs, we then inoculated PBMCs from pediatric SLE patients or from normal subjects. Figure 3 demonstrated that the single-stranded RNA (ssRNA) sensitized the lupus PBMCs more significantly than the normal PBMCs, the transfection with 100, 200 or 500 nM ssRNA promoted higher levels of IL-1β (P<0.05 for 100 or 500 nM, P<0.01 for 200 nM; Figure 3A), IL-6 (P<0.05 for 100 nM or P<0.01 for 200 nM; Figure 3B), or IFN-α (P<0.05 for 100 or 200 nM; Figure 3C). However, the level of IL-8 production was not significantly promoted by the transfection with ssRNA in either lupus or normal subjects. Therefore, the PBMCs from the pediatric SLE patients were more sensitive to the TLR-7/8 agonist.
Figure 3.
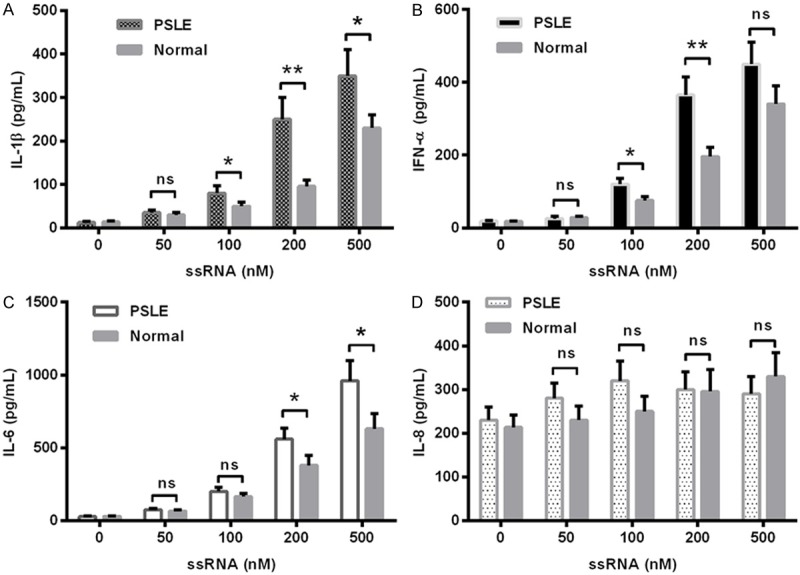
Levels of IL-1β, IL-6, IFN-α and IL-8 produced from pediatric SLE PBMCs, upon the stimulation with ssRNA. IL-1β (A), IFN-α (B), IL-6 (C), or IL-8 (D) in the supernatant of PBMCs post the transfection with 50, 100, 200, or 500 nM ssRNA was assayed with the ELISA kit for each cytokine. The significance was calculated by the t test, and the statistical significantce was indicated as *P<0.05, **P<0.01, ns: no significance.
Then the TLR-7/8 agonist R848 was utilized to stimulate PBMCs from pediatric SLE patients or from normal subjects. Figure 4A indicated that there was higher level of IL-1β produced from the PBMCs from pediatric SLE patients, upon the stimulation with R848 (P<0.05 for 0.5 μg/mL or P<0.01 for 1 μg/mL). And such significant difference was also recognized in the production of IFN-α and IL-6 between the pediatric SLE and normal groups of cells (P<0.05 for IFN-α induction by either 0.5 or 1 μg/mL R848, Figure 4B; P<0.01 for for IL-6 induction by either 0.5 or 1 μg/mL R848, Figure 4C). Therefore, there was a significant difference in the sensitivity to ssRNA or to TLR-7/8 agonist R848 of PBMCs from pediatric SLE patients and from normal subjects.
Figure 4.
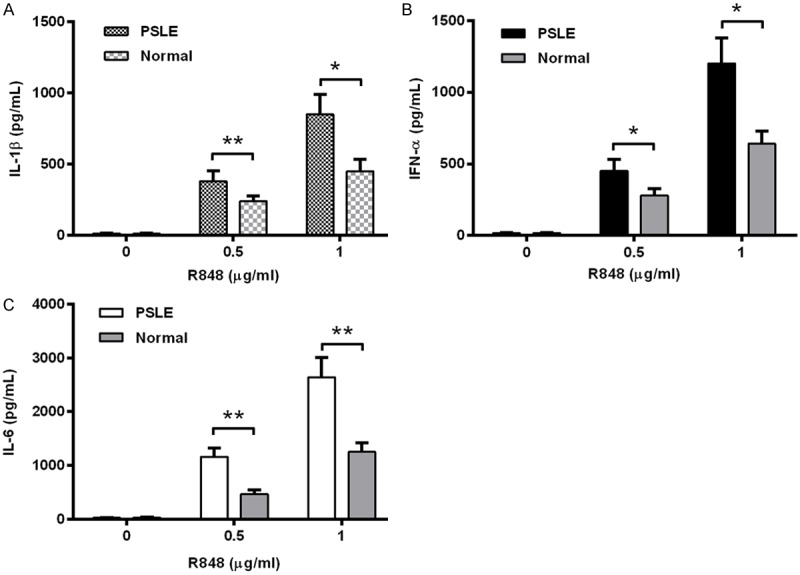
Levels of IL-1β, IL-6, IFN-α and IL-8 produced from pediatric SLE PBMCs, upon the stimulation with R848. IL-1β (A), IFN-α (B), or IL-6 (C) in the supernatant of PBMCs post the treatment with 0, 0.5 or 1 μg/mL R848 (TLR-7/8 agonist) was assayed with the ELISA kit for each cytokine. The significance was calculated by the t test, and the statistical significance was indicated as *P<0.05, **P<0.01.
Discussion
The pathophysiology of SLE is influenced by hormonal as well as environmental factors, including ultraviolet light (UVB), drugs, pollutants, vaccinations, smoking, and microbial infection [30]. Generally, these factors can adversely affect immune reactivity or can modulate gene expression and function of immune cells [31]. Infection with microbial agents such as bacteria or viruses might deregulate the human immune system to cause autoimmunity [32]. In particular, infection with viruses has been confirmed to potentiate autoimmune conditions [33,34], via interacting with or modifying innate and acquired immune responses that could facilitate autoimmunity [35]. Such viruses as Epstein-Barr Virus (EBV) [36] and Cytomegalovirus (CMV) [37]. And the Influenza vaccination can induce new-onset of autoimmune responses in SLE [38]. Thus, virus was considered as potential pathogenic agent in SLE [35].
In the present study, we firstly recognized the upregulation of serum cytokines, such as IL-1β, IL-6, IFN-α and IL-8 in patients with pediatric SLE, the serum levels of such pro-inflammatory cytokines were significantly higher in pediatric SLE patients than in normal subjects. And the pro-inflammatory cytokine promotion was accompanied with the upregulation of TLRs, such as TLR-7, TLR-8 and TLR-9 in PBMCs from patients with pediatric SLE, the percentage of PBMCs with the positivity of TLR-7, TLR-8 or TLR-9 was significantly higher in the pediatric SLE patients than in normal subjects. And the expression levels of the above-mentioned TLRs were quantitatively higher in the PBMCs from pediatric SLE patients than from normal subjects, though the levels of TLR-2 and TLR-4 were not significantly higher in the pediatric SLE group. Moreover, the stimulation with ssRNA, the TLR-7/8 stimulator, promoted higher levels of proinflammatory cytokines, in the PBMCs from pediatric SLE patients than in the PBMCs from normal subjects. Thus, we recognized the association of the promoted proinflammatory cytokines with the TLR-7/8/9 promotion in PBMCs from pediatric SLE patients. TLR-7, -8 and -9 are multiple pathogen recognition sensors, particularly detect viral RNAs [27,39]. The endosome-localized TLR-7/8/9 and their ligands determine the production of inflammatory cytokines in such host cells as PBMCs. Thus, the present study implies a potential association of the promotion of inflammatory cytokines with the virus infection via the TLR-7/8 activation.
Acknowledgements
The present study was supported by the grant from the Affiliated Hospital of Inner Mongolia Medical University.
Disclosure of conflict of interest
None.
References
- 1.Liu CC, Kao AH, Manzi S, Ahearn JM. Biomarkers in systemic lupus erythematosus: challenges and prospects for the future. Ther Adv Musculoskelet Dis. 2013;5:210–233. doi: 10.1177/1759720X13485503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotz PH. The autoantibody repertoire: searching for order. Nat Rev Immunol. 2003;3:73–78. doi: 10.1038/nri976. [DOI] [PubMed] [Google Scholar]
- 3.Takazoe K, Shimada T, Nakano H, Kawamura T, Utsunomiya Y, Kanai T, Kitajima T, Yamaguchi Y, Joh K, Mitarai T, Sakai O. Massive uncomplicated vascular immune complex deposits in the kidney of a patient with systemic lupus erythematosus. Clin Nephrol. 1997;48:195–198. [PubMed] [Google Scholar]
- 4.Kozora E, Filley CM, Zhang L, Brown MS, Miller DE, Arciniegas DB, Pelzman JL, West SG. Immune function and brain abnormalities in patients with systemic lupus erythematosus without overt neuropsychiatric manifestations. Lupus. 2012;21:402–411. doi: 10.1177/0961203311429116. [DOI] [PubMed] [Google Scholar]
- 5.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 6.Crow MK. Collaboration, genetic associations, and lupus erythematosus. N Engl J Med. 2008;358:956–961. doi: 10.1056/NEJMe0800096. [DOI] [PubMed] [Google Scholar]
- 7.Lee HM, Sugino H, Nishimoto N. Cytokine networks in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:676284. doi: 10.1155/2010/676284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora V, Verma J, Marwah V, Kumar A, Anand D, Das N. Cytokine imbalance in systemic lupus erythematosus: a study on northern Indian subjects. Lupus. 2012;21:596–603. doi: 10.1177/0961203311434937. [DOI] [PubMed] [Google Scholar]
- 9.Chang DM, Su WL, Chu SJ. The expression and significance of intracellular T helper cytokines in systemic lupus erythematosus. Immunol Invest. 2002;31:1–12. doi: 10.1081/imm-120003217. [DOI] [PubMed] [Google Scholar]
- 10.Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001;3:136–141. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 13.Davis LS, Hutcheson J, Mohan C. The role of cytokines in the pathogenesis and treatment of systemic lupus erythematosus. J Interferon Cytokine Res. 2011;31:781–789. doi: 10.1089/jir.2011.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugos B, Kiss E, Dul C, Gubisch W, Szegedi G, Sipka S, Zeher M. Measurement of interleukin-1 receptor antagonist in patients with systemic lupus erythematosus could predict renal manifestation of the disease. Hum Immunol. 2010;71:874–877. doi: 10.1016/j.humimm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Peterson E, Robertson AD, Emlen W. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus. 1996;5:571–575. doi: 10.1177/096120339600500603. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CY, Wu TH, Yu CL, Lu JY, Tsai YY. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85:207–214. doi: 10.1159/000045663. [DOI] [PubMed] [Google Scholar]
- 18.Malide D, Russo P, Bendayan M. Presence of tumor necrosis factor alpha and interleukin-6 in renal mesangial cells of lupus nephritis patients. Hum Pathol. 1995;26:558–564. doi: 10.1016/0046-8177(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 19.Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, Fleisher T, Balow JE, Lipsky PE. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Wang X, Zhang F, Yin H. Toll-like receptors as therapeutic targets for autoimmune connective tissue diseases. Pharmacol Ther. 2013;138:441–451. doi: 10.1016/j.pharmthera.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 22.Theofilopoulos AN, Kono DH, Beutler B, Baccala R. Intracellular nucleic acid sensors and autoimmunity. J Interferon Cytokine Res. 2011;31:867–886. doi: 10.1089/jir.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruse K, Janko C, Urbonaviciute V, Mierke CT, Winkler TH, Voll RE, Schett G, Munoz LE, Herrmann M. Inefficient clearance of dying cells in patients with SLE: anti-dsDNA autoantibodies, MFG-E8, HMGB-1 and other players. Apoptosis. 2010;15:1098–1113. doi: 10.1007/s10495-010-0478-8. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocytederived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing realtime PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 27.Han X, Li X, Yue SC, Anandaiah A, Hashem F, Reinach PS, Koziel H, Tachado SD. Epigenetic regulation of tumor necrosis factor alpha (TNFalpha) release in human macrophages by HIV-1 single-stranded RNA (ssRNA) is dependent on TLR8 signaling. J Biol Chem. 2012;287:13778–13786. doi: 10.1074/jbc.M112.342683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 29.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, Lee KD, Coffman RL, Barrat FJ. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarzi-Puttini P, Atzeni F, Iaccarino L, Doria A. Environment and systemic lupus erythematosus: an overview. Autoimmunity. 2005;38:465–472. doi: 10.1080/08916930500285394. [DOI] [PubMed] [Google Scholar]
- 31.Tugnet N, Rylance P, Roden D, Trela M, Nelson P. Human Endogenous Retroviruses (HERVs) and Autoimmune Rheumatic Disease: Is There a Link? Open Rheumatol J. 2013;7:13–21. doi: 10.2174/1874312901307010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87:385–395. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 33.Francis L, Perl A. Infection in systemic lupus erythematosus: friend or foe? Int J Clin Rheumtol. 2010;5:59–74. doi: 10.2217/ijr.09.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigante D, Mazzoni MB, Esposito S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun Rev. 2014;13:96–102. doi: 10.1016/j.autrev.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Nelson P, Rylance P, Roden D, Trela M, Tugnet N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus. 2014;23:596–605. doi: 10.1177/0961203314531637. [DOI] [PubMed] [Google Scholar]
- 36.James JA, Robertson JM. Lupus and Epstein-Barr. Curr Opin Rheumatol. 2012;24:383–388. doi: 10.1097/BOR.0b013e3283535801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed AE, Hasen AM, Mohammed GF, Elmaraghy NN. Real-Time PCR of cytomegalovirus and Epstein-Barr virus in adult Egyptian patients with systemic lupus erythematosus. Int J Rheum Dis. 2015;18:452–8. doi: 10.1111/1756-185X.12261. [DOI] [PubMed] [Google Scholar]
- 38.Vista ES, Crowe SR, Thompson LF, Air GM, Robertson JM, Guthridge JM, James JA. Influenza vaccination can induce new-onset anticardiolipins but not beta2-glycoprotein-I antibodies among patients with systemic lupus erythematosus. Lupus. 2012;21:168–174. doi: 10.1177/0961203311429554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu SL, Chan PK, Wong CK, Szeto CC, Ho SC, So K, Yu MM, Yim SF, Cheung TH, Wong MC, Cheung JL, Yeung AC, Li EK, Tam LS. Antagonist-mediated down-regulation of Tolllike receptors increases the prevalence of human papillomavirus infection in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R80. doi: 10.1186/ar3803. [DOI] [PMC free article] [PubMed] [Google Scholar]


