Abstract
Background: The aim of this study was to investigate the expression of PH domain leucine-rich repeat protein phosphatase 1 (PHLPP1) in gastric cancer (GC), and its potential influence on the prognosis of GC patients. Methods: At present study, we examined the immunohistochemical expression of PHLPP1 on tissue microarrays (TMAs) containing 135 gastric adenocarcinoma tissues and 135 matched adjacent non-tumor tissues. In addition, both semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) and western blotting analysis (WB) were adopted to detect of the expression of PHLPP1 in the GC cell lines (AGS, SUN-1, KATO-III, BGC-823, MGC-803, SGC-7901, and HGC-27) and the normal gastric cell line GES-1. Survival analysis was used to investigate the efficiency of the prognostic evaluation of PHLPP1 expression in GC patients. Results: Positive expression rate of PHLPP1 in the primary GC tissues was significantly lower than that in adjacent non-tumor tissues (55.6% vs. 87.4%, P<0.001). Both gene transcription (mRNA) and Protein expression of PHLPP1 in the GC cell lines were significantly lower than those in the GES-1 cell line, respectively. The Kaplan-Meier analysis showed that patients presented PHLPP1 negative expression in the GC tissues had significantly lower overall survival rate than those presented PHLPP1 positive expression in the GC tissues (P=0.008). With the multivariate survival analysis (Cox regression), PHLPP1 expression in the GC tissue was identified as an independent predictor of the survival of patients. Conclusions: This study indicated that aberrant PHLPP1 expression was observed in GC tissues, which was significantly associated with the poor prognostic outcomes of GC patients.
Keywords: PH domain leucine-rich repeat protein phosphatase 1, stomach, neoplasm, prognosis, immunohistochemical
Introduction
Despite decreasing incidence and mortality rates in developed countries, gastric cancer (GC) remains the fourth most common cancer and the second leading cause of cancer mortality [1]. So far, surgical resection remains the unique method of primary curative treatment of GC. Although many improvements of GC treatments were come out in recent years, none of them provided a significantly promising outcome for patients. Therefore, the 5-year survival rate of GC is still at 20-25% [2]. In China, most of GC patients who were originally diagnosed in the advance stage presented the dismal prognoses. Several molecule biomarkers (including cyclin E, E-cadherin, HER2, p53, Ring finger protein 180 (RNF180), Protocadherin-10 (PCDH10), ras association domain protein 10 (RASSF10) and so on) have been identified to be closely correlate to the prognosis of GC up to now [3-9]. Unfortunately, none of above-mentioned biomarkers has been widely used in clinic owing to the low specificity. Therefore, detection of a novel biomarker with high specificity should be considered as a promising way to enhance the accurately prognostic prediction for GC. PHLPP1 (PH domain leucine-rich repeat protein phosphatase 1) is a protein-Ser/Thr phosphatase, which acts as a negative regulator of the PI3-kinase/Akt pathway in canceration. It contains an N-terminus Ras association domain, followed by an N-terminal PH domain, a leucine-rich repeat (LRR) region, a PP2C phosphatase domain, and a C-terminal PDZ ligand [10]. PHLPP1 play an important role in diverse cellular activities, such as proliferation, cell survival, migration, and cell death, to exert their antitumor and metastasis suppressor functions [11-14]. Although several authors reported that the expression of PHLPP1 was frequently decreased in diverse cancer tissues, there is no investigation to identify whether PHLPP1 expression in the GC tissues was aberrant [14-20].
The aim of this study was to explore whether the PHLPP1 expression might be considered as a potential biomarker for accurate prediction the prognosis of GC. To address this issue, we examined the PHLPP1 expression in the GC and the adjacent non-tumor tissues by using immunohistochemical staining. Survival analysis was used to investigate the prognostic prediction effect of PHLPP1 expression in the GC patients. In addition, RT-PCR and WB were adopted to detect the PHLPP1 expression in the GC cell lines and the GES-1 line for elucidation the practicability of PHLPP1 expression for specially prognostic prediction of GC.
Patients and methods
Data source
After approval from the Tianjin Medical University Cancer Hospital institutional review board, data from the cancer registry of the Tianjin Cancer Institute was obtained. Data obtained from the registry were listed as follows: age at surgery; gender, tumor location, tumor size, depth of tumor invasion (T stage, according to the Seventh Edition of UICC TNM Classification for GC), number of metastatic lymph nodes (N stage, according to the Seventh Edition of UICC TNM Classification for GC), Lauren classification, differentiation, and follow-up vital status. Oral and written informed consents were also obtained from patients who were included in this study.
Cell lines
Human GC cell lines (AGS, SUN-1, KATO-III, BGC-823, MGC-803, SGC-7901, and HGC-27) were purchased from the Type Culture Collection of the Chinese Academy of Sciences, (Shanghai, China). Human normal gastric mucosa cell GES-1 line was purchased from Biowit Technologies Corporation (Shenzhen, China). All GC cell lines and GES-1 cell line were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air in RPMI 1640 (Thermo Electron Corporation, Beijing, China). Media were supplemented with 10% (v/v) FBS (Life Tech, Mulgrave Vic, Australia) and penicillin/streptomycin (10,000 IU/ml penicillin, 20 mg/ml streptomycin; Roche, Swiss). The medium was changed twice a week.
Tissue samples
After the curative gastrectomy, 135 gastric adenocarcinoma tissues and 135 matched adjacent non-tumor tissues were retrieved from the Department of Gastroenterology, Tianjin Medical University Cancer Hospital (Tianjin, China), between August 2004 and December 2007 were enrolled in this study and then sent to Shanghai Outdo Biotech Company (Shanghai China) for microarrays (TMAs) (Cat No. T14-501 TMA1-3). The tumor and adjacent non-tumor tissue samples were histologically verified. The patients were not subjected to radiation, chemical or biological treatment before potentially curative gastrectomy was performed. Adjuvant chemotherapy or radiotherapy was not routinely administered to the patients. The clinicopathological characteristics of the two cohorts are summarized in Table 1. The patients’ consent was obtained for the use of the tissue samples and records, and the study protocol was approved and permission for use of the clinical data was given by the Institutional Research Ethics Committee of Tianjin Medical University Cancer Hospital.
Table 1.
Correlation between PHLPP1 expression and clinicopathological characteristics of GC patients
| Characteristics | PHLPP1 expression Negative (%) | PHLPP1 expression Positive (%) | P |
|---|---|---|---|
| Gender | 0.113 | ||
| Male | 39 (65.0) | 58 (77.3) | |
| Female | 21 (35.0) | 17 (22.7) | |
| Age at surgery | 0.063 | ||
| <58 | 32 (53.3) | 28 (37.3) | |
| ≥58 | 28 (46.7) | 47 (62.7) | |
| Tumor size | 0.345 | ||
| <5 | 17 (28.3) | 27 (36.0) | |
| ≥5 | 43 (71.7) | 48 (64.0) | |
| Tumor location | 0.539 | ||
| Upper third | 11 (18.3) | 18 (24.0) | |
| Middle third | 6 (10.0) | 10 (13.3) | |
| Lower third | 29 (48.4) | 36 (48.0) | |
| More than 2/3 stomach | 14 (23.3) | 11 (14.7) | |
| Depth of tumor invasion | 0.638 | ||
| T1 | 0 (0) | 0 (0) | |
| T2 | 5 (8.3) | 4 (5.3) | |
| T3 | 3 (5.0) | 6 (8.0) | |
| T4 | 52 (86.7) | 65 (86.7) | |
| N stage | 0.510 | ||
| N0 | 12 (20.0) | 15 (20.0) | |
| N1 | 6 (10.0) | 8 (8.0) | |
| N2 | 10 (16.7) | 20 (26.7) | |
| N3 | 32 (53.3) | 32 (42.7) | |
| Lauren classification | 0.377 | ||
| Intestinal | 10 (16.7) | 20 (26.7) | |
| Diffuse | 46 (76.6) | 51 (68.0) | |
| Mixed | 4 (6.7) | 4 (5.3) | |
| Differentiation | 0.085 | ||
| Well-differentiated | 10 (16.7) | 22 (29.3) | |
| Poorly-differentiated | 50 (83.3) | 53 (70.7) |
Surgical treatment
Curative resection was defined as a complete lack of grossly visible tumor tissue and metastatic lymph nodes remaining after resection, with pathologically negative resection margins. Primary tumors were resected en bloc with limited or extended lymphadenectomy (D1 or D2-3 according to the Japanese Gastric Cancer Association (JGCA)). Surgical specimens were evaluated as recommended by the seventh UICC TNM classification for GC.
Immunohistochemical staining
Immunohistochemistry was performed on formalin-fixed paraffin embedded on TMAs of resected specimens. Tissue cores with a diameter of 1.5 mm from randomly selected adenocarcinoma tissues and matched adjacent non-tumor tissues were used for the preparation of the TMAs. The TMAs were deparaffinized, and rehydrated. Then the slides were heated in an auto clave at 130°C for 3 min for PHLPP1 in 0.01 M citric acid buffer following deparaffinization for antigen retrieval before being immersed in 3% hydrogen peroxide to block endogenous peroxidase for 20 minutes. The immunohistochemistry procedure was performed according to the manufacturer’s instructions. The sections were then incubated with a polyclonal primary antibody against PHLPP1 (1:100 dilution; ab71972, abcam. Co. Ltd) at 4°C overnight. After incubation with a Peroxidase-conjugated AffiniPure Goat Anti-Rabbit secondary antibody (ZSGB-BIO; Beijing, China) and DAB, the slides were counterstained with Mayer’s hematoxylin. A known positive tissue sample (normal colonic mucosa slide) was used as a positive control. And PBS buffer was used to replace the primary antibodies in negative control staining.
The percentage of immunopositive cells was scored on a scale of 0 (none), 1 (<10%), 2 (11-50%), and 3 (>50%). The semi-quantification for immunostaining intensity was scored according to the following scale: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The total score of cell was obtained by adding the immunostaining score and the immunointensity score (range, 0-9). Scores from 2 to 9 were regarded as positive, whereas scores from 0 to 1 were regarded as negative. The immunohistochemical expression was independently reviewed by two of the authors.
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR)
For the PHLPP1 semi-quantitative RT-PCR, RNA was extracted from seven GC cell lines and GES-1 cell line using Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. Total RNA was reverse transcribed to cDNA in a 20 ul volume using Reverse Transcription kit (Invitrogen, Carlsbad, CA, USA). Primers designed and utilized for PHLPP1 was: forward sequence: 5’-AGTGAACCGATGGACAAGACG-3’, and reverse sequence: 5’-TGTTGGTGCTTTTTCACTTCTTCT-3’. The GAPDH gene was used as an endogenous control for quantitative DNA-PCR. Primers designed and utilized for GAPDH were listed as follows: forward sequence 5’-TGGGTGTGAACCATGAGAAGT-3’ and reverse sequence 5’-TGAGTCCTTCCACGATACCAA-3’. Annealing was performed at 72°C for PHLPP1. All PCR product electrophoreses were performed on a 2% agarose gel with ethidium bromide and visualized using the Gel Imager system (Asia Xingtai Mechanical Equipment Co., Beijing, China). The relative expression values of PHLPP1 mRNA were expressed by ratio between target mRNA gray scale value and GAPDH gray scale value.
Western blotting analysis
Cell lines (seven GC cell lines and GES-1 cell line) were respectively added to 1 mL of 100 mmol/L Tris/HCl (pH 7.5), 100 mmol/L NaCl, 0.5% sodium deoxycholate, 1 mmol/L ethylenediaminetetraacetic acid, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, and protease inhibitor. Protein (50 ug) per lane was then resolved using a 4% to 12% bis-Tris gel (Invitrogen) and transferred onto a nitrocellulose membrane (Invitrogen). The membrane was blocked for 1 h using Starting Block buffer (Pierce Biotechnology) and incubated at 4°C overnight with a primary antibody followed by incubation for 1 h at room temperature with secondary antibodies (Molecular Probes). The following antibodies were used for Western blots: PHLPP1 (1:2000; ab71972, abcam. Co. Ltd). Gel Imager system (Asia Xingtai Mechanical and Electrical Equipment Company, Beijing, China) to analyze images and to determine gray values.
Follow-up
After curative surgery, all patients were followed every 3-6 months for 2 year, then every year or until death. The follow-up of all patients who were included in this study was completed in September 2012. B ultrasonography, CT scans, chest X-ray, and endoscopy were obtained with every visit.
Statistical analysis
Statistical software package SPSS 19.0 was employed for all analysis. Differences in the different variables of GC patients were estimated using the x2 test for categorical data and independent-paired Student’s t test for continuous variables. Median overall survival (OS) was determined using Kaplan-Meier method, and log-rank test was performed to determine significance. Potentially important factors in univariate analyses (P<0.05) were included in multivariate analyses. OS was subjected to multivariate analysis by using Cox proportional hazard model with forward step procedures. Hazard ratios (HR) and 95% confidence interval were calculated. Significance was set at P<0.05.
Results
Patient demographics
The clinicopathological characteristics of the GC patients and PHLPP1 expression were listed in Table 1. The median OS of all patients was 34 months, ranging from 2 months to 77 months. Only 29 (21.5%) patients with GC are alive even after follow up was completed.
Immunohistochemical analysis for the PHLPP1 expression in the GC tissues and the adjacent non-tumor tissues
With the immunohistochemistry staining, we found that PHLPP1 protein expression was observed mainly in the cytoplasm of the GC cells and the adjacent non-tumor tissues cells. The majority of the adjacent non-tumor tissues showed positive expression of PHLPP1 was 87.4% (118/135). In contrast, the positive expression rate of PHLPP1 in the primary GC tissues was only 55.6% (75/135), which was significantly lower than that in the adjacent non-tumor tissues (shown in Table 2 and Figure 1). The results revealed that the PHLPP1 expression was significantly decreased in the GC tissues, compared to the adjacent non-tumor tissues.
Table 2.
PHLPP1 expression in GC and adjacent non-tumor tissues
| Expression of PHLPP1 | ||||
|---|---|---|---|---|
|
|
||||
| Tissue sample | n | Negative (%) | Positive (%) | P |
| Adjacent non-tumor tissues | 135 | 17 (22.6) | 118 (87.4) | <0.001 |
| GC tissues | 135 | 60 (44.4) | 75 (55.6) | |
Figure 1.
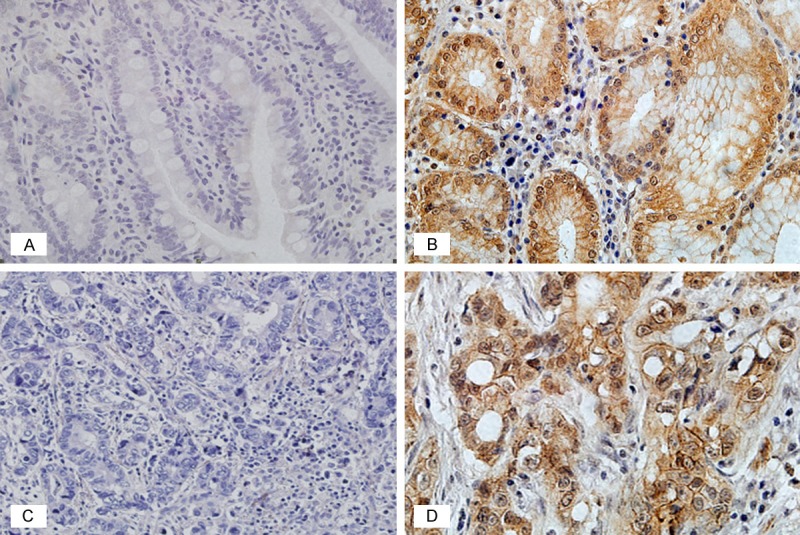
Immunohistochemical staining of PHLPP1 expression in the adjacent non-tumor tissues and the GC tissues (400-fold). A. Negative PHLPP1 expression in the adjacent non-tumor tissues; B. Positive PHLPP1 expression in the adjacent non-tumor tissues; C. Negative PHLPP1 expression in the GC tissues; D. Positive PHLPP1 expression in the GC tissues.
RT-PCR detection of PHLPP1 expression in the GC and the GES-1 cell lines
The mRNA expression of PHLPP1 was detected in GC cells and GES-1 cell by RT-PCR (Figure 2). The mRNA expression of PHLPP1 in the GC cell lines was significantly lower than that in the GES-1 cell line (0.305 ± 0.012 vs. 0.693 ± 0.010, PHGC-27<0.001; 0.399 ± 0.018 vs. 0.693 ± 0.010, PSGC-7901<0.001; 0.407 ± 0.010 vs. 0.693 ± 0.010, PMGC-803<0.001; 0.501 ± 0.016 vs. 0.693 ± 0.010, PBGC-823<0.001; 0.525 ± 0.016 vs. 0.693 ± 0.010, PAGS<0.001; 0.268 ± 0.012 vs. 0.693 ± 0.010, PSNU-1<0.001; 0.0261 ± 0.008 vs. 0.693 ± 0.010, PKATO-III<0.001).
Figure 2.
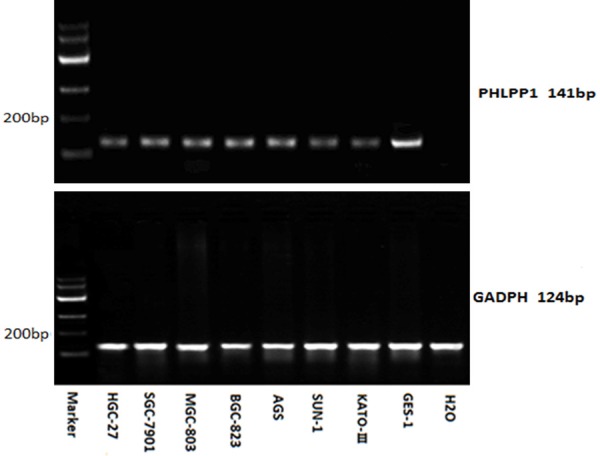
PHLPP1 mRNA expression (RT-PCR) in the GC cell lines and that in the GES-1 cell line. (0.305 ± 0.012 vs. 0.693 ± 0.010, PHGC-27<0.001; 0.399 ± 0.018 vs. 0.693 ± 0.010, PSGC-7901<0.001; 0.407 ± 0.010 vs. 0.693 ± 0.010, PMGC-803<0.001; 0.501 ± 0.016 vs. 0.693 ± 0.010, PBGC-823<0.001; 0.525 ± 0.016 vs. 0.693 ± 0.010, PAGS<0.001; 0.268 ± 0.012 vs. 0.693 ± 0.010, PSNU-1<0.001; 0.0261 ± 0.008 vs. 0.693 ± 0.010, PKATO-III<0.001).
Western blot analysis of PHLPP1 expression in the GC and the GES-1 cell lines
The protein expression of PHLPP1 was also simultaneously detected in the GC cells and the GES-1 cell by WB (Figure 3). The protein expression of PHLPP1 in the GC cell lines was significantly lower than that in the GES-1 cell line (0.398 ± 0.050 vs. 1.217 ± 0.030, PHGC-27<0.001; 0.510 ± 0.046 vs. 1.217 ± 0.030, PSGC-7901<0.001; 0.177 ± 0.012 vs. 1.217 ± 0.030, PSUN-1<0.001; 0.650 ± 0.062vs.1.217 ± 0.030, PBGC-823<0.001; 0.620 ± 0.045 vs. 1.217 ± 0.030, PMGC-803<0.001; 0.712 ± 0.065 vs. 1.217 ± 0.030, PAGS<0.001; 0.376 ± 0.020 vs. 1.217 ± 0.030, PKATO-III<0.001).
Figure 3.
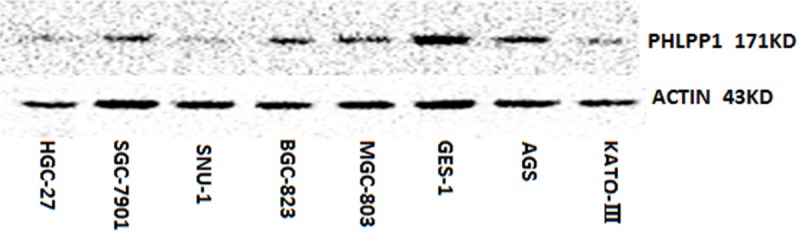
Western Blot analysis for PHLPP1 protein expression in the GC cell lines and that in the GES-1 cell line. (0.398 ± 0.050 vs. 1.217 ± 0.030, PHGC-27<0.001; 0.510 ± 0.046 vs. 1.217 ± 0.030, PSGC-7901<0.001; 0.177 ± 0.012 vs. 1.217 ± 0.030, PSUN-1<0.001; 0.650 ± 0.062vs.1.217 ± 0.030, PBGC-823<0.001; 0.620 ± 0.045 vs. 1.217 ± 0.030, PMGC-803<0.001; 0.712 ± 0.065 vs. 1.217 ± 0.030, PAGS<0.001; 0.376 ± 0.020 vs. 1.217 ± 0.030, PKATO-III<0.001).
Correlation between PHLPP1 expression and clinicopathological characteristics of GC patients
Table 1 showed that the correlations between PHLPP1 expression and clinicopathologic characteristics, including gender, age at surgery, tumor size, tumor location, depth of tumor invasion, N stage, Lauren classification differentiation. However, above-mentioned clinicopathologic characteristics did not showed any statistically significant association with PHLPP1 expression.
Survival analysis
The prognostic value of PHLPP1 expression in GC patients was determined. The Kaplan-Meier analysis showed that the OS of patients with PHLPP1 negative expression was significantly lower than that of patients with PHLPP1 positive expression (P=0.008) (Figure 4). In univariate survival analysis, N stage, tumor size, and PHLPP1 expression were confirmed as prognostic factors for OS, whereas other clinicopathological characteristics, such as gender, age at surgery, tumor location, T stage, Lauren classification and differentiation had no prognostic significance for OS (Table 3). With the Cox regression analysis, N stage (HR=1.476, P<0.001) and PHLPP1 expression (HR=1.763, P=0.005) were identified as the independent predictors of the prognosis of GC patients.
Figure 4.
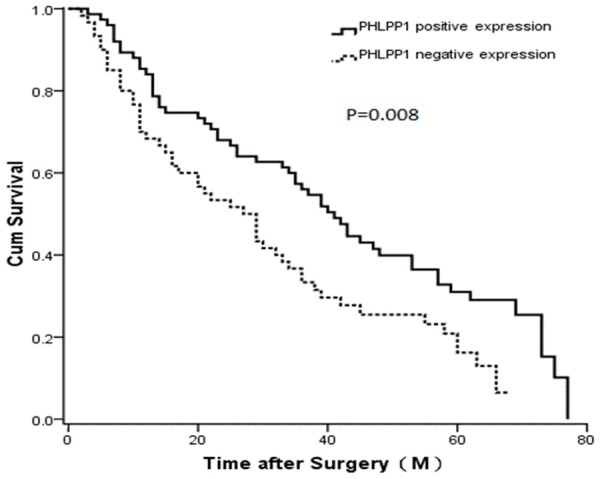
Kaplan-Meier survival curves comparing months of survival in gastric cancer patients are shown for PHLPP1 expression (P=0.008).
Table 3.
Univariate and multivariate Cox proportional Hazard models for OS of GC patients after surgery
| Characteristics | Median OS (mo) | χ2 value | Univariate P value | HR value | Multivariate P value |
|---|---|---|---|---|---|
| Gender | 2.583 | 0.108 | |||
| Male | 36 | ||||
| Female | 29 | ||||
| Age at surgery | 1.729 | 0.189 | |||
| <58 | 36 | ||||
| ≥58 | 33 | ||||
| Tumor size (cm) | 5.721 | 0.017 | 1.290 (1.071-1.509) | 0.244 | |
| <5 | 41 | ||||
| ≥5 | 27 | ||||
| Tumor location | 4.079 | 0.253 | |||
| Upper third | 40 | ||||
| Middle third | 36 | ||||
| Lower third | 36 | ||||
| More than 2/3 stomach | 16 | ||||
| Depth of tumor invasion | 0.017 | 0.991 | |||
| T1 | 0 | ||||
| T2 | 36 | ||||
| T3 | 42 | ||||
| T4 | 34 | ||||
| N stage | 20.388 | <0.001 | 1.476 (1.386-1.566) | <0.001 | |
| N0 | 60 | ||||
| N1 | 48 | ||||
| N2 | 25 | ||||
| N3 | 21 | ||||
| Lauren classification | 3.231 | 0.199 | |||
| Intestinal | 36 | ||||
| Diffuse | 32 | ||||
| Mixed | 33 | ||||
| Differentiation | 1.655 | 0.198 | |||
| Well-differentiated | 36 | ||||
| Poorly-differentiated | 32 | ||||
| PHLPP1 expression | 7.126 | 0.008 | 1.763 (1.501-1.965) | 0.005 | |
| Negative | 27 | ||||
| Positive | 41 |
Discussion
In recent years, many studies reported that loss of PHLPP1 expression was closely associated with tumor progression in several kinds of human cancers [14,16]. Overexpression of PHLPP1 in glioblastoma and colon cancer cells inhibits tumorigenesis in xenografted nude mice, which confirms the role of PHLPP1 as a tumor suppressor [11,16]. PHLPP1 expression is also markedly reduced in several cancer cell lines that have been identified to fulfill the role of a negative regulator for Akt (high preference for Akt2 and 3 isoforms) by direct dephosphorylation [12,14]. Apart from Akt dephosphorylation, PHLPP1 has other substrates such as mammalian sterile 20-like kinase 1 (MST1) [21], protein kinase C (PKC) [22], and ribosomal protein S6 kinase 1 (S6K1) [23], which are identified to be critical for PHLPP1 tumor suppressor function. The results of PHLPP1 mediate dephosphorylation of these substrates can promote apoptosis and suppress proliferation of diverse cancer cell lines. However, some studies reported that PHLPP1-dependent inhibition of cell growth and apoptosis may be cancer-type specific [11,12,16,21].
In this study, the rate of PHLPP1 expression in the primary GC tissues was significantly lower than that in the adjacent non-tumor tissues, which was consistent with previously finding by other authors. [19]. Moreover, we found that both mRNA and protein expression of PHLPP1 in the GC cell lines were significantly lower than those in the GES-1 cell line, respectively. Therefore, we considered that these findings showed that the PHLPP1 should be considered as a potential biomarker for prognostic prediction of GC. However, the mechanisms of low expression of PHLPP1 in GC still remains unclear and need to be further investigated.
Some researchers reported that negative PHLPP1 expression was significantly associated with the poor survival of patients with cancers [14,17]. In this study, we also demonstrated that there was a significantly negative correlation between the PHLPP1 expression and OS of GC patients after the curative gastrectomy. The patients with negative expression PHLPP1 had a shorter median OS than those with positive PHLPP1 expression (P=0.008). Multivariate survival analysis showed that PHLPP1 was an independent predictor of the OS of GC patients, representing PHLPP1 expression of a potentially prognostic predictor for GC. However, our results did not showed any statistically significant association with between PHLPP1 expression and clinicopathological characteristics. We did not know why GC patients with negative expression PHLPP1 have a dismal survival and need to be further investigated from the molecular mechanism.
In a summary, this study indicates that aberrant expression of PHLPP1 was observed in GC and loss of PHLPP1 might identify patients with poorly prognostic outcomes. Our study also showed that PHLPP1 was an independent prognostic factor for patients with GC. Therefore, PHLPP1 may be deemed as a promising prognostic factor for GC.
Acknowledgements
This study was supported in part by grants from the Program of National Natural Science Foundation of China (No. 81572372), the Application Foundation and Advanced Technology Program of Tianjin Municipal Science and Technology Commission (No. 15JCYBJC24800), the Key Program of Tianjin Municipal Science and Technology Commission (No. 13ZCZCSY20300) and the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (No. 2013ZX09303001).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int. 2011;108:698–705. doi: 10.3238/arztebl.2011.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsina M, Landolfi S, Aura C, Caci K, Jimenez J, Prudkin L, Castro S, Moreno D, Navalpotro B, Taberner J, Scaltriti M. Cyclin E amplification/overexpression is associated with poor prognosis in gastric cancer. Ann Oncol. 2015;26:438–9. doi: 10.1093/annonc/mdu535. [DOI] [PubMed] [Google Scholar]
- 4.Di Bartolomeo M, Pietrantonio F, Pellegrinelli A, Martinetti A, Marian L, Daidone MG, Bajetta E, Pelosi G, de Braud F, Floriani I, Miceli R. Osteopontin, E-cadherin, and beta-catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric cancer. 2015 doi: 10.1007/s10120-015-0495-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Zheng G, Chen L, Xiong B. Effect of HER-2/neu over-expression on prognosis in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2011;12:1417–23. [PubMed] [Google Scholar]
- 6.Yildirim M, Kaya V, Demirpence O, Gunduz S, Bozcuk H. Prognostic significance of p53 in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2015;16:327–32. doi: 10.7314/apjcp.2015.16.1.327. [DOI] [PubMed] [Google Scholar]
- 7.Deng J, Liang H, Ying G, Zhang R, Wang B, Yu J, Fan D, Hao X. Methylation of CpG sites in RNF180 DNA promoter prediction poor survival of gastric cancer. Oncotarget. 2014;5:3173–83. doi: 10.18632/oncotarget.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng J, Liang H, Ying G, Dong Q, Zhang L, Yu J, Fan D, Hao X. Clinical significance of the methylated cytosine-phosphate-guanine sites of protocadherin-10 promoter for evaluating the prognosis of gastric cancer. J Am Coll Surg. 2014;219:904–13. doi: 10.1016/j.jamcollsurg.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Deng J, Liang H, Ying G, Li H, Xie X, Yu J, Fan D, Hao X. Methylation of ras association domain protein 10 (RASSF10) promoter negative association with the survival of gastric cancer. Am J Cancer Res. 2014;4:916–23. [PMC free article] [PubMed] [Google Scholar]
- 10.Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19:223–30. doi: 10.1016/j.tem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao TY, Furnari F, Newton AC. PHLPP: A phosphatase that directly dephosphorylates akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Brognard J, Sierecki E, Gao TY, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Stevens PD, Yang H, Gulhati P, Wang W, Evers BM, Gao T. The deubiquitination enzyme USP46 functions as a tumor suppressor by controlling PHLPP-dependent attenuation of Akt signaling in colon cancer. Oncogene. 2013;32:471–8. doi: 10.1038/onc.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitsche C, Edderkaoui M, Moore RM, Eibl G, Kasahara N, Treger J, Grippo PJ, Mayerle J, Lerch MM, Gukovskaya AS. The phosphatase PHLPP1 regulates Akt2, promotes pancreatic cancer cell death, and inhibits tumor formation. Gastroenterology. 2012;142:377–87. e1–5. doi: 10.1053/j.gastro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Pratt CP, Zeeman ME, Schultz N, Taylor BS, O’Neill A, Castillo-Martin M, Nowak DG, Naguib A, Grace DM, Murn J, Navin N, Atwal GS, Sander C, Gerald WL, Cordon-Cardo C, Newton AC, Carver BS, Trotman LC. Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell. 2011;20:173–86. doi: 10.1016/j.ccr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Yu X, Wang J, Li T, Jin T, Lei D, Pan X. Aberrant Expression of PHLPP1 and PHLPP2 Correlates with Poor Prognosis in Patients with Hypopharyngeal Squamous Cell Carcinoma. PLoS One. 2015;10:e0119405. doi: 10.1371/journal.pone.0119405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Shu H, Wang Z, Li G, Cui J, Wu H, Cai S, He W, He Y, Zhan W. Loss expression of PHLPP1 correlates with lymph node metastasis and exhibits a poor prognosis in patients with gastric cancer. J Surg Oncol. 2013;108:427–32. doi: 10.1002/jso.23419. [DOI] [PubMed] [Google Scholar]
- 20.Molina JR, Agarwal NK, Morales FC, Hayashi Y, Aldape KD, Cote G, Georgescu MM. PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene. 2012;31:1264–74. doi: 10.1038/onc.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao M, Wang Y, Xu X, Lu J, Dong Y, Tao W, Stein J, Stein GS, Iglehart JD, Shi Q, Pardee AB. Mst1 is an interacting protein that mediates PHLPPs’ induced apoptosis. Mol Cell. 2010;38:512–23. doi: 10.1016/j.molcel.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283:6300–11. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Stevens PD, Li X, Schmidt MD, Gao T. PHLPP-mediated dephosphorylation of S6K1 inhibits protein translation and cell growth. Mol Cell Biol. 2011;31:4917–27. doi: 10.1128/MCB.05799-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


